Abstract
Background
Total joint arthroplasty (TJA) has been one of the most rewarding interventions for treating patients suffering from joint disorders. However, periprosthetic joint infection (PJI) is a serious complication that frequently accompanies TJA. Our study aimed to investigate the application of the leukocyte esterase (LE) strip in the diagnosis of PJI.
Material/Methods
From October 2014 to July 2015, 72 patients who had undergone joint puncture after arthroplasty in our hospital were enrolled in this trial. One drop of synovial fluid from each available patient was applied to the LE strip, and the results were observed after 1–3 min. If the color turned to dark purple, we recognized this as a positive result, while other colors were regarded as negative results. Centrifugation was used when the synovial fluid was mixed with blood. The Musculoskeletal Infection Society (MSIS) definition was used as the standard reference to identify whether PJI was found in patients or not. The results of diagnosis and LE strips test were compared, and indicators reflecting diagnostic value were calculated. Correlation of the LE data with erythrocyte sedimentation rate (ESR), elevated C-reactive protein (CRP), synovial white blood cell (WBC) counts, and polymorphonuclear neutrophil (PMN) percentage was calculated.
Results
By MSIS criteria, 38 patients were diagnosed with PJI and 34 patients were not infected. Two types of LE strip presented the same results with sensitivity of 84.21% (95% confidence interval [CI]: 68.75~93.98%), specificity of 97.06% (95% CI: 84.67~99.93%), positive predictive value (PPV) of 96.97% (95% CI: 84.24~99.92%), and negative predictive value (NPV) of 84.62% (95% CI: 69.47~94.14%). There were one false-positive case and six false-negative cases in this trial. There is a strong correlation between LE strip and synovial fluid PMN percentage.
Conclusions
The sensitivity and specificity of the LE strip in the diagnosis of PJI are quite high, which means the LE strip might be used as an alternative to diagnose PJI in clinical practice.
MeSH Keywords: Arthritis, Infectious; Arthroplasty, Replacement; Esterases; Leukocytes
Background
Periprosthetic joint infection (PJI) has been considered as one of the most overwhelming complications after total joint arthroplasty (TJA). It has been demonstrated that 1%–3% patients may suffer from PJI [1–3], which is a critical issue challenging clinical practitioners. The symptoms of PJI are often nonspecific, therefore making the diagnosis of PJI filled with challenges [4]. In caring for a painful joint arthroplasty, the ability to distinguish between septic and aseptic failure of the prosthesis is critical, as the treatment for PJI necessitates unique surgical strategies that aim to eradicate the infecting organism(s) [5].
The Musculoskeletal Infection Society (MSIS) came up with a consensus statement providing a concise definition of a PJI for both clinical practice and research publications [6] that has been more widely used. Diagnosis of PJI according to the MSIS definition requires either one of two major criteria (sinus tract communication with a prosthesis or a pathogen isolated by culture from two separate fluid samples), or four of six minor criteria including elevated erythrocyte sedimentation rate (ESR), elevated C-reactive protein (CRP), elevated white blood cell (WBC) count, elevated percentage of polymorphonuclear neutrophils (PMN), presence of purulence, and more than five neutrophils per high-power field on frozen section [7]. Requiring a large amount of money and at least one week to make a definite diagnosis, the MSIS standard is quite complicated to be used in clinical work. Therefore, exploring a precise, convenient, and cost-effective method is of critical urgency for the diagnosis of PJI.
Parvizi et al. [8] first proved that LE strip test can be used as a novel and rapid diagnostic method for PJI in 2011. However, this method has not been reported in any Asian population and has never been used in domestic hospitals. Therefore, in our prospective study, we used two common LE strips in hospitals and regarded the MSIS definition as the reference standard to further evaluate the diagnostic value of this convenient strip in hip and knee PJI.
Material and Methods
Research subjects
We prospectively performed the LE strip test on 72 patients who had revised total knee or hip arthroplasty for either aseptic failure or PJI from October 2014 to September 2015 in PLA General Hospital. We got intraoperative synovial fluid from joints undergoing knee and hip arthroplasty revision. Synovial aspiration was performed just prior to the arthrotomy with an 18-gauge needle. Inclusion criteria were the following: patients after TJA (knee and hip) with sustained swelling of the joint and fever, with unexplainable pain in the joint after surgery, with increased ESR and CRP for consecutive days, or with the requirement of joint cavity paracentesis to identify the infection.
