Abstract
Introduction. Staphylococcus pseudintermedius, an opportunistic pathogen of dogs and cats, is rarely reported to cause infection in humans. Here, we describe a case of severe skin infection caused by S. pseudintermedius, in a 47-year-old male, a dog owner; to the best of our knowledge, this is the first such case reported from Scotland.
Case presentation. The patient presented with a short history of a severe ecthyma-like lesion on his forehead, with smaller lesions on his abdomen and legs. Bacterial culture revealed Clostridium perfringens, thought to be colonizing the wound, and a Staphylococcus species, identified as S. pseudintermedius by matrix-assisted laser desorption/ionization-time of flight MS and confirmed by molecular methods using a PCR-RFLP approach. The patient was treated with flucloxacillin, penicillin V and Fucibet cream, and recovered fully. Zoonotic infection was considered likely; however, screening swabs from his dogs grew S. pseudintermedius of a different clonal type. Both patient and dog strains carried Staphylococcus intermedius exfoliative toxin and leucocidin I, closely related to Panton–Valentine leucocidin, possibly contributing to the severity of the infection. S pseudintermedius, although coagulase positive, is normally negative by rapid slide clumping and latex agglutination tests routinely used to identify Staphylococcus aureus. Hence, S. pseudintermedius may easily be misidentified as a coagulase-negative staphylococcus and considered insignificant.
Conclusion. This is, to the best of our knowledge, the first reported case of a human S. pseudintermedius infection in Scotland. Zoonotic transmission of S. pseudintermedius between pets and owners has been shown. However, in this case zoonosis could not be confirmed.
Keywords: zoonosis, Staphylococcus pseudintermedius, Siberian husky, ecthyma-like, flucloxacillin
Abbreviations
CNS, coagulase-negative staphylococcus; MALDI-TOF, matrix-assisted laser desorption/ionization-time of flight; PVL, Panton–Valentine leukocidin; ST, sequence type.
Introduction
Staphylococcus pseudintermedius belongs to a group of three closely related staphylococcal species (S. pseudintermedius, Staphylococcus intermedius and Staphylococcus delphini) known as the S. intermedius group [1]. S. pseudintermedius is a member of the normal flora, colonizing up to 90 %, of dogs, and is also a major opportunistic pathogen responsible for a wide range of infections including pyoderma, otitis, wound and urinary-tract infections in dogs, cats and horses, but has only rarely been isolated from humans [2]. However, there have been an increasing number of case reports documenting serious invasive infections caused by S. pseudintermedius in humans, including infected dog bite wounds, bacteraemia, pneumonia, brain abscesses and septic arthritis, most of which have been related to dog exposure [3]. While S. pseudintermedius is a coagulase-positive organism, the characteristics of its coagulase differs from Staphylococcus aureus coagulase [4]. The majority of medical microbiology departments use rapid latex slide agglutination tests to screen for S. aureus. These tests differentiate S. aureus from other staphylococcal species by detecting clumping factor and cell bound protein A. As these two factors are rarely present in S. pseudintermedius, up to 90 % of isolates tested will give a negative result. Therefore, the true incidence of human infection is unknown, but is likely to be higher than that reported. This is due to the pathogen being frequently misidentified, either as a coagulase-negative staphylococcus (CNS) and regarded as a contaminant, or as S. aureus [5]. Since the implementation of the matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) MS (Bruker), rapid and accurate identification of S. pseudintermedius is now possible, whereas previously it may have been misidentified as S. aureus or discarded based on a negative latex agglutination test [6].
Similar to S. aureus, S. pseudintermedius produces numerous virulence factors including toxins, such as haemolysins, exfoliative toxins, coagulase, thermonuclease, clumping factor and protein A, enterotoxins and a leucotoxin I (Luk-I), which is very similar to Panton–Valentine leucocidin (PVL) from S. aureus. However, as many of these virulence factors have yet to be characterized in detail, knowledge of the pathogenesis of S. pseudintermedius is very limited [7]. This case report details the clinical features, diagnosis and the phenotypic and molecular characterization of S. pseudintermedius isolates from an infected human and his colonized dogs to determine whether zoonotic transmission had occurred.
