Abstract
Receptors that activate the heterotrimeric G protein Gαq are thought to play a role in the development of heart failure. Dysregulation of autophagy occurs in some pathological cardiac conditions including heart failure, but whether Gαq is involved in this process is unknown. We used a cardiomyocyte-specific transgenic mouse model of inducible Gαq activation (termed GαqQ209L) to address this question. After 7 days of Gαq activation, GαqQ209L hearts contained more autophagic vacuoles than wild type (WT) hearts. Increased levels of proteins involved in autophagy, especially p62 and LC3-II, were also seen. LysoTracker staining and western blotting showed that the number and size of lysosomes and lysosomal protein levels were increased in GαqQ209L hearts, indicating enhanced lysosomal degradation activity. Importantly, an autophagic flux assay measuring LC3-II turnover in isolated adult cardiomyocytes indicated that autophagic activity is enhanced in GαqQ209L hearts. GαqQ209L hearts exhibited elevated levels of the autophagy initiation complex, which contains the Class III phosphoinositide 3-kinase Vps34. As a consequence, Vps34 activity and phosphatidylinositol 3-phosphate levels were higher in GαqQ209L hearts than WT hearts, thus accounting for the higher abundance of autophagic vacuoles. These results indicate that an increase in autophagy is an early response to Gαq activation in the heart.
Keywords: Gαq, autophagy, heart failure, electron microscopy, Vps34 activity, p62 aggregation
Introduction
Autophagy is a degradation pathway in which double-membrane vesicles called autophagosomes enclose bulk intracellular materials and send them to lysosomes for degradation (1). Under physiological conditions, a basal level of autophagy maintains cellular homeostasis by removing damaged proteins and organelles and recycling their constituent building blocks for other metabolic uses (2). Autophagy is also an essential mechanism for cells to cope with stress. Nutrient deprivation, hypoxia, oxidative stress, hormones and exercise are all reported to induce autophagy, which allows the cell to produce nutrients and ATP that are necessary for survival (2–4).
Autophagy starts with the assembly of an initiation complex that contains Vps34, p150, Atg14 and Beclin1. Vps34 and p150 are the catalytic and regulatory subunits, respectively, of Class III phosphatidylinositol (PI) 3-kinase. Atg14 binds to both Vps34-p150 and Beclin1 and directs the complex to intracellular membranes where nucleation of autophagosome vesicles occurs (5). Binding to Beclin1 and Atg14 greatly increases the lipid kinase activity of Vps34, which phosphorylates PI at the 3′ hydroxyl position to generate PI 3-phosphate [PI(3)P] at the nucleation site. This leads to recruitment of proteins that participate in two pathways that promote membrane elongation, curvature, and closure into an autophagosome (6). Atg7 acts in both pathways and thus is critical for autophagosome formation. In one of the pathways, LC3-I is lipidated to produce LC3-II, which inserts into the lipid bilayer to facilitate membrane elongation. Recognition and delivery of cargo into the forming autophagosome is mediated by the adaptor protein p62 (7). Autophagosomes then fuse with lysosomes and the contents are degraded by hydrolases.
The use of knockout mice has demonstrated that autophagy is essential for normal cardiac function. Cardiac-specific Vps34 knockout mice developed cardiac hypertrophy and died between 5 and 13 weeks of age (8). Cardiac-specific deletion of Atg5 (a protein that acts in one of the membrane elongation pathways mentioned above) led to mitochondrial dysfunction, development of dilated cardiomyopathy, and early death starting at 6 months of age (9). Moreover, inducible knockout of Atg5 in the adult mouse heart led to left ventricular dilatation and contractile dysfunction, with a disorganized sarcomere structure and mitochondrial misalignment and aggregation (10). These results indicate that a deficiency in autophagy can lead to heart failure.
Accumulating evidence suggests that autophagy is dysregulated in cardiovascular diseases. Increased autophagic activity was reported in the ischemic myocardium (3, 11) and preconditioned heart (3). Another study described increased autophagy as a response to hemodynamic stress in a model of load-induced heart failure (12). It is well established that the heterotrimeric G protein α subunit Gαq mediates heart failure induced by pressure overload. Transgenic expression of Gαq triggered pathological phenotypes (13, 14), and blockade of Gαq signaling prevented pressure overload-induced heart failure (15). Gαq is activated by a number of receptors whose agonists are upregulated in the progression to heart failure, including angiotensin II and catecholamines. It was reported that angiotensin II increases autophagy in the heart (16), and some molecules in the Gαq signaling pathway were shown to have positive or negative effects on autophagy in different studies (17–21). However, direct evidence that Gαq impacts autophagy in the heart has not been reported. In this study, we used our transgenic mouse model of inducible Gαq to address this question (14, 22–24). We observed that Gαq activation in cardiac myocytes leads to increased formation of the autophagy initiation complex and thus stimulates autophagic activity prior to the onset of heart failure.
Methods
Animals
C57BL/6J mice were purchased from the Jackson Laboratory and used as wild type (WT) controls. Generation and characterization of the transgenic mouse models of inducible Gαq activation in the C57BL/6J background (termed GαqQ209L and GαqQ209L-AA) were described previously (14, 22–24). All protocols for animal experiments were approved by the Stony Brook University Animal Care and Use Committee.
Materials
Antibodies were obtained from the following sources: p62 (Abnova H00008878-M01), LC3 (Cell Signaling 2775), Vps34 (Cell Signaling 4263), Beclin1 (Santa Cruz sc-10086), Atg7 (Cell Signaling 2631), Atg14 (a gift from Dr. Zhenyu Yue, Icahn School of Medicine at Mount Sinai), GAPDH (Sigma G8795), Gαq/11 (Santa Cruz sc-392), normal goat IgG (Santa Cruz sc-2028), normal goat serum (Jackson Immuno Research 005-000-121), cathepsin D (Santa Cruz sc-6486), and Alexa Fluor 647-conjugated goat anti-mouse IgG secondary antibody (Invitrogen A21236). The monoclonal antibodies against Lamp-1 (1D4B) and Lamp-2 (ABL-93), developed by J.T. August, were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at the University of Iowa, Department of Biology, Iowa City, IA 52242. Specialty chemicals were obtained from the following sources: blebbistatin (Sigma B0560), bafilomycin A1 (Enzo BML-CM110), protease inhibitor cocktail (Sigma P8340), tamoxifen (Sigma T5648), Medium 199 (Sigma M4530), LysoTracker Red (Invitrogen L7528), laminin (Invitrogen 23017-015), insulin-transferrin-selenium (Gibco 41400), and Liberase TM Research Grade (Roche 05401127001).
Tamoxifen treatment
Tamoxifen was sonicated in autoclaved peanut oil at 10 mg/ml. WT, GαqQ209L or GαqQ209L-AA mice 8 to 12 weeks old were injected with tamoxifen intraperitoneally at 1 mg per day for 3, 7 or 14 days as indicated.
Echocardiography
Mice were anesthetized with 1–2% isoflurane, and M-mode transthoracic echocardiography was performed and fractional shortening was calculated as previously described (25).