PJI diagnosis based on MSIS
Patients were divided into two groups (PJI group and non-PJI group) based on the reference standard of MSIS. Patients meeting one of the three following criteria were classified in the PJI group: (1) sinus tract communication with a prosthesis; (2) the same pathogen isolated by culture from two separate fluid samples or tissues; and (3) four of the following six criteria were positive: (i) increased ESR and CRP (ESR >30 mm/h, CRP >10 mg/L); (ii) increased synovial fluid white blood cells (>3000/μL); (iii) increased synovial fluid percentage of polymorphonuclear neutrophils (>65%); (iv) presence of purulence in synovial fluid; (v) positive culture in synovial fluid or tissue; and (vi) histopathological analysis of perisprosthetis showed more than 5 neutrophils per high-power field on frozen section in more than 5 high-power fields (×400). Patients who could not meet these above-mentioned criteria were classified in the non-PJI group.
LE strip test
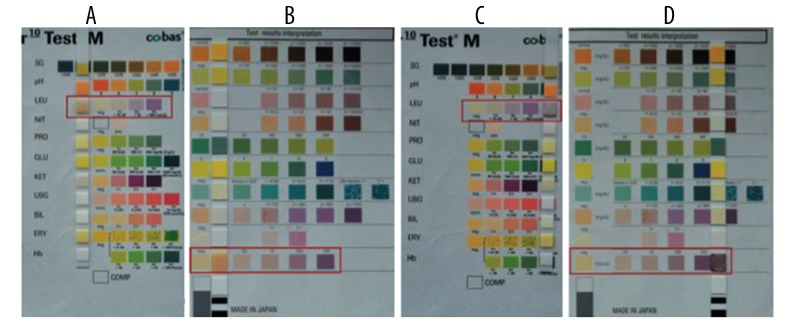
Immediately following aspiration, one drop of synovial fluid was applied to the leukocyte test pad of two standard chemical test strips (Combur10 TestM Roche, Germany, and AUTION Sticks 10PA Arkray) to detect the presence of LE. Results were recorded after 60 to 180 seconds [9–11]. Two LE strips were used per aspiration to ensure the reliability of the strip result. The changing color of the test strip was interpreted as negative (white), trace (slightly purple), + (light purple), or ++ (dark purple). Only dark purple was considered as a positive result; otherwise, the result was negative (Figure 1). All strips were read and interpreted by three different trained orthopedic research fellows, and a conclusion was made based on the major result if there is a disparity. Samples contaminated with blood were centrifuged [12] and supernatant was applied to the strip (5000 r/min, 3 min). In addition, there were 9 patients whose synovial fluid was insufficient for LE strip test but who could be diagnosed through using the MSIS criteria. These patients were regarded as false-negative.
Figure 1.
LE strip test of synovial fluid with two different strips. Synovial fluid from four different patients was dropped on two strips. (A, B) Showed that the LE strip tests were negative. (C, D) Showed that LE strip tests were positive. In (A, C) Combur10 TestM Roche strips were used, while in (B, D), AUTION Sticks 10PA Arkray strips were used.
Statistical analysis
The data were processed with the statistical software package SPSS. A diagnostic 2×2 table was established based on the LE test and MSIS standard. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. Pearson’s correlation coefficient between the LE reading and the CRP, ESR, WBC counts, and PMN percentage was calculated.
Results
General information about included patients
Based on the MSIS reference standard, 38 patients (26 knee replacement and 12 hip replacement) were diagnosed as having PJI. Among them were 19 males and 19 females, with ages ranging from 31 to 80 years (63.35±12.8 years) and body mass index (BMI) of 19.10~32.04 kg/m2 (25.07±3.01 kg/m2). Among the 34 uninfected patients (23 knee replacement and 11 hip replacement), there were 15 males and 18 females, with ages ranging from 22 to 79 years (56.89+13.76 years) and BMI of 18.36~33.28 kg/m2 (23.91±2.89 kg/m2). There was no statistical difference between these two groups in terms of age, gender, and BMI (p>0.05).