Case Report
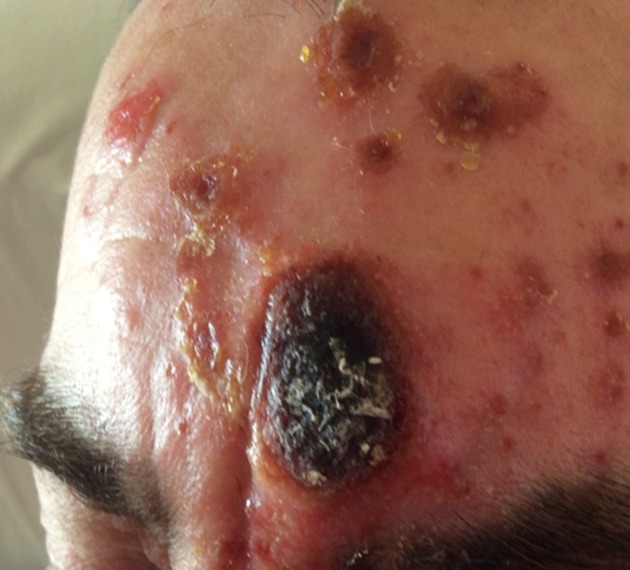
A 47-year-old sawmill worker presented with a 1 week history of an ecthyma-like, painful, enlarging crusting lesion on his forehead, with several smaller satellite lesions (Fig. 1). Initially, a small red mark had appeared over the glabellar region of his forehead, which rapidly developed into the large eschar. There was no history of trauma to the area. He then developed very itchy satellite lesions on the forehead, abdomen and limbs. He had two reactive lymph nodes palpable in the left cervical chain, but was neither systemically unwell nor febrile. He reported a similar lesion on his forehead two years previously, which had responded rapidly to oral flucloxacillin. A swab from the main lesion grew Clostridium perfringens and a CNS identified by MALDI-TOF MS as S. pseudintermedius. No fungi were isolated from the lesion. The patient’s anterior nares were screened for colonization with S. pseudintermedius, which were negative.
Fig. 1.
Appearance of the skin lesion at presentation.
The isolation of C. perfringens from the lesion was concerning and its significance uncertain. There were no features of clostridial soft tissue infection, no loss of tissue viability around the lesion and no evidence of skin necrosis, no bullae, blisters, bruising nor numbness, and no systemic toxicity. It was concluded that this organism was colonizing the lesion and not contributing to the infection.
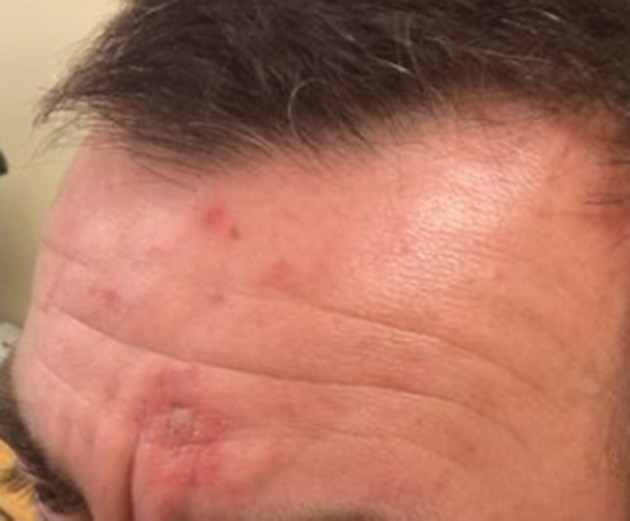
The patient was treated with oral penicillin V, 500 mg, and flucloxacillin, 500 mg, four times daily for 2 weeks. When these antibiotics were started, the S. pseudintermedius had not been fully identified and the significance of the C. perfringens was yet to be determined. It was, therefore, felt reasonable to include penicillin V with flucloxacillin. Fucibet cream was included to reduce inflammation, irritation and wound discharge. This was applied topically twice daily for several weeks, in conjunction with warm bathing with water/white vinegar twice daily until the eschar separated. The patient was given oral chlorphenamine, 4 mg, as required, for the itching. Five weeks later, his skin lesion had almost completely resolved (Fig. 2) with some residual areas of post-inflammatory hyperpigmentation. He was given a rescue course of oral flucloxacillin, 500 mg, four times daily, to use in the event of a further infection.