Adult mouse cardiomyocyte isolation and culture
Mice were anesthetized under 3% isoflurane, and hearts were removed and mounted on a Langendorff apparatus. Retrograde perfusion was performed by gravity pressure. Hearts were first perfused with MyoBuffer (137 mM NaCl, 5.4 mM KCl, 2 mM MgSO4, 0.33 mM NaH2PO4, 10 mM HEPES, 10 mM taurine, 10 mM glucose, and 100 U-100 μg/ml penicillin-streptomycin, pH 7.4) for 3 min and then with 5 μg/ml Liberase TM in MyoBuffer plus 0.1 mM CaCl2. When the hearts became soft, they were dismounted and perfused manually with Stop Buffer (MyoBuffer plus 1% bovine serum albumin (BSA)) with 0.2 mM CaCl2. The tissue was minced and disrupted in Stop Buffer with 0.2 mM CaCl2 using a Pasteur pipet. The suspension was filtered through 200 μm nylon mesh into a 15 ml centrifuge tube. Cardiomyocytes of good quality quickly settled to the bottom and the supernatant was removed. Cardiomyocytes were washed sequentially with Stop Buffer containing 0.5 mM CaCl2 and 1.0 mM CaCl2, then resuspended in Plating Medium (ACCIT medium (Medium 199, 2 mM L-carnitine, 5 mM creatine, 5 mM taurine, 2 mg/ml BSA, 100 U-100 μg/ml penicillin-streptomycin, 10 μl/ml insulin-transferrin-selenium, 2 mM glutamine, and 25 μM blebbistatin) (26) with 5% fetal bovine serum). Cardiomyocytes were plated onto laminin-coated cell culture dishes and incubated in a 5% CO2 atmosphere at 37°C for 2 h. After incubation, healthy, rod-shaped cardiomyocytes adhered to the dish; dead and unhealthy cells were aspirated and fresh ACCIT medium was added. Treatments were carried out after a 2-h incubation in ACCIT medium.
Transmission electron microscopy (TEM)
Mice were anesthetized under 3% isoflurane, and hearts were quickly removed and retrograde perfused manually with 7 ml MyoBuffer and then 7 ml fixative (2% paraformaldehyde (PFA) and 2.5% glutaraldehyde in phosphate-buffered saline (PBS), pH 7.4). The endocardium was cut into ~1 mm3 cubes in fixative at room temperature and stored overnight in fixative at 4°C. Fixed samples were placed in 2% osmium tetroxide in PBS, pH 7.4, for 1 h, then dehydrated in a graded series of ethanol, and embedded in Embed 812 resin. Ultra-thin sections of 80 nm were placed on formvar-coated copper grids or mesh. Sections were counterstained with uranyl acetate and lead citrate and viewed with an electron microscope (FEI Tecnai12 BioTwinG2) at 80 kV. Images were acquired with a CCD digital camera system (model XR-60; Advanced Microscopy Techniques Corp.). 10–15 pictures were taken from each section using a uniform random sampling method at a magnification of 9300× (27). Images to show detailed structure were taken at a magnification of 30,000× or higher. Organelles were counted using 9300× images and normalized by an area of 156.75 μm2 per image.
Western blotting
Mice were anesthetized under 3% isoflurane and killed by cervical dislocation. Hearts were quickly removed and the atria and aorta were trimmed off. Ventricles were homogenized in General Lysis Buffer (10 mM sodium pyrophosphate, 50 mM HEPES, pH 7.5, 1% Triton X-100, 50 mM NaCl, 50 mM NaF, 5 mM EDTA, pH 8.0, 200 μM sodium orthovanadate, 0.2 mM phenylmethanesulfonyl fluoride (PMSF), and 1 μl/ml protease inhibitor cocktail) using a glass dounce homogenizer on ice. Crude lysates were centrifuged at 13,500 rpm for 20 min at 4°C. Supernatants were collected and protein concentration was quantified using a Bradford assay (Bio-Rad). Proteins were separated by SDS-PAGE and electrophoretically transferred onto PVDF. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline plus 0.1% Tween 20 for 1 h, incubated with primary antibody overnight at 4°C, and incubated with horse radish peroxidase-conjugated secondary antibody for 1–2 h at room temperature. Enhanced chemiluminescence substrate (Thermo Pierce) was then applied to the blot, and signals were captured using a FluorChem E system (ProteinSimple). The density of each band was analyzed using AlphaView software (ProteinSimple).
Cultured cardiomyocytes were lysed in Lysis Buffer B (1% sodium deoxycholate, 1% SDS, 1% Triton X-100, 10 mM Tris, pH 8.0, and 0.14 M NaCl) and sonicated to fragment DNA. Crude lysates were centrifuged at 13,500 rpm for 15 min at 4°C, and the protein concentration of the supernatant was quantified using a BCA assay (Thermo Scientific). Proteins were then analyzed by western blotting.
RNA extraction and real-time quantitative PCR (RT-qPCR)
RNA was isolated from pieces of fresh ventricle weighing 40–50 mg using the RNeasy kit (QIAGEN) following the manufacturer’s protocol. cDNA was made from 1.6 μg of RNA using the iScript cDNA synthesis kit (Bio-Rad), and cDNA made from 40 ng of RNA was added to each qPCR reaction. Reactions were done in triplicate. RT-qPCR was carried out using TaqMan gene expression assays (Applied Biosystems), and gene expression relative to GAPDH was calculated using the ΔΔCq method (28). For each gene, the value obtained for one of the WT animals was assigned as 1, and values obtained for the other WT and GαqQ209L animals were normalized to it. Quality controls were performed following MIQE guidelines (29). Relative standard curves were performed for each TaqMan assay to ensure that PCR efficiency was between 90% and 110% and that the cDNA did not contain factors that inhibit PCR. The TaqMan assays used were: p62, Mm00448091_m1; LC3, Mm00782868_sH; Vps34, Mm00619489_m1; Beclin1, Mm01265461_m1; Atg14, Mm00553733_m1, Atg7, Mm00512209_m1; and p150, Mm00661451_m1.
PI(3)P level
Fresh ventricles were snap frozen in liquid nitrogen and stored at -80°C. The tissue was pulverized using a liquid nitrogen-cooled stainless steel mortar and pestle, and phospholipids were extracted following the protocol in the PI(3)P Mass ELISA Kit (Echelon) with modifications (30). The powder was transferred to a 15 ml glass tube and incubated on ice for 5 min in 7 ml 0.5 M trichloroacetic acid (TCA). The powder was then washed with 5 ml of 5% TCA containing 1 mM EDTA and then with 5 ml water. 8% of the suspension was then removed for the purpose of protein amount normalization. The powder was centrifuged down, sonicated in Lysis Buffer B, and the protein was quantified using the BCA assay. The rest of the powder was pelleted and depleted of neutral lipids by extracting with 6 ml of methanol:chloroform (2:1) for 10 min at room temperature. Acidic lipids were obtained by extracting the tissue with 4.5 ml of methanol:chloroform:HCl (80:40:1) for 15 min at room temperature. PI(3)P in this fraction was then extracted by adding 1.5 ml of chloroform and 2.7 ml of 0.1 M HCl. The lower organic phase was transferred into a 1.5 ml tube, dried under vacuum, and stored at -20°C. PI(3)P was measured using the PI(3)P Mass ELISA Kit following the manufacturer’s protocol. The amount of PI(3)P was normalized to the amount of protein extracted from tissue of an equal mass.
Beclin1 immunoprecipitation (IP) and Vps34 activity assay
Freshly collected ventricles were homogenized on ice in Vps34 Lysis Buffer (20 mM Tris, pH 7.5, 137 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 100 mM NaF, 10 mM sodium pyrophosphate, 1% NP-40, 10% glycerol, 0.1 mM sodium orthovanadate, 0.2 mM PMSF, and 1 μl/ml protease inhibitor cocktail) and centrifuged at 13,500 rpm for 20 min at 4°C. The protein concentration of the supernatants was quantified by Bradford assay. Samples containing 50 μg of protein were used for western blotting, and samples containing 2 mg of protein were used for Beclin1 IP/western blots and Vps34 activity assays. Vps34 was co-precipitated with anti-Beclin1 antibody overnight at 4°C, and the precipitates were pulled down with protein G agarose beads for 1–2 h at 4°C. For Beclin1 IP/western blots, the beads were washed with Vps34 Lysis Buffer, boiled in SDS sample buffer, and subjected to western blotting. For Vps34 assays, the beads were washed 3 times with Vps34 Lysis Buffer and then twice with a buffer containing 50 mM Hepes, pH 7.5, 100 mM NaCl and 1 mM EGTA. 25% of each sample was boiled in SDS sample buffer and subjected to western blotting. The remainder was assayed for Vps34 activity by measuring incorporation of 32P into L-α-phosphatidylinositol as previously described (31). For each heart sample, duplicate IPs were performed and average radioactivity from both Vps34 assays was calculated.
