Abstract
Introduction
Patients with severe community-acquired pneumonia (CAP) are believed to have an exaggerated inflammatory response to bacterial infection. Therapies aiming to modulate the inflammatory response have been largely unsuccessful, perhaps reflecting that CAP is a heterogeneous disorder that cannot be modulated by a single anti-inflammatory approach. We hypothesize that the host inflammatory response to pneumonia may be characterized by distinct cytokine patterns, which can be harnessed for personalized therapies.
Methods
Here, we use hierarchical cluster analysis of cytokines to examine if patterns of inflammatory response in 13 hospitalized patients with CAP can be defined. This was a secondary data analysis of the Community-Acquired Pneumonia Inflammatory Study Group (CAPISG) database. The following cytokines were measured in plasma and sputum on the day of admission: interleukin (IL)-1β, IL-1 receptor antagonist (IL-1ra), IL-6, CXCL8 (IL-8), IL-10, IL-12p40, IL-17, interferon (IFN)γ, tumor necrosis factor (TNF)α, and CXCL10 (IP-10). Hierarchical agglomerative clustering algorithms were used to evaluate clusters of patients within plasma and sputum cytokine determinations.
Results
A total of thirteen patients were included in this pilot study. Cluster analysis identified distinct inflammatory response patterns of cytokines in the plasma, sputum, and the ratio of plasma to sputum.
Conclusions
Inflammatory response patterns in plasma and sputum can be identified in hospitalized patients with CAP. Characterization of the local and systemic inflammatory response may help to better discriminate patients for enrollment into clinical trials of immunomodulatory therapies.
Keywords: Cytokines, pneumonia, outcomes, inflammatory response, steroids, immunomodulation
Introduction
Even with appropriate antibiotic therapy, some hospitalized patients with community-acquired pneumonia (CAP) progress to clinical failure and death. High cytokine levels produce an exaggerated inflammatory response, known as the cytokine storm, and may drive poor clinical outcomes. As a result, investigators have evaluated the use of immunomodulatory agents in an attempt to control this exaggerated inflammatory response and reduce patient mortality. Corticosteroids are the primary immunomodulatory agents considered for patients with CAP, and results of several investigations [1-10] have been evaluated in recent systematic reviews and meta-analyses [11,12] Although steroids are considered important immunomodulatory agents for these patients, other considerations include macrolides [13-18] statins [19-29] and tissue factor pathway inhibitor [30].
Severe pneumonia at the time of hospital admission is the primary criterion for patient enrollment into clinical trials of immunomodulatory therapies [10,31]. This is due to a general consensus that severity of disease and the inflammatory response are positively correlated. If this is the case, patients with more severe pneumonia should present with an exaggerated inflammatory response and would be ideal candidates for enrollment into trials of immunomodulatory therapies. Although this inflammation-severity correlation may be true at the population level, increased disease severity at the individual level may also be impacted by factors unrelated to the inflammatory response. For example, a patient hospitalized with CAP with a prior history of congestive heart failure who develops severe respiratory deterioration due to cardiogenic pulmonary edema may not have an exaggerated inflammatory response. Any patient without an exaggerated inflammatory response, regardless of disease severity, would not be an appropriate candidate for a trial of an immunomodulatory therapy. Several experts in the field have indicated that the failure of many recent investigations into immunomodulation in hospitalized patients with CAP is likely to be related to our inability to properly characterize the patients who would benefit from these therapies prior to enrollment [32,33]. To address this concern, methods to better identify patients with and without an exaggerated inflammatory response are needed. We hypothesize that patients with CAP can be clustered into distinct inflammatory response groups at the time of hospital admission using cytokine levels in plasma and/or sputum.
The objective of this study was to evaluate the utility of hierarchical agglomerative cluster analysis of plasma and sputum cytokines to examine if inflammatory patterns can be defined in hospitalized patients with CAP.
2. Methods
2.1 Study design and setting
This was a secondary data analysis of data previously collected from the Community-Acquired Pneumonia Inflammatory Study Group (CAPISG). An in-depth description of the CAPISG study and specimen collection has been previously published [34]. Briefly, we prospectively enrolled non-consecutive adult patients hospitalized with CAP at the University of Louisville Hospital, Louisville, Kentucky, USA, from 01 April 2011 to 01 August 2012. The study protocol required enrolled patients to provide blood and sputum specimens on the day of admission and at regular intervals during hospitalization.
2.2 Study population
Adult patients with CAP in whom both blood and sputum specimens were obtained on the day of admission were included in the current pilot study. CAP was defined as a new pulmonary infiltrate (within 24 hours of admission), associated with at least one of the following criteria: a new or increased cough, an abnormal temperature (37.8 °C), or an abnormal leukocyte count (leukocytosis, leukopenia or the presence of immature neutrophils). Pneumonia was defined as community-acquired if a patient had no history of hospitalization during the 2 weeks prior to admission. Patients were excluded if they had a medical history that, in the investigator's opinion, precluded subject compliance with the protocol.