Fig. 2.
Appearance at 5 weeks post-treatment.
Three pet Siberian husky dogs resided with the male owner. The dogs were healthy, well cared for and had no skin lesions nor infection. The patient had no other close contact with dogs. Other members of the family had no current or past skin problems or infections.
Investigations
Admission blood tests revealed a white cell count of 12.8×109 cells l−1, CRP <3 mgl−1, and normal urea and electrolytes, liver function tests, glucose, IgG, IgA and IgM immunoglobulins, and a negative human immunodeficiency virus antibody test. The patient's wound swabs were cultured on Columbia blood and Sabouraud agar incubated in air and neomycin blood agar incubated anaerobically, for 48 h.
Each dog was screened for S. pseudintermedius colonization. Swabs were taken from the nostril, mouth, ear, forelimb and hind limb axillae, between the toes, and the anal margin. Swabs taken from the dogs were positive for S. pseudintermedius, identified by MALDI-TOF MS. Species identification was confirmed by PCR and PCR-RFLP as described elsewhere [8, 9].
PFGE and multi-locus sequence typing (MLST) was performed as described elsewhere [10, 11]. The PFGE analysis, using Bionumerics software version 7.6.1 (Applied Mathematics), with a similarity cut off of 80 % showed that the patient and dog isolates were unrelated (data not shown). This was confirmed by MLST, where the patient and dog isolates belonged to novel unrelated sequence types (STs) (ST673 allelic profile 4-72-3-1-8-4-2 and ST686 allelic profile 5-7-2-23-8-1-1, respectively).
Genes encoding toxins PVL, toxic shock syndrome toxin 1 and exfoliative toxins a, b were not detected by PCR [12]. However, both the patient and dog strains carried S. intermedius exfoliative toxin (SIET) and leucocidin I (lukF/S-PV), which is closely related to PVL [13, 14]. Antibiotic-susceptibility testing performed with the Vitek 2 system revealed the patient’s strain to be resistant to penicillin, confirmed by the presence of the blaZ gene [15], and susceptible to oxacillin and fusidic acid. The canine strains were susceptible to penicillin, confirmed by the absence of the blaZ gene, oxacillin and fusidic acid.
Outcome and follow-up
The patient completed his treatment and recovered fully.
Discussion
There have been relatively few studies on the rate of S. pseudintermedius colonization in humans and in those that have been performed low levels of colonization have been reported. In a study of humans and their pet dogs in Ontario, Canada, S. pseudintermedius was isolated from 4.1 % of healthy subjects [16], and a study from Japan, investigating rates of staphylococci in the oral cavities of healthy adults, found members of the S. intermedius group in 8.9 % of subjects [17].
S. pseudintermedius is a coagulase-positive organism, which is normally negative by rapid slide clumping test and by commercial latex agglutination tests that detect clumping factor, protein A and/or surface antigens in S. aureus. As a consequence, the risk that S. pseudintermedius is misidentified as a CNS is high in human diagnostic laboratories, as these commercial tests are routinely used to screen for rapid discrimination of S. aureus and CNS [7].
Clinical details concerning the nature of an infection are, of course, very significant for determining optimal microbiological laboratory processing of specimens. In this case, clinical information on the request form was lacking, and had it not been for the presence of a potentially significant pathogen, C. perfringens, and the severity of the lesion, the unknown staphylococcus would not have been fully identified. Identification of this isolate as S. pseudintermedius prompted the history of dog contact to be elicited from the patient. The significance of the C. perfringens remains uncertain; polymicrobial cultures from humans and dogs infected with S. pseudintermedius are common [18, 19]. While it has been reported that the virulence of S. pseudintermedius infections in humans may differ to those of companion animals, where the organism is often the primary pathogen, this organism certainly has the potential to be virulent in the human host.