Diagnostic value of LE strip when compared with MSIS reference standard
Among the 72 patients included in our study, we could not obtain enough synovial fluid for the LE strip test in 9 (12.5%) of them, leaving 63 samples for the LE strip test. Among the 63 samples, 61 were readable and three doctors differed on 2 of the results, which were considered to be between light purple (negative) and dark purple (positive). We finally made the decision based on the major opinions, one positive and the other negative. As for other 17 samples that were contaminated with blood, all results were readable after centrifugation. A total of 33 samples were defined as dark purple (positive), while 30 samples were defined as light purple, slightly purple, or white. The final result of the two chosen strips was the same in all samples.
After comparing the LE strip test with the MSIS reference standard, there were 1 false-positive sample and 6 false-negative samples among all 72 samples (Table 1). Sensitivity and specificity of the two strips were 84.21% (95% confidence interval [CI]: 68.75~93.98%) and 97.06% (95% CI: 84.67~99.93%), respectively. PPV and NPV were 96.97% (95% CI: 84.24~99.92%) and 84.62% (95% CI: 69.47~94.14%), respectively (Table 2).
Table 1.
Comparison of LE strips test results and MSIS diagnostic criteria (n=63).
| LE strip test | MSIS diagnostic criteria | Total | |
|---|---|---|---|
| Infection group | Non-infection group | ||
| Positive | 32 | 1 | 33 |
| Negative | 3 | 27 | 30 |
| Total | 35 | 28 | 63 |
Two types of LE strips sharing the same results.
Table 2.
Sensitivity, specificity, positive predictive value and negative predictive value of LE strips.
| Items | Results | 95%CI |
|---|---|---|
| Sensitivity (%) | 91.4 | 75.8–97.8 |
| Specificity (%) | 96.4 | 79.8–99.8 |
| Positive predictive value (%) | 97.0 | 82.5–99.8 |
| negative predictive value (%) | 90.0 | 72.3–97.4 |
Comparison of LE strip with CRP, ESR, and PMN percentage in diagnosing PJI
ESR, CRP, white blood cell (WBC) count and ploymorphonuclear (PMN) percentage were calculated and compared between the PJI and non-PJI group (Table 3). In addition, we analyzed the correlation of the LE data with the corresponding data for parameters that increased in serum or synovial fluid for diagnosing PJI: ESR, CRP, synovial WBC count, and synovial PMN percentage included (Table 4). The LE level was significantly correlated with all those parameters (p<0.001), and the strongest correlation was with the percentage of PMN in the synovial fluid (r=0.845).
Table 3.
Comparison of the mean result of laboratory test for the cohorts with positive and negative findings for infection according to MSIS.
| Laboratory test | Mean value | P value | |
|---|---|---|---|
| PJI group (n=35) | Non-PJI group (n=28) | ||
| ESR (mm/hr) | 42.1 | 20.8 | <0.001 |
| CRP (mg/dl) | 4.33 | 1.02 | <0.001 |
| WBC count (/μL) | 34987 | 10983 | <0.001 |
| PMN percentage | 87.22 | 48.91 | <0.001 |
Table 4.
Correlation between the LE reading and the ESR, CRP, WBC and PMN. The correlation coefficient for each pair of values is listed.
| LE | ESR | CRP | WBC | PMN | |
|---|---|---|---|---|---|
| LE | 1 | ||||
| ESR | 0.663 | 1 | |||
| CRP | 0.604 | 0.658 | 1 | ||
| WBC | 0.578 | 0.428 | 0.563 | 1 | |
| PMN | 0.854 | 0.589 | 0.521 | 0.612 | 1 |
Discussion
PJI is currently one of the most serious complications that our patients, clinicians, and our healthcare system are confronted with [13]. The reasons for the diagnostic difficulty include the absence of specific clinical signs and symptoms, the relative lack of accurate laboratory tests, and low culture rate in isolation of pathogens due to prior therapy and formation of biofilms [14,15]. The MSIS recently responded to this diagnostic difficulty by developing a definition for PJI [16]. Although clinically useful, this definition remains complex and time consuming due to the subjective observation of purulence and interpretation of the frozen section histology and the low culture rate, all of which cause delay in diagnosis. Therefore, it is urgent to search for a precise, rapid, and cost-effective detection method.