LysoTracker Red staining and confocal microscopy
Cardiomyocytes isolated as described above were plated on laminin-coated glass-bottom dishes (MatTek Corporation). After incubation in ACCIT medium for 2 h, cardiomyocytes were loaded with 67 nM LysoTracker Red for 30 min. Cells were washed twice with ACCIT medium and images were taken using an Olympus Fluoview FV1000 system. The same voltage and magnification were used for each image. Images were exported into TIFF files using FluoView2.0 software (Olympus). The number and area of LysoTracker Red-positive puncta in each cardiomyocyte were analyzed using CellProfiler 2.0 (Broad Institute) (32), and statistical analysis was done using GraphPad Prism 4.0.
Immunofluorescence (IF) microscopy
Cardiomyocytes were isolated as described above, except that Ca2+-free Tyrode Buffer (137.7 mM NaCl, 2.3 mM NaOH, 1 mM MgCl2, 10 mM glucose, 5 mM HEPES, and 5.4 mM KCl) was used instead of MyoBuffer solutions during the Langendorff perfusions, and Ca2+-free KB solution (83 mM KCl, 30 mM K2HPO4, 5 mM MgSO4, 5 mM sodium pyruvate, 5 mM β-hydroxy-butyric acid (sodium salt), 5 mM creatine, 20 mM taurine, 10 mM glucose, 0.5 mM EGTA, and 5 mM HEPES) was used instead of Stop Buffer solutions for the manual perfusion, mincing and washing steps. Cardiomyocytes were plated on laminin-coated glass coverslips. After 45 min at room temperature, the KB solution was aspirated and the cells were fixed using 4% PFA in PBS for 20 min. The cells were then permeablized using 0.1% Triton X-100 in PBS, blocked using 5% normal goat serum in PBS, and incubated with mouse anti-p62 antibody in 5% goat serum in PBST (PBS with 0.1% Tween 20) at 4°C overnight. The samples were then incubated with goat anti-mouse IgG secondary antibody conjugated to Alexa Fluor 647 (1:300) in PBST at room temperature for 1 h. Nuclei were stained with DAPI (1 μg/ml in PBS) for 5 min at room temperature. Coverslips were mounted using Immu-Mount and dried overnight. Images were acquired and analyzed using the same method as for LysoTracker Red staining.
For IF microscopy of tissue sections, hearts were retrograde perfused with Ca2+-free Tyrode Buffer and then with 4% PFA. Tissue was fixed for 24 h at 4°C, and then the PFA was removed by four PBS washes over 3–4 h. The tissue was then soaked in 30% sucrose in PBS overnight, equilibrated in 30% sucrose and OCT (1:1) for 30 min, and then embedded in OCT and stored at -80°C. IF staining of 7 μm cryosections was then performed.
Statistical analysis
Student’s t-test was performed in MS Excel 2013; Mann-Whitney test was performed in GraphPad4.0.
Results
Autophagic protein levels are increased in GαqQ209L hearts
We previously established and characterized a transgenic mouse model of cardiac-specific inducible Gαq. These mice express constitutively active GαqQ209L fused to a modified hormone-binding domain of the estrogen receptor (hbER). Use of the α myosin heavy chain promoter drives expression selectively in cardiac myocytes. The GαqQ209L-hbER protein is inactive under normal conditions but becomes active when the animals (referred to as GαqQ209L mice) are injected with tamoxifen, leading to heart failure within 21–28 days (14).
We first investigated whether autophagic protein levels are changed upon Gαq activation in the heart. After 3 days of tamoxifen injections, p62 and LC3-II protein levels increased moderately (Fig. 1A & Fig. 1B). After 7 days of injections, GαqQ209L hearts consistently exhibited large increases in the protein levels of both p62 and LC3-II (Fig. 1C & Fig. 1D). Furthermore, we found that the levels of other proteins that participate in the autophagic machinery, i.e., Vps34, Beclin1, Atg7 and Atg14, were increased in GαqQ209L hearts after 7 days of tamoxifen injections (Fig. 1C & Fig. 1D), but not after 3 days (Fig. 1A & Fig. 1B). RT-qPCR showed 2-fold and 4-fold upregulation in p62 mRNA after 3 days and 7 days of tamoxifen injections, respectively, which could account for the increased level of p62 protein in GαqQ209L hearts (Fig. 1B & Fig. 1DE). Atg7 mRNA increased slightly after 7 days (Fig. 1E), while LC3, Vps34, Beclin1 and Atg14 mRNA levels were not increased at either time point (data not shown).
Figure 1. Autophagic protein levels in GαqQ209L (QL) hearts.
Mice were injected with tamoxifen for 3 days (A, B & E) or 7 days (C–H). (A & C) Immunoblots of autophagy-related proteins in heart lysates. GAPDH is a loading control. (B & D) Summary graphs of bands in (A) and (C) quantified by densitometry and normalized to GAPDH, respectively. (E) Normalized expression of p62 and Atg7 mRNA levels in ventricular tissue. n=6 mice per group. Data shown in bar graphs are mean ± SE. * signifies p<0.05, ** p<0.01, and ***, p<0.001 by Student’s t-test. (F) Immunofluorescence microscopy of p62 in isolated cardiomyocytes (63x). (G) The number of p62 aggregates quantified using CellProfiler 2.0 in cardiomyocytes isolated from 3 WT and 4 QL mice. Bars, mean values. p<0.0001, Student’s t-test. (H) Images of heart cryosections (40x). Upper panels, differential interference contrast images. Lower panels, immunofluorescence microscopy of p62. Nuclei were counterstained with DAPI.
p62 transcription has been shown to increase in response to a number of cellular stresses (33), as p62 protein plays a critical role in maintaining cellular homeostasis by forming aggregates to sequester harmful proteins such as the negative redox regulator Keap-1 (34). To investigate the subcellular distribution of p62, we performed IF microscopy using isolated cardiomyocytes. The signal was very faint in WT cells, but we found that p62 formed large aggregates throughout the sarcoplasm in GαqQ209L myocytes (Fig. 1F & Fig. 1G). p62 aggregates were also observed in cryosections of GαqQ209L hearts, confirming that they were not an artifact of cardiomyocyte isolation (Fig. 1H). Thus, despite increased autophagy, p62 expression and aggregation are induced, which may be an adaptive response to fight against stress conditions such as elevated levels of reactive oxygen species (ROS) that can be caused by Gαq activation (35, 36).
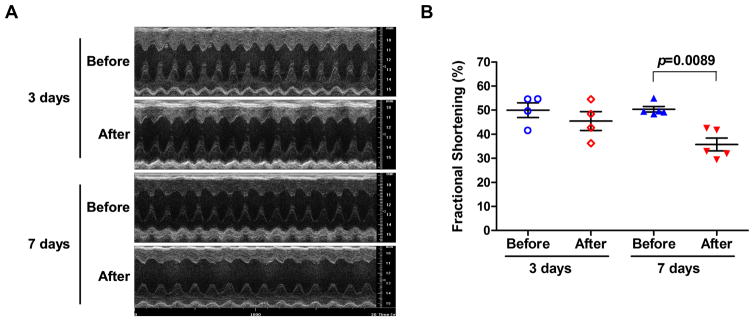
Reduced cardiac contractility in GαqQ209L mice
Previous studies showed that GαqQ209L mice developed relatively mild contractile dysfunction after 7 days of injections and a more pronounced contractile defect after 14 days (14, 24). Heart failure occurred after 21–28 days of tamoxifen treatment (14, 22). WT mice did not develop abnormal cardiac contractility, even after 28 days of tamoxifen injections (14, 24).To ensure that any effects on autophagy we observed are not secondary to heart failure, we reexamined cardiac contractile function in GαqQ209L mice injected for ≤ 7 days. Serial echocardiography showed that fractional shortening tended to be lower but was not significantly changed in GαqQ209L hearts after 3 days of tamoxifen injections (50.0% ± 3.1 before vs. 45.5% ± 3.9 after; Fig. 2). However, consistent with our previous results (14, 22, 24), mice injected for 7 days showed a significant but moderate decrease in fractional shortening (50.4% ± 1.2 before vs. 35.7% ± 2.7 after; Fig. 2). This defect is not as severe as that seen in mice injected for 14 days (fractional shortening = 20.9% ± 1.3) (24). Values for systolic and diastolic left ventricular posterior wall thickness were larger in GαqQ209L mice after 3 days of tamoxifen treatment but not at 7 days (Table 1). Thus, there may be an early and transient phase of hypertrophy in this model that was not detected in previous studies (14, 24). Heart rate and left ventricular end systolic diameter were significantly altered in GαqQ209L hearts after 7 days of tamoxifen injections but not after 3 days (Table 1).