2.3 Data sources and measurements
After patient consent, trained study coordinators and/or research associates collected clinical data from the patient's medical record onto a paper case report form. A separate research associate entered these data into a secure, web-based data management system hosted by the University of Louisville Division of Infectious Diseases. Data quality issues identified by the research associate entering the data were fixed prior to submission of the case to the database. The database also contains several data quality checks to limit out-of-range errors and inappropriate data types. These data quality structures were built in based on our decades of experience collecting and entering clinical data into electronic databases. After all data queries were resolved, the case was accepted into the database for analysis. The following clinical variables were collected and evaluated:
Demographics: age and sex
Comorbid conditions: chronic obstructive pulmonary disease (COPD), cerebrovascular disease, congestive heart failure, chronic renal disease, diabetes mellitus, active coronary artery disease, neoplastic disease, neurologic disease/mental illness, acute renal disease, hyperlipidemia, HIV status, ACE inhibitor use, aspirin use, beta blocker use, heparin use, statin use, hospitalization for 2 or more days in the prior 90 days, home infusion therapy, home oxygen therapy, nursing home resident, and atrial fibrillation.
Physical examination: oral temperature, heart rate, respiratory rate, systolic blood pressure, and diastolic blood pressure.
Laboratory tests: pH, FiO2, PaCO2, serum bicarbonate, serum blood urea nitrogen (BUN), serum creatinine, serum glucose, hematocrit, hemoglobin, serum potassium, serum sodium, platelet count, procalcitonin, and white blood cell count (WBC).
Severity of CAP: need for ICU admission on arrival, altered mental status, vasopressors, IV steroids on admission, and the Pneumonia Severity Index.
All microbiological evaluations were done according to clinical needs per hospital microbiology protocols.
2.4 Specimen collection and processing
2.4.1 Plasma
Blood samples were obtained on the day of admission to the hospital. Venous blood was collected using sodium citrate BD Vacutainer® tubes and processed within 2 hours of collection. The tubes were centrifuged at 300 × g for 10 minutes and the plasma separated by aspiration, aliquoted (0.5 ml each) and stored frozen at -80 °C until assayed.
2.4.2 Sputum
Sputum samples were obtained on the day of admission to the hospital and processed within 2 hours of collection, according to the method described by Pizzichini et al. [35]. Briefly, samples were diluted with an equal amount of a 0.1% Dithiothreitol solution in phosphate buffered saline (PB and incubated in a rocking platform for 15 minutes in order to digest the mucus. An equal volume of sterile saline was added, followed by an additional 5 minutes of incubation. The samples were then filtered through nylon gauze and centrifuged at 790 × g for 10 minutes. The cell-free supernatants were aliquoted and stored frozen at -80 &8451; until used for the measurement of cytokine levels.
2.5 Cytokine measurements
Laboratory methods for cytokine measurements have been previously published [34]. The concentrations of interleukin (IL)-1β, IL-1 receptor antagonist (IL-1ra), IL-6, CXCL8 (IL-8), IL-10, IL-12p40, IL-17, interferon (IFN)γ, tumor necrosis factor (TNF)α, and CXCL10 (IP-10) in plasma and sputum samples were determined using Milliplex MAP Multiplex kits (EMD Millipore, Billerica, MA). Following thawing, plasma and processed sputum samples were centrifuged at 10,000 × g for 5 min and the supernatants used in the assay according to the manufacturer's instructions.
2.6 Compliance with Ethical Standards
The University of Louisville Human Subjects Program Protection Office approved this study prior to any data collection (approval number: 07.0182). Informed consent was obtained from all individual participants included in the study.
2.7 Statistical analysis
For each patient, cytokine values were scaled by subtracting the mean cytokine value across all patients from each particular patient cytokine level. This value was then divided by the standard deviation of the particular cytokine. The scale function in Rv3.2.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for scaling purposes. Hierarchical agglomerative clustering algorithms were then used to evaluate clusters of patients within the plasma and sputum determinations separately. Finally, the plasma determination was divided by the sputum determination and the same clustering methods were used to evaluate clusters from the plasma/sputum ratio. Ensemble clustering methods were used to identify the most appropriate distance metric and cluster linkage function for hierarchical agglomerative cluster analysis [36]. From these results, the distance metric and linkage function providing the most clinically meaningful clustering were chosen. For each clustering algorithm, dendrograms and heatmaps were created to visualize the clusters and scaled cytokine values. Dark blue colors on the heatmaps represent high cytokine values, white colors represent average values, and dark red represent low values. To compare plasma and sputum clustering dendrograms, we used the Pearson cophenetic correlation statistic. Briefly, cophenetic correlation statistics are similar to traditional correlation measures, ranging from -1 (perfect negative correlation) to +1 (perfect positive correlation), with a zero being no correlation.