At present, the true incidence of S. pseudintermedius as a human zoonotic pathogen is unknown [20]. However, reports of zoonotic transmission of meticillin-susceptible and -resistant S. pseudintermedius between dogs and humans have been published [21]. Guardabassi et al. [22] have investigated the occurrence of S. pseudintermedius in infected dogs, their owners and subjects with no daily contact with dogs. In the study, the dog owners were significantly more likely to carry S. pseudintermedius than the control group and they often carried the same strain as their dog. In this case, the strain infecting the patient was different to that of his colonized dogs, indicating that zoonotic transmission, between the patient and his dog, had not occurred. However, it is possible that the dogs may have been colonized by more than one strain, but due to the limitations of our typing methodology, multiple strain colonization could not be determined. Polyclonal S. aureus colonization in humans has been shown, caused by exposure to diverse strains from exogenous sources, such as other individuals or pets [23]. Therefore, in cases of suspected zoonotic transmission, multiple isolates should be selected for testing to limit missing potential cases of multiple strain colonizations. Alternatively, it is also possible that the patient could have been infected from another unknown source.
All isolates in this case report were positive for the genes encoding toxins LukI and SIET, which is consistent with other reports on the detection of virulence genes in S. pseudintermedius [15]. These genes share significant homology with PVL and other exfoliatins detected in S. aureus. While S. intermedius exfoliative toxin has been shown to cause symptoms similar to canine pyoderma and human scalded skin syndrome, and LukI to be leucotoxic to polymorphonuclear cells, their role in pathogenesis among strains of S. pseudintermedius remains unclear [4]. While zoonotic transmission between the patient and his dogs could not be confirmed, to our knowledge, this is the first report of a S. pseudintermedius infection in a human from Scotland.
Funding information
The authors received no specific grant from any funding agency.
Acknowledgements
We thank the patient for giving his permission to publish this case and the photographs, and for allowing us to screen his dogs.
Conflicts of interes
The authors declare that there are no conflicts of interest.
Ethical statement
No ethical approval was required for this study.
References
- 1.Bannoehr J, Ben Zakour NL, Waller AS, Guardabassi L, Thoday KL, et al. Population genetic structure of the Staphylococcus intermedius group: insights into agr diversification and the emergence of methicillin-resistant strains. J Bacteriol. 2007;189:8685–8692. doi: 10.1128/JB.01150-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruscher C, Lübke-Becker A, Wleklinski CG, Soba A, Wieler LH, et al. Prevalence of methicillin-resistant Staphylococcus pseudintermedius isolated from clinical samples of companion animals and equidaes. Vet Microbiol. 2009;136:197–201. doi: 10.1016/j.vetmic.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Wang N, Neilan AM, Klompas M. Staphylococcus intermedius infections: case report and literature review. Infect Dis Rep. 2013;5:e3. doi: 10.4081/idr.2013.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald JR. The Staphylococcus intermedius group of bacterial pathogens: species re-classification, pathogenesis and the emergence of meticillin resistance. Vet Dermatol. 2009;20:490–495. doi: 10.1111/j.1365-3164.2009.00828.x. [DOI] [PubMed] [Google Scholar]
- 5.Börjesson S, Gómez-Sanz E, Ekström K, Torres C, Grönlund U. Staphylococcus pseudintermedius can be misdiagnosed as Staphylococcus aureus in humans with dog bite wounds. Eur J Clin Microbiol Infect Dis. 2015;34:839–844. doi: 10.1007/s10096-014-2300-y. [DOI] [PubMed] [Google Scholar]
- 6.Silva MB, Ferreira FA, Garcia LN, Silva-Carvalho MC, Botelho LA, et al. An evaluation of matrix-assisted laser desorption ionization time-of-flight mass spectrometry for the identification of Staphylococcus pseudintermedius isolates from canine infections. J Vet Diagn Invest. 2015;27:231–235. doi: 10.1177/1040638715573297. [DOI] [PubMed] [Google Scholar]