Figure 2. Cardiac contractility of GαqQ209L mice.
Serial M-mode echocardiographic measurements were done on 2 cohorts of GαqQ209L mice before and after tamoxifen injection for 3 days (n=4) or 7 days (n=5). (A) Representative M-mode transthoracic views. (B) Percent fractional shortening calculated from measurements in M-mode. Each point represents the average calculated from multiple cardiac cycles for one animal. Bars show mean values with standard error of the mean. Significance determined by Student’s t-test.
TABLE 1.
Echocardiographic Measurements of GαqQ209L Mice
| 3 days
|
7 days
|
|||
|---|---|---|---|---|
| Tamoxifen injection | Before | After | Before | After |
| Number of mice | 4 | 4 | 5 | 5 |
| LVEDD (mm) | 3.86 ± 0.14 | 3.88 ± 0.12 | 3.65 ± 0.09 | 3.61 ± 0.18 |
| dLVPWT (mm) | 0.50 ± 0.01 | 0.56 ± 0.02* | 0.58 ± 0.05 | 0.66 ± 0.07 |
| LVESD (mm) | 1.94 ± 0.19 | 2.12 ± 0.17 | 1.81 ± 0.07 | 2.31 ± 0.11* |
| sLVPWT (mm) | 1.06 ± 0.03 | 1.18 ± 0.03* | 1.05 ± 0.04 | 0.99 ± 0.11 |
| Heart rate (bpm) | 483 ± 11 | 466 ± 4 | 465 ± 15 | 445 ± 31* |
Serial echocardiographic examinations were done on GαqQ209L mice before and after tamoxifen injection for 3 days or 7 days. LVEDD, left ventricular end diastolic diameter; dLVPWT, diastolic left ventricular posterior wall thickness; LVESD, left ventricular end systolic diameter; sLVPWT, systolic left ventricular posterior wall thickness; bpm, beats per minute. Values are mean ± SE.
significantly different from the Before value in the same group (p<0.05, Student’s t-test).
We decided to perform subsequent experiments on mice injected with tamoxifen for 7 days because their hearts showed a relatively mild contractile defect and the molecular changes in autophagy were consistent.
Abundance of autophagic vacuoles is increased in hearts of GαqQ209L mice
The autophagy process consists of three essential steps: 1) formation of autophagosomes; 2) fusion of autophagosomes with endosomes or lysosomes; and 3) degradation and recycling of the contents. Autophagic vacuoles can be characterized into two categories: early or initial autophagic vacuoles (AVi or autophagosomes), which are surrounded by a double membrane and contain undigested cytoplasmic contents; and late or degradative autophagic vacuoles (AVd), which result from the fusion of autophagosomes with endosomes or lysosomes, have a single membrane, and contain electron-dense digested material (37). We next investigated autophagic vacuole formation in tamoxifen-injected GαqQ209L vs. WT mice using TEM.
Low-magnification TEM images showed that the sarcomere structure of the majority of GαqQ209L cardiomyocytes was abnormal to various degrees: some were slightly misaligned (Fig. 3A(c), upper part of section), while others were severely disrupted (Fig. 3A(b) & Fig. 3A(c), lower part of section). These changes in ultrastructure are consistent with the contractile dysfunction observed by echocardiography.
Figure 3. Autophagic vacuoles are increased in GαqQ209L (QL) hearts.
Mice were injected with tamoxifen for 7 days. Heart sections were imaged by TEM. Error bars in bar graphs show standard error of the mean. (A) (a–d) Representative images from one WT (a) and three QL (b–d) hearts. Cyan arrow head, lipid droplet; white arrow head, lysosome; cyan arrow, deformed mitochondrion; white arrows, AVd. Bars, 2 μm. (e–i) Higher magnification views of QL hearts. (e) and (i) are boxed areas in (b); h is boxed area in (d). *, mitochondrion in autophagic vacuole; yellow arrows, AVi; white arrows, AVd; yellow arrow heads, developing autophagosome membrane. Bars, 500 nm. (B) Quantification of autophagic vacuoles (AVi and AVd). 51 images from 5 sections of 3 WT hearts and 102 images from 8 sections of 4 QL hearts were analyzed. “Profile” stands for the object being counted. (C) Quantification of AVi and AVd in each section. The horizontal axis indicates animal identification. (D) Representative images of a normal mitochondrion in a WT heart and a deformed mitochondrion in a QL heart. Bars, 500 nm. Bar graph, quantification of deformed mitochondria. *, p<0.05, Student’s t-test. (E) Quantification of lipid droplets. **, p<0.01, Student’s t-test. (F) Quantification of lysosomes.
We observed many more autophagic vacuoles in GαqQ209L hearts than in WT hearts (Fig. 3A and Fig. 3B). AVi’s containing mitochondria and glycogen were observed in GαqQ209L hearts (Fig. 3A(e,h)). Fig. 3A(f) shows an AVi containing a mitochondrion fusing with an AVd. One type of very large empty vacuole seemed to have resulted from digestion of several mitochondria (Fig. 3A(c,g)) and thus was categorized as AVd. There were also smaller AVd’s containing digested cytosolic material (Fig. 3A(b,i)). In GαqQ209L hearts, the numbers of AVi and AVd increased proportionally as compared to WT, which indicates that autophagosome maturation is normal in GαqQ209L hearts (Fig. 3B and Fig. 3C). We also observed an increased number of deformed mitochondria in sections of GαqQ209L hearts (Fig. 3A(b) and Fig. 3D), which accounted for 1.7% ± 0.5 of the mitochondria counted. Another striking observation is that while WT hearts contained many lipid droplets, GαqQ209L hearts were deficient in this ultrastructure (Fig. 3A(a) and Fig. 3E). By contrast, the number of lysosomes tended to be larger in GαqQ209L hearts than in WT hearts (Fig. 3A(b) and Fig. 3F).
Lysosomal activity is enhanced in GαqQ209L hearts
The increased number of autophagosomes and LC3-II upregulation observed in GαqQ209L hearts could be due to impaired clearance of autophagosomes, although the presence of digested material in AVd’s and autophagosomes containing mitochondria fusing with AVd’s (Fig. 3A) would seem to be against this possibility. To confirm that clearance of autophagosome cargo is not impaired in GαqQ209L hearts, we next examined lysosomes by using LysoTracker Red to stain the acidic organelles in isolated cardiomyocytes. In WT cells, most of the acidic vesicles were small and clustered around the nuclei (Fig. 4A). In GαqQ209L cardiomyocytes, enlarged vesicles appeared around the nuclei and throughout the length of the cell (Fig. 4A). The number of acidic vesicles per myocyte was larger in GαqQ209L cells than in WT cells (Fig. 4B). Furthermore, the average size of the lysosomes in GαqQ209L myocytes was larger than in WT cells (Fig. 4B). Western blotting showed that levels of two lysosome-associated membrane proteins (Lamp-1 and Lamp-2) that are important for lysosomal function were elevated in GαqQ209L hearts (Fig. 4C). Importantly, in an active lysosome, immature pro-cathepsin D (52 kDa) is proteolytically cleaved to generate an intermediate form (48 kDa), which is further cleaved to yield the two subunits of the mature form (34 kDa and 14 kDa). In GαqQ209L hearts, both the intermediate and mature forms of cathepsin D were increased (Fig. 4C). Collectively, these data indicate that lysosomal activity is enhanced in GαqQ209L hearts.