The LE strip has been used to detect urinary tract infection for more than three decades [17–19], and its diagnostic value has been reported [20]. Parvizi et al. first proved that the LE strip could be used in the diagnosis of PJI. They reported that ++ was considered as the positive result, and the sensitivity and specificity were 80.6% and 100%, respectively, with a PPV and NPV of 100% and 93.3%, respectively [8]. Since then, Aggarwal et al. [12], Wetters et al. [21], Nelson et al. [22], Colvin et al. [10], and De Vecchi et al. [23] have reported the use of the LE strip in the diagnosis of PJI. However, two cut-off values (++ or ++/+) have been used in these above-mentioned studies, although they used the same kind of LE strip. This turns out to generate different sensitivities and specificities, even in the same study. In our study, we also found that two samples were hard to define since three doctors’ opinions were not identical and we made the decision based on the majority of them. Indeed, there is currently no standard cut-off value for the LE strip in the diagnosis of PJI. In addition, some studies did not use the same reference standard for the diagnosis of PJI, although MSIS has been considered as the golden standard. All these factors lead to the difficulty of using the LE strip. Therefore, it might be better to use the automated reader to define the final result instead of using naked eye, which has been used in clinical labs in diagnosing the urinary tract infection. In our current study, we used MSIS as the reference standard and ++ as the positive result, and these generated the sensitivity and specificity of 84.21% (95% CI: 68.75~93.98%) and 97.06% (95% CI: 84.67~99.93%), respectively.
Another critical issue that aroused our attention was that since the result was based on color change of the strip, samples contaminated with blood might interfere with the reading result. Parvizi et al. reported that among the 177 included samples, there were 47 (26.6%) bloody samples that could not be read [8]. Wetters et al. also found that 29.2% of synovial fluid samples were mixed with blood and could not be read directly [21]. Similar to these studies, we also found 17 (23.61%) bloody samples in our study. Through centrifugation, the red cells were sedimentated to the bottom of the tube and the supernatant was used to drop on the strip, which generated the readable result. This method was also used in these published papers. We also found that after centrifugation, there were still some samples with slight red or insoluble cloud matter, which exerted a slight influence on the reading. These samples were defined as negative and were all in accordance with the MSIS standard.
There are still several limitations to our study. Firstly, any debris or blood in the synovial aspirate had the potential to influence the results of the colorimetric test in one-third of the aspirates. These samples could not be tested directly with colorimetric reagent strips. We addressed this concern by using centrifugation to separate the bloody contaminant from the synovial fluid in cases of bloody aspirate, a protocol that preserves the accuracy of the colorimetric test for LE. Although all individuals were trained to evaluate the LE strip test results, a possible bias might be introduced because of the subjectivity of LE strip interpretation. In addition, due to the colorimetric analysis of this reagent strip, subjective opinions might exist when making the decision. Therefore, in our study, we took three doctors’ opinions into account when confronted with samples that were hard to define. It might be more precise if the colorimetric analysis of the strip is performed by the automated reader, which generates semi-quantitative readings, rather than by the naked eye, so that subjective variations in determining color can be avoided. Thirdly, we found that the color of the strip might be deepened with a longer interval of time, which might change the final definition. We speculated that this might be related to the thickness of the synovial fluid, which might take a longer time to penetrate through the membrane. Therefore, the appropriate time for reading the final result is worthy of further exploration in the future research. Last but not least, although results of samples after centrifugation were dependable, the number was still quite small, which means a larger number of samples are required to verify the efficiency of this method.
Conclusions
The future for LE as a marker for PJI is promising. In revision arthroplasty, LE may be a more accurate and efficient predictor of infection at re-implantation than current markers such as ESR and CRP, which have a low specificity for PJI. Detection of LE in synovial fluid is considered to be simple, rapid, and valuable, demonstrating a high specificity and NPV for diagnosing PJI when compared with MSIS criteria.