- 7.Bannoehr J, Guardabassi L. Staphylococcus pseudintermedius in the dog: taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet Dermatol. 2012;23:253–e52. doi: 10.1111/j.1365-3164.2012.01046.x. [DOI] [PubMed] [Google Scholar]
- 8.Bannoehr J, Franco A, Iurescia M, Battisti A, Fitzgerald JR. Molecular diagnostic identification of Staphylococcus pseudintermedius. J Clin Microbiol. 2009;47:469–471. doi: 10.1128/JCM.01915-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki T, Tsubakishita S, Tanaka Y, Sakusabe A, Ohtsuka M, et al. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J Clin Microbiol. 2010;48:765–769. doi: 10.1128/JCM.01232-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bannerman TL, Hancock GA, Tenover FC, Miller JM. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–555. doi: 10.1128/jcm.33.3.551-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solyman SM, Black CC, Duim B, Perreten V, van Duijkeren E, et al. Multilocus sequence typing for characterization of Staphylococcus pseudintermedius. J Clin Microbiol. 2013;51:306–310. doi: 10.1128/JCM.02421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrotra M, Wang G, Johnson WM, Manisha M, Gehua W. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J Clin Microbiol. 2000;38:1032–1035. doi: 10.1128/jcm.38.3.1032-1035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Futagawa-Saito K, Sugiyama T, Karube S, Sakurai N, Ba-Thein W, et al. Prevalence and characterization of leukotoxin-producing Staphylococcus intermedius in isolates from dogs and pigeons. J Clin Microbiol. 2004;42:5324–5326. doi: 10.1128/JCM.42.11.5324-5326.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lautz S, Kanbar T, Alber J, Lämmler C, Weiss R, et al. Dissemination of the gene encoding exfoliative toxin of Staphylococcus intermedius among strains isolated from dogs during routine microbiological diagnostics. J Vet Med B. 2006;53:434–438. doi: 10.1111/j.1439-0450.2006.00999.x. [DOI] [PubMed] [Google Scholar]
- 15.Gharsa H, Ben Slama K, Gómez-Sanz E, Lozano C, Klibi N, et al. Antimicrobial resistance, virulence genes, and genetic lineages of Staphylococcus pseudintermedius in healthy dogs in Tunisia. Microb Ecol. 2013;66:363–368. doi: 10.1007/s00248-013-0243-y. [DOI] [PubMed] [Google Scholar]
- 16.Hanselman BA, Kruth SA, Rousseau J, Weese JS. Coagulase positive staphylococcal colonization of humans and their household pets. Can Vet J. 2009;50:954–958. [PMC free article] [PubMed] [Google Scholar]
- 17.Ohara-Nemoto Y, Haraga H, Kimura S, Nemoto TK. Occurrence of staphylococci in the oral cavities of healthy adults and nasal–oral trafficking of the bacteria. J Med Microbiol. 2008;57:95–99. doi: 10.1099/jmm.0.47561-0. [DOI] [PubMed] [Google Scholar]
- 18.Abrahamian FM, Goldstein EJ. Microbiology of animal bite wound infections. Clin Microbiol Rev. 2011;24:231–246. doi: 10.1128/CMR.00041-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buriko Y, van Winkle TJ, Drobatz KJ, Rankin SC, Syring RS. Severe soft tissue infections in dogs: 47 cases (1996-2006) J Vet Emerg Crit Care. 2008;18:608–618. doi: 10.1111/j.1476-4431.2008.00370.x. [DOI] [Google Scholar]
- 20.Frank MG, Keniston A, Madinger N, Price C, Bessesen MT. Staphylococcus intermedius group infections in humans: report of four cases and a literature review. J Med Microbiol Case Rep. 2015;2 [Google Scholar]
- 21.Somayaji R, Priyantha MA, Rubin JE, Church D. Human infections due to Staphylococcus pseudintermedius, an emerging zoonosis of canine origin: report of 24 cases. Diagn Microbiol Infect Dis. 2016;85:471–476. doi: 10.1016/j.diagmicrobio.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Guardabassi L, Loeber ME, Jacobson A. Transmission of multiple antimicrobial-resistant Staphylococcus intermedius between dogs affected by deep pyoderma and their owners. Vet Microbiol. 2004;98:23–27. doi: 10.1016/j.vetmic.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Votintseva AA, Miller RR, Fung R, Knox K, Godwin H, et al. Multiple-strain colonization in nasal carriers of Staphylococcus aureus. J Clin Microbiol. 2014;52:1192–1200. doi: 10.1128/JCM.03254-13. [DOI] [PMC free article] [PubMed] [Google Scholar]