Figure 4. Lysosomal activity is enhanced in GαqQ209L (QL) hearts and cardiomyocytes.
Mice were injected with tamoxifen for 7 days. (A) Isolated cardiomyocytes loaded with LysoTracker Red were imaged by confocal microscopy (63x magnification). Representative cells are shown. (B) LysoTracker-positive puncta were analyzed using CellProfiler 2.0. Left panel, lysosomes per cell; n=41 for WT and n=64 for QL. Right panel, median lysosome area per cell; n=43 for WT and n=64 for QL. Bars, mean values. p<0.0001, Mann-Whitney test (both panels). (C) Lysosomal proteins in ventricular lysates were detected by western blotting. GAPDH is a loading control.
Autophagic flux is increased in GαqQ209L cardiomyocytes
A standard biochemical method used to show autophagic activity is the autophagic flux assay, in which protein accumulates when bafilomycin A1 is used to block lysosomal degradation. This assay was performed on cardiomyocytes isolated from WT and GαqQ209L hearts. Consistent with our observations in the whole heart (Fig. 1C), cardiomyocytes from GαqQ209L mice showed increased levels of LC3-II as compared to WT in the absence of bafilomycin A1 (Fig. 5). More importantly, when the cells were treated with bafilomycin A1, LC3-II increased in both the WT and GαqQ209L myocytes, indicating that autolysosomes are functional in GαqQ209L cells (Fig. 5). The average increase in LC3-II in QL myocytes (1.7 arbitrary units) was larger than in WT cells (1.1 arbitrary units), indicating an increase in autophagic flux (p=0.0246, Student’s t-test).
Figure 5. Autophagic flux is increased in GαqQ209L (QL) cardiomyocytes.
Cardiomyocytes isolated from mice injected with tamoxifen for 7 days were treated with vehicle or 100 nM bafilomycin A1 (Baf A1) in ACCIT medium for 6 h. Cell lysates were analyzed by western blotting (upper panels). The bar graph shows LC3-II band density normalized to GAPDH. Averages and standard error of the mean from four experiments are shown. n=4 for WT and n=5 for QL. *, p<0.05, Student’s t-test.
Vps34 activity is increased in GαqQ209L hearts
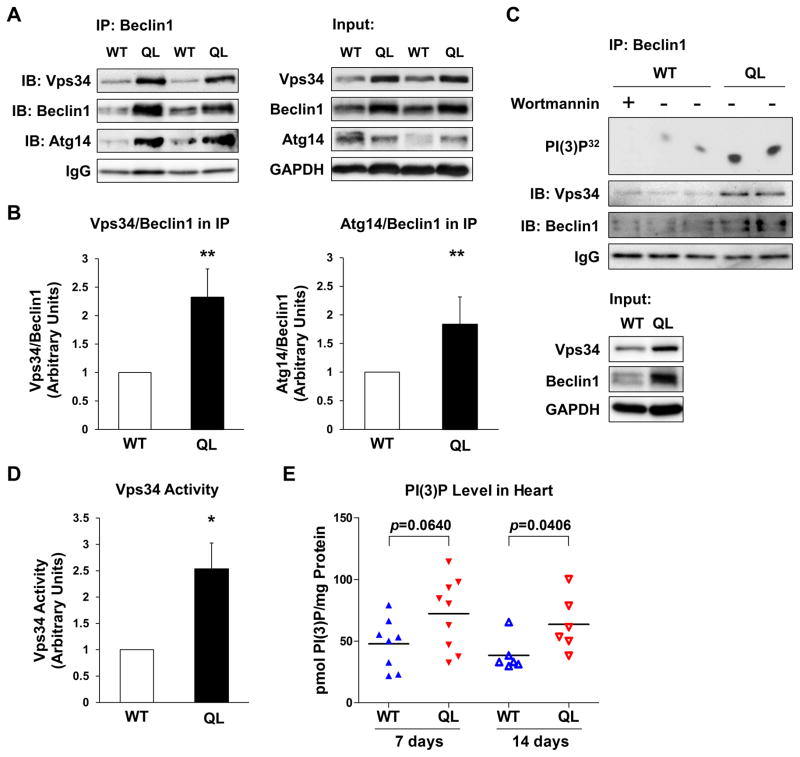
The increase in protein expression of Beclin1, Vps34, and Atg14 in GαqQ209L hearts suggests that the autophagy initiation complex that contains these components is also upregulated. On the other hand, Beclin1 and Vps34 can be found in other functionally distinct complexes (38). To further explore the mechanism of increased autophagic activity in GαqQ209L hearts, we checked the formation of the autophagy-promoting Beclin1/Vps34/Atg14 complex using coimmunoprecipitation. We found that more Vps34 and Atg14 co-immunoprecipitated with Beclin1 from GαqQ209L hearts than from WT hearts (Fig. 6A and Fig. 6B). In vitro lipid kinase assays demonstrated that Vps34 activity was also enhanced in Beclin1 immunoprecipitates from GαqQ209L hearts (Fig. 6C and Fig. 6D). To determine if these in vitro assays correlate with in vivo PI(3)P levels, we isolated phospholipids from hearts and used an ELISA assay to measure PI(3)P. PI(3)P levels were higher in GαqQ209L hearts as compared to WT hearts in mice injected with tamoxifen for 7 days, and the difference was statistically significant after 14 days of injections (Fig. 6E). It should be noted that the relatively severe contractile defect in animals injected for 14 days might cause systemic or intrinsic changes that impact cardiac autophagy independently of the GαqQ209L transgene. These results suggest that activation of GαqQ209L in the heart leads to increases in assembly of the autophagy-initiation Beclin1/Vps34/Atg14 complex, Vps34 activity, and production of PI(3)P, which culminates in autophagosome formation.
Figure 6. Increased formation of the autophagy initiation complex and Vps34 activity in GαqQ209L (QL) hearts.
Mice were injected with tamoxifen for 7 days except where otherwise noted. (A) Left panels, immunoblots (IB) of Beclin1 immunoprecipitates (IP) of heart lysates. Right panels (“Input”), immunoblots of the same lysates without IP. IgG and GAPDH are loading controls. (B) Protein bands from Beclin1 IP/IBs were quantified by densitometry. Normalized Vps34/Beclin1 (n=5) and Atg14/Beclin1 (n=6) ratios (mean ± SE) are shown. **, p<0.01, Student’s t-test. (C) Top panel, Vps34 activity in Beclin1 IPs of heart lysates. An autoradiograph detecting the assay product PI(3)P32 is shown. One assay contained 50 μM wortmannin to inhibit Vps34. Lower panels, immunoblots of the same Beclin1 IPs. Bottom panels (“Input”), immunoblots of the same lysates. (D) PI(3)P32 produced in Vps34 activity assays (n=3 mice per group) was quantified by scintillation counting. Mean ± SE values normalized to the WT mean are shown. *, p<0.05, Student’s t-test. (E) Ventricular PI(3)P levels normalized to protein in mice injected with tamoxifen for 7 or 14 days. Each point represents one heart. Bars, mean values. Significance determined by Mann-Whitney test.