Footnotes
Source of support: There is no source of financial support for the current study
References
- 1.Phillips JE, Crane TP, Noy M, et al. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: A 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–48. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz SM, Lau E, Watson H, et al. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27:61–65e61. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Jamsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25:87–92. doi: 10.1016/j.arth.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med. 2009;361:787–94. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garvin KL, Konigsberg BS. Infection following total knee arthroplasty: prevention and management. Instr Course Lect. 2012;61:411–19. [PubMed] [Google Scholar]
- 6.Alijanipour P, Adeli B, Hansen EN, et al. Intraoperative purulence is not reliable for diagnosing periprosthetic joint infection. J Arthroplasty. 2015;30:1403–6. doi: 10.1016/j.arth.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–94. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: The utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011;93:2242–48. doi: 10.2106/JBJS.J.01413. [DOI] [PubMed] [Google Scholar]
- 9.Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: Matched for musculoskeletal infection society criteria. J Bone Joint Surg Am. 2014;96:1917–20. doi: 10.2106/JBJS.M.01591. [DOI] [PubMed] [Google Scholar]
- 10.Colvin OC, Kransdorf MJ, Roberts CC, et al. Leukocyte esterase analysis in the diagnosis of joint infection: Can we make a diagnosis using a simple urine dipstick? Skeletal Radiol. 2015;44:673–77. doi: 10.1007/s00256-015-2097-5. [DOI] [PubMed] [Google Scholar]
- 11.Tischler EH, Plummer DR, Chen AF, et al. Leukocyte esterase: Metal-on-metal failure and periprosthetic joint infection. J Arthroplasty. 2016 doi: 10.1016/j.arth.2016.03.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal VK, Tischler E, Ghanem E, Parvizi J. Leukocyte esterase from synovial fluid aspirate: A technical note. J Arthroplasty. 2013;28:193–95. doi: 10.1016/j.arth.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Beaule PE, Shea B, Abedlbary H, et al. A protocol for a systematic review of the diagnostic accuracy of blood markers, synovial fluid, and tissue testing in periprosthetic joint infections (PJI) Syst Rev. 2015;4:148. doi: 10.1186/s13643-015-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parvizi J, Adeli B, Zmistowski B, et al. Management of periprosthetic joint infection: The current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94:e104. doi: 10.2106/JBJS.K.01417. [DOI] [PubMed] [Google Scholar]
- 15.Liao YY, Lin YM. The value of intraoperative Gram stain in revision total knee arthroplasty. J Bone Joint Surg Am. 2010;92:1323. author reply 1323–24. [PubMed] [Google Scholar]
- 16.Shahi A, Deirmengian C, Higuera C, et al. Premature therapeutic antimicrobial treatments can compromise the diagnosis of late periprosthetic joint infection. Clin Orthop Relat Res. 2015;473:2244–49. doi: 10.1007/s11999-015-4142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bingham J, Clarke H, Spangehl M, et al. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472:4006–9. doi: 10.1007/s11999-014-3900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gollwitzer H, Dombrowski Y, Prodinger PM, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95:644–51. doi: 10.2106/JBJS.L.00205. [DOI] [PubMed] [Google Scholar]
- 19.Jin X, Beguerie JR, Zhang W, et al. Circulating C reactive protein in osteoarthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2015;74:703–10. doi: 10.1136/annrheumdis-2013-204494. [DOI] [PubMed] [Google Scholar]
- 20.Cox N, Pilling D, Gomer RH. DC-SIGN activation mediates the differential effects of SAP and CRP on the innate immune system and inhibits fibrosis in mice. Proc Natl Acad Sci USA. 2015;112:8385–90. doi: 10.1073/pnas.1500956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wetters NG, Berend KR, Lombardi AV, et al. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27:8–11. doi: 10.1016/j.arth.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Nelson GN, Paxton ES, Narzikul A, et al. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24:1421–26. doi: 10.1016/j.jse.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 23.De Vecchi E, Villa F, Bortolin M, et al. Leukocyte esterase, glucose and C-reactive protein in the diagnosis of prosthetic joint infections: A prospective study. Clin Microbiol Infect. 2016 doi: 10.1016/j.cmi.2016.03.020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]