GαqQ209L regulates autophagy through phospholipase Cβ (PLCβ)
In the canonical Gαq signaling pathway, Gαq activates PLCβ, which hydrolyzes PI 4,5-bisphosphate to produce inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds to the IP3 receptor (IP3R) to trigger a release of Ca2+ into the cytosol, and DAG activates conventional and novel isoforms of protein kinase C. A second target of Gαq is the Class 1A phosphoinositide 3-kinase PI3Kα, which is inhibited by binding to GαqQ209L (39, 40). To determine which of these pathways contributes to the increase in autophagy in GαqQ209L hearts, we used a second cardiomyocyte-specific transgenic mouse line (termed GαqQ209L-AA) that expresses a tamoxifen-regulated GαqQ209L mutant in which Arg256 and Thr257 are changed to Ala. This protein cannot activate PLCβ but still inhibits PI3Kα (14, 23). There were minimal changes in left ventricular chamber and wall dimensions with a small increase in end systolic diameter (Table 2). Importantly, GαqQ209L-AA mice did not develop a contractility defect after 7 days of tamoxifen injections (Fig. 7A & Fig. 7B), and also did not show a reduced heart rate (Table 2), consistent with our previous observations at 14 days (24) and 28 days (14). We compared autophagic protein levels in hearts of WT and GαqQ209L-AA mice injected with tamoxifen for 7 days. There was no difference in the levels of p62, LC3-II, Vps34, Beclin1, Atg7 or Atg14 between the two groups, in contrast to the increased protein levels seen in GαqQ209L samples (Fig. 7C). This result suggests that activation of autophagy requires Gαq activation of PLCβ.
TABLE 2.
Echocardiographic Measurements of GαqQ209L-AA Mice
| Tamoxifen injection | Before | After |
|---|---|---|
| Number of mice | 8 | 8 |
| LVEDD (mm) | 3.38 ± 0.20 | 3.73 ± 0.04 |
| dLVPWT (mm) | 0.47 ± 0.01 | 0.53 ± 0.02 |
| LVESD (mm) | 1.78 ± 0.05 | 2.02 ± 0.07* |
| sLVPWT (mm) | 0.89 ± 0.02 | 0.92 ± 0.01 |
| Heart rate (bpm) | 407 ± 8 | 424 ± 4 |
Serial echocardiographic examinations were done on GαqQ209L-AA mice before and after tamoxifen injection for 7 days. LVEDD, left ventricular end diastolic diameter; dLVPWT, diastolic left ventricular posterior wall thickness; LVESD, left ventricular end systolic diameter; sLVPWT, systolic left ventricular posterior wall thickness; bpm, beats per minute. Values are mean ± SE.
significantly different from the Before value in the same group (p<0.05, Student’s t-test).
Figure 7. Upregulation of autophagy is mediated by PLCDβ.
(A & B) Serial M-mode echocardiographic measurements were done on GαqQ209L-AA (AA) mice before and after tamoxifen injection for 7 days. (A) Representative M-mode transthoracic views. (B) Percent fractional shortening. (mean values with standard error of the mean). Each point represents the average calculated from multiple cardiac cycles for one animal. No significant difference between the mean values (bars, with standard error of the mean) by Student’s t-test. (C) Heart lysates from WT, GαqQ209L (QL) and AA mice injected with tamoxifen for 7 days were analyzed by western blotting. GAPDH is a loading control. (D) Bands in (C) were quantified by densitometry and normalized to GAPDH. Data shown are mean ± SE.
Discussion
According to the World Health Organization, cardiovascular diseases are the leading cause of death, contributing to ~31% of deaths worldwide. Heart failure is the major cause of mortality and morbidity in patients with cardiovascular diseases (41). Despite the development of multiple treatments, therapy for heart failure remains unsatisfactory, with a 5-year mortality rate of ~50% (42). Thus, a full understanding of the mechanisms of progression in heart failure is critical if we are to produce more effective methods to prevent and treat this condition.
Following cardiac injury, up-regulation of hormones leads to the activation of G protein-coupled receptors (GPCR) that promote progression to heart failure. Many reports have shown that activation of receptors coupled to Gαi, Gαs or Gαq can modulate autophagy (43). The study of how GPCRs regulate autophagy is complicated by the fact that receptors can couple to multiple types of Gα proteins, and both the Gα and Gβγ dimers that are released upon receptor activation signal to downstream effectors. The transgenic approach we used here studied the effect of activated Gαq in isolation and revealed that Gαq stimulates autophagy in the heart. These results support the hypothesis that GPCR activation of Gαq signaling contributes to the increase in autophagy during the pathophysiological progression to heart failure.
In contrast to our finding that Gαq stimulates autophagy, Zhang et al. used siRNAs to knock down Gαq/11 in HeLa cells and saw increased autophagy, thus concluding that Gαq inhibits this process (44). The discrepancy between our finding and those of Zhang et al. (44) may be due to cell type-specific differences.
An increase in cardiac autophagy has been observed in other models of cardiac injury and heart failure (3, 11, 12, 45, 46). Our GαqQ209L mice exhibit relatively mild contractile dysfunction after 7 days of tamoxifen injections that develops into heart failure with 2–3 weeks of further treatment (14, 22). We found that autophagy was significantly increased in mice treated for 7 days, well before the onset of heart failure. The increased levels of p62 and LC3-II seen after 3 days of GαqQ209L activation, when contractile dysfunction was not detected, suggest that the increase in autophagy may be a direct result of Gαq signaling. However, we cannot rule out the possibility that the increase in autophagy is a secondary response to cellular injury. Cardiac contractility in GαqQ209L mice injected with tamoxifen for 7 days is reduced because of a disorganized T-tubule network that causes abnormal Ca2+ handling (24), raising the possibility that an increase in autophagy affects T-tubule remodeling.
Our results using the GαqQ209L-AA mouse suggest that Gαq stimulates autophagy through the PLCβ pathway. Studies of molecules in the canonical Gαq signaling pathway have reported mixed effects on autophagy. In non-cardiac cells, elevation of intracellular IP3 decreased autophagic flux by an unknown mechanism, while the IP3R was shown to block autophagy by binding to and sequestering Beclin1 in a complex that cannot initiate autophagosome formation (19). Similar observations were made in neonatal rat cardiomyocytes (21). Increased cytosolic Ca2+ concentration has been reported to induce or inhibit autophagy, depending on cell type (17, 18, 20). It is not clear how the increases in IP3 and the changes in Ca2+ handling that occur in GαqQ209L hearts contribute to the increase in autophagy seen here. Gαq may also induce mitochondrial oxidative stress (47). Although the number of damaged mitochondria in GαqQ209L hearts was small, we cannot rule out the possibility that damaged mitochondria generate oxidative stress to induce autophagy. Due to the multiple effects that are initiated by activation of Gαq signaling, determining which signaling pathway downstream of PLCβ is the dominant driver of autophagy will need further investigation.
We found that GαqQ209L activation increased the level of autophagy-related proteins and the Vps34-Beclin1-Atg14 complex. Several studies have shown that the Vps34-p150-Beclin1-Atg14 interaction stabilizes each component: knockdown of Vps34, Vps15 (the yeast homolog of p150), Beclin1/Atg6 (the Beclin1 homolog in yeast), or Atg14 resulted in decreased levels of other proteins in the complex (48–50). It is possible that release of sequestered Beclin1 from
non-autophagic complexes (19) upon activation of GαqQ209L permits the formation of new autophagy initiation complexes. Stabilization of the proteins in the new Vps34-Beclin1-Atg14 complexes would explain why we observed an increase in protein but not mRNA levels in the GαqQ209L heart. In addition to the autophagy initiation complex, we observed a small increase in Atg7 transcription in GαqQ209L hearts after injection of tamoxifen for 7 days. Other studies have described a stimulatory effect of Gαq on Atg7 transcription. Mining a dataset deposited to Gene Expression Omnibus, we found that uveal melanoma cell lines harboring a constitutively active Gαq mutant had a 2.1-fold higher level of Atg7 expression than WT cell lines (51). In the mouse heart, Atg7 mRNA levels increased 2.3-fold after four weeks of angiotensin II infusion (which activates the Gαq-coupled AT1 receptor) (52). Moreover, we observed increased protein expression of Lamp-2 and increased cathepsin D proteolytic cleavage. These data suggest that Gαq enhances the expression of key proteins in organelles at every level of the autophagy pathway.
Very interestingly, we observed both increased p62 mRNA and protein levels in GαqQ209L hearts. As an important mediator and substrate of autophagy, p62 is degraded upon autophagy activation in most cell systems. However, other studies have shown that the p62 protein level in the heart increases in response to autophagic stimuli such as starvation (53). Another study described upregulation of p62 expression and its localization in aggregates in two mouse models of cardiomyopathy caused by transgenic expression of misfolded proteins (54). As far as we know, this is the first report of a cell signaling pathway that upregulates p62 mRNA expression in the heart. A major function of p62 is to self-oligomerize and form protein aggregates to sequester harmful or toxic proteins such as Keap-1, a negative regulator of the master antioxidant protein Nrf2 (55). p62 aggregation plays a critical role in promoting cellular antioxidant responses (34). It is possible that p62 upregulation and aggregation function as a response mechanism to alleviate the ROS stress induced by pressure overload or Gαq activation (35, 36).
In summary, it is well accepted that sustained activation of Gαq signaling contributes to cardiac remodeling that leads to heart failure. Using our conditional GαqQ209L mouse model, we previously elucidated the contractile and electrophysiological abnormalities caused by hyperactivation of Gαq that result in dilated cardiomyopathy and heart failure. The present study demonstrates that Gαq/PLCβ signaling initiates molecular changes in autophagy that occur well before the onset of heart failure. We speculate that the upregulation in autophagic flux observed in cardiomyocytes of our GαqQ209L mice is a compensatory response to the increased demand for protein and organelle recycling necessary for Gαq-induced cardiac remodeling. It is possible that when this compensatory mechanism is overwhelmed by continued Gαq signaling, the inability to remove potentially toxic protein accumulations such as p62, accentuates the progression to heart failure. It is known that the loss of critical autophagy regulators such as Atg5 or Vps34 by gene deletion results in heart failure. Future studies that allow fine-tuning of the level of autophagic flux will greatly improve our understanding of this critical cellular process in the development and progression of this deadly cardiac disease.
Conclusions
Activation of GαqQ209L in the heart causes increased formation of autophagosomes and lysosomes, resulting in increased autophagic flux. We speculate that similar processes are triggered by chronic exposure to angiotensin II, catecholamines and other hormones whose blood concentrations are elevated in hypertension and heart failure and that activate Gαq-coupled receptors. Clinical studies are ongoing to pharmacologically activate autophagy for treatment of neurodegenerative diseases (20, 56). Future studies that investigate the consequences of manipulating autophagy in heart failure may lead to novel therapeutic strategies to treat this deadly disease.
Acknowledgments
Supported by grants from the Department of Veterans Affairs Merit Review Program (BX002263 to R.Z.L.) and the National Institutes of Health (DK108989 to R.Z.L. and GM97355 to W.X.Z.).
We thank the Transmission Electron Microscopy Facility in the Central Microscopy Imaging Center at Stony Brook University, Stony Brook, New York 11794 for their contribution towards the TEM preparation. We thank Dr. Barbara Rosati for guidance on real-time qPCR.
Footnotes
Conflict of Interest Disclosure: none
References
- 1.Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol. 2008;3:427–55. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- 2.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010 Dec 3;330(6009):1344–8. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Essick EE, Sam F. Oxidative stress and autophagy in cardiac disease, neurological disorders, aging and cancer. Oxid Med Cell Longev. 2010 May-Jun;3(3):168–77. doi: 10.4161/oxim.3.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012 Jan 26;481(7382):511–5. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obara K, Ohsumi Y. Atg14: a key player in orchestrating autophagy. Int J Cell Biol. 2011;2011:713435. doi: 10.1155/2011/713435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatica D, Chiong M, Lavandero S, Klionsky DJ. Molecular mechanisms of autophagy in the cardiovascular system. Circ Res. 2015 Jan 30;116(3):456–67. doi: 10.1161/CIRCRESAHA.114.303788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007 Aug 17;282(33):24131–45. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 8.Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, Zong WX. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A. 2012 Feb 7;109(6):2003–8. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Takuji Shirasawa TS, Mizushima N, Otsu K. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010 Jul;6(5):600–6. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 10.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007 May;13(5):619–24. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 11.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005 Sep 27;102(39):13807–12. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007 Jul;117(7):1782–93. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW., 2nd Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997 Jul 22;94(15):8121–6. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan G, Jiang YP, Lu Z, Martin DW, Kelly DJ, Zuckerman JM, Ballou LM, Cohen IS, Lin RZ. A transgenic mouse model of heart failure using inducible Gαq. J Biol Chem. 2005 Dec 2;280(48):40337–46. doi: 10.1074/jbc.M506810200. [DOI] [PubMed] [Google Scholar]
- 15.Wettschureck N, Rutten H, Zywietz A, Gehring D, Wilkie TM, Chen J, Chien KR, Offermanns S. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Gαq/Gα11 in cardiomyocytes. Nat Med. 2001 Nov;7(11):1236–40. doi: 10.1038/nm1101-1236. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Ma B, Han X. The role of autophagy in angiotensin II induced pathological cardiac hypertrophy. J Mol Endocrinol. 2016 Sep 12; doi: 10.1530/JME-16-0086. [DOI] [PubMed] [Google Scholar]
- 17.Harr MW, McColl KS, Zhong F, Molitoris JK, Distelhorst CW. Glucocorticoids downregulate Fyn and inhibit IP3-mediated calcium signaling to promote autophagy in T lymphocytes. Autophagy. 2010 Oct;6(7):912–21. doi: 10.4161/auto.6.7.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law BY, Wang M, Ma DL, Al-Mousa F, Michelangeli F, Cheng SH, Ng MH, To KF, Mok AY, Ko RY, Lam SK, Chen F, Che CM, Chiu P, Ko BC. Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis. Mol Cancer Ther. 2010 Mar;9(3):718–30. doi: 10.1158/1535-7163.MCT-09-0700. [DOI] [PubMed] [Google Scholar]
- 19.Vicencio JM, Ortiz C, Criollo A, Jones AW, Kepp O, Galluzzi L, Joza N, Vitale I, Morselli E, Tailler M, Castedo M, Maiuri MC, Molgo J, Szabadkai G, Lavandero S, Kroemer G. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 2009 Jul;16(7):1006–17. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- 20.Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J Biol Chem. 2010 Mar 19;285(12):9100–13. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong A, Grubb DR, Cooley N, Luo J, Woodcock EA. Regulation of autophagy in cardiomyocytes by Ins(1,4,5)P3 and IP3-receptors. J Mol Cell Cardiol. 2013 Jan;54:19–24. doi: 10.1016/j.yjmcc.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Jiang YP, Ballou LM, Lu Z, Li W, Kelly DJ, Cohen IS, Lin RZ. Reversible heart failure in Gαq transgenic mice. J Biol Chem. 2006 Oct 6;281(40):29988–92. doi: 10.1074/jbc.M604699200. [DOI] [PubMed] [Google Scholar]
- 23.Lu Z, Jiang YP, Ballou LM, Cohen IS, Lin RZ. Gαq inhibits cardiac L-type Ca2+ channels through phosphatidylinositol 3-kinase. J Biol Chem. 2005 Dec 2;280(48):40347–54. doi: 10.1074/jbc.M508441200. [DOI] [PubMed] [Google Scholar]
- 24.Wu CY, Chen B, Jiang YP, Jia Z, Martin DW, Liu S, Entcheva E, Song LS, Lin RZ. Calpain-dependent cleavage of junctophilin-2 and T-tubule remodeling in a mouse model of reversible heart failure. J Am Heart Assoc. 2014 Jun;3(3):e000527. doi: 10.1161/JAHA.113.000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Z, Jiang YP, Xu XH, Ballou LM, Cohen IS, Lin RZ. Decreased L-type Ca2+ current in cardiac myocytes of type 1 diabetic Akita mice due to reduced phosphatidylinositol 3-kinase signaling. Diabetes. 2007 Nov;56(11):2780–9. doi: 10.2337/db06-1629. [DOI] [PubMed] [Google Scholar]
- 26.Ellingsen O, Davidoff AJ, Prasad SK, Berger HJ, Springhorn JP, Marsh JD, Kelly RA, Smith TW. Adult rat ventricular myocytes cultured in defined medium: phenotype and electromechanical function. Am J Physiol. 1993 Aug;265(2 Pt 2):H747–54. doi: 10.1152/ajpheart.1993.265.2.H747. [DOI] [PubMed] [Google Scholar]
- 27.Ylä-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL. Chapter 10 Monitoring Autophagy by Electron Microscopy in Mammalian Cells. Methods in Enzymology. 2009;452:143–64. doi: 10.1016/S0076-6879(08)03610-0. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001 Dec;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009 Apr;55(4):611–22. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 30.Chilvers ER, Batty IH, Challiss RA, Barnes PJ, Nahorski SR. Determination of mass changes in phosphatidylinositol 4,5-bisphosphate and evidence for agonist-stimulated metabolism of inositol 1,4,5-trisphosphate in airway smooth muscle. Biochem J. 1991 Apr 15;275(Pt 2):373–9. doi: 10.1042/bj2750373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dou Z, Chattopadhyay M, Pan JA, Guerriero JL, Jiang YP, Ballou LM, Yue Z, Lin RZ, Zong WX. The class IA phosphatidylinositol 3-kinase p110β subunit is a positive regulator of autophagy. J Cell Biol. 2010 Nov 15;191(4):827–43. doi: 10.1083/jcb.201006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamentsky L, Jones TR, Fraser A, Bray MA, Logan DJ, Madden KL, Ljosa V, Rueden C, Eliceiri KW, Carpenter AE. Improved structure, function and compatibility for CellProfiler: modular high-throughput image analysis software. Bioinformatics. 2011 Apr 15;27(8):1179–80. doi: 10.1093/bioinformatics/btr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010 Jul 16;285(29):22576–91. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010 Mar;12(3):213–23. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 35.Date MO, Morita T, Yamashita N, Nishida K, Yamaguchi O, Higuchi Y, Hirotani S, Matsumura Y, Hori M, Tada M, Otsu K. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J Am Coll Cardiol. 2002 Mar 6;39(5):907–12. doi: 10.1016/s0735-1097(01)01826-5. [DOI] [PubMed] [Google Scholar]
- 36.Satoh M, Matter CM, Ogita H, Takeshita K, Wang CY, Dorn GW, 2nd, Liao JK. Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation. 2007 Jun 26;115(25):3197–204. doi: 10.1161/CIRCULATIONAHA.106.657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eskelinen EL. Maturation of autophagic vacuoles in Mammalian cells. Autophagy. 2005 Apr;1(1):1–10. doi: 10.4161/auto.1.1.1270. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan KL. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013 Jan 17;152(1–2):290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ballou LM, Chattopadhyay M, Li Y, Scarlata S, Lin RZ. Gαq binds to p110α/p85α phosphoinositide 3-kinase and displaces Ras. Biochem J. 2006 Mar 15;394(Pt 3):557–62. doi: 10.1042/BJ20051493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballou LM, Lin HY, Fan G, Jiang YP, Lin RZ. Activated Gαq inhibits p110α phosphatidylinositol 3-kinase and Akt. J Biol Chem. 2003 Jun 27;278(26):23472–9. doi: 10.1074/jbc.M212232200. [DOI] [PubMed] [Google Scholar]
- 41.Mendis S, Puska P, Norrving B. [Accessed October 6, 2015];Global Atlas on cardiovascular disease prevention and control - Policies, strategies and interventions. 2011 Available at: http://www.who.int/cardiovascular_diseases/publications/atlas_cvd/en/
- 42.Taylor CJ, Roalfe AK, Iles R, Hobbs FD. Ten-year prognosis of heart failure in the community: follow-up data from the Echocardiographic Heart of England Screening (ECHOES) study. Eur J Heart Fail. 2012 Feb;14(2):176–84. doi: 10.1093/eurjhf/hfr170. [DOI] [PubMed] [Google Scholar]
- 43.Wauson EM, Dbouk HA, Ghosh AB, Cobb MH. G protein-coupled receptors and the regulation of autophagy. Trends Endocrinol Metab. 2014 May;25(5):274–82. doi: 10.1016/j.tem.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang T, Dong K, Liang W, Xu D, Xia H, Geng J, Najafov A, Liu M, Li Y, Han X, Xiao J, Jin Z, Peng T, Gao Y, Cai Y, Qi C, Zhang Q, Sun A, Lipinski M, Zhu H, Xiong Y, Pandolfi PP, Li H, Yu Q, Yuan J. G-protein-coupled receptors regulate autophagy by ZBTB16-mediated ubiquitination and proteasomal degradation of Atg14L. Elife. 2015;4:e06734. doi: 10.7554/eLife.06734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fidzianska A, Bilinska ZT, Walczak E, Witkowski A, Chojnowska L. Autophagy in transition from hypertrophic cardiomyopathy to heart failure. J Electron Microsc (Tokyo) 2010;59(2):181–3. doi: 10.1093/jmicro/dfp048. [DOI] [PubMed] [Google Scholar]
- 46.Tannous P, Zhu H, Nemchenko A, Berry JM, Johnstone JL, Shelton JM, Miller FJ, Jr, Rothermel BA, Hill JA. Intracellular protein aggregation is a proximal trigger of cardiomyocyte autophagy. Circulation. 2008 Jun 17;117(24):3070–8. doi: 10.1161/CIRCULATIONAHA.107.763870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Gαq overexpression-induced heart failure. Circ Res. 2011 Apr 1;108(7):837–46. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fogel AI, Dlouhy BJ, Wang C, Ryu SW, Neutzner A, Hasson SA, Sideris DP, Abeliovich H, Youle RJ. Role of membrane association and Atg14-dependent phosphorylation in beclin-1-mediated autophagy. Mol Cell Biol. 2013 Sep;33(18):3675–88. doi: 10.1128/MCB.00079-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol. 2001 Feb 5;152(3):519–30. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thoresen SB, Pedersen NM, Liestol K, Stenmark H. A phosphatidylinositol 3-kinase class III sub-complex containing VPS15, VPS34, Beclin 1, UVRAG and BIF-1 regulates cytokinesis and degradative endocytic traffic. Exp Cell Res. 2010 Dec 10;316(20):3368–78. doi: 10.1016/j.yexcr.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Ambrosini G, Pratilas CA, Qin LX, Tadi M, Surriga O, Carvajal RD, Schwartz GK. Identification of unique MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance. Clin Cancer Res. 2012 Jul 1;18(13):3552–61. doi: 10.1158/1078-0432.CCR-11-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang BY, Hu C, Ryu S, Khan JA, Biancolella M, Prayaga S, Seung KB, Novelli G, Mehta P, Mehta JL. Genomics of cardiac remodeling in angiotensin II-treated wild-type and LOX-1-deficient mice. Physiol Genomics. 2010 Jun;42(1):42–54. doi: 10.1152/physiolgenomics.00009.2010. [DOI] [PubMed] [Google Scholar]
- 53.Reichelt ME, Mellor KM, Curl CL, Stapleton D, Delbridge LM. Myocardial glycophagy - a specific glycogen handling response to metabolic stress is accentuated in the female heart. J Mol Cell Cardiol. 2013 Dec;65:67–75. doi: 10.1016/j.yjmcc.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Q, Su H, Ranek MJ, Wang X. Autophagy and p62 in cardiac proteinopathy. Circ Res. 2011 Jul 22;109(3):296–308. doi: 10.1161/CIRCRESAHA.111.244707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004 Dec;24(24):10941–53. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, O'Kane CJ, Floto RA, Rubinsztein DC. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008 May;4(5):295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]