To compare differences in patient characteristics between groups defined as having a higher inflammatory response versus a lower inflammatory response from the cluster analysis, frequencies and percentages for categorical variables and medians with interquartile ranges were presented for continuous variables. Statistical hypothesis testing was not done due to the probability of error in the multiple tests and the limited number of patients included in the study. R v3.2.0 (R Foundation for Statistical Computing, Austria) was used for all analyses. The following R packages and were used: gplots [37], dendextend [38], dendextendRcpp [39], clue [36], and RColorBrewer [40].
3. Results
3.1 Demographic, clinical and laboratory findings
A total of 13 patients were included in this pilot study. Baseline patient characteristics and clinical outcomes for each of the 13 patients can be found in Table 1. No patients were started on vasopressors on admission or during hospitalization. For higher vs lower inflammatory phenotype groups identified via cluster analysis of the sputum specimens patients with a higher inflammatory phenotype had a lower rate of ICU admission (0% vs 67%, respectively). Similarly, when evaluating plasma cytokine profiles, patients with a higher inflammatory phenotype had a lower rate of ICU admission versus those with a lower inflammatory phenotype (12% vs 60%, respectively). This difference did not remain when evaluating the plasma/sputum ratio. No other clinically meaningful differences were identified. A pathogen was identified in 6 of the 13 patients. Streptococcus pneumoniae was identified in patients 1, 4, and 12, influenza in patient 2, Pseudomonas aeruginosa in patient 3, and methicillin-resistant Staphylococcus aureus in patient 6.
Table 1. Patient Characteristics.
| Patient Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | Male | Male | Female | Female | Female | Male | Male | Male | Male |
| Age (years) | 78 | 54 | 40 | 51 | 63 | 62 | 72 | 57 | 53 | 78 | 85 | 65 | 59 |
| Obese (BMI>30 kg/M2) | No | Yes | No | Yes | No | No | Yes | Yes | No | No | No | No | No |
| Immunocompromized | No | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| ICU admission | Yes | No | No | No | No | No | No | No | Yes | No | Yes | No | Yes |
| Nursing liome resident | No | No | No | No | No | No | No | Yes | No | No | No | No | Yes |
| Active cancer | No | No | No | No | No | Yes | Yes | No | No | Yes | No | No | No |
| Liver disease | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Renal disease | No | No | No | No | Yes | No | No | No | No | No | No | No | No |
| Congestive heart failure | No | No | No | Yes | No | No | Yes | Yes | No | Yes | No | No | No |
| Cerebrovascular disease | No | No | No | No | No | No | No | Yes | No | No | Yes | No | No |
| Altered mental status | No | No | No | No | No | No | No | No | No | Yes | No | No | No |
| Heart rate, (beats/nib) | 91 | 130 | 98 | 78 | 93 | 87 | 118 | 108 | 77 | 94 | 156 | 92 | 102 |
| Respiratory rate breaths/rain | 18 | 22 | 24 | 16 | 22 | 20 | 25 | 24 | 23 | 20 | 40 | 25 | 25 |
| Systolic blood pressure (mmHg) | 95 | L44 | 114 | 135 | 99 | 115 | 187 | 160 | 185 | 133 | 99 | 151 | 9D |
| Oral temperature (F) | 99 | 100 | 100 | 98.7 | 100.4 | 98.6 | 102 | 101 | 101 | 99.2 | 100 | 97.5 | 98 |
| Hematocrit (%) | 41.4 | 48.8 | 38 | 40.3 | 42.5 | 35 | 34 | 43.5 | 36.1 | 31.8 | 43.2 | 34.2 | 37.7 |
| Blood urea nitrogen mg/dl | 13 | 16 | 13 | 22 | 36 | 40 | 10 | 31 | 5 | 70 | 21 | 10 | 13 |
| Sodium mmol/litre | 137 | 134 | 140 | 133 | 133 | 129 | 140 | 135 | 140 | 146 | 140 | 139 | 138 |
| Arterial pH | No ABG | 7.36 | No ABG | 7.46 | No ABG | 7.41 | No ABG | 7.39 | 7.44 | No ABG | 7.41 | 7.414 | No ABG |
| Pa02 mmHg | No ABG | 59 | No ABG | 60 | No ABG | 67 | No ABG | 94 | 66 | No ABG | 7B.4 | 65.9 | No ABG |
| Glucose mg/dl | 116 | 97 | 101 | 295 | 88 | 102 | 105 | 1S5 | 107 | 108 | 179 | 111 | 174 |
| Pleural effusion | No | Yes | No | No | No | No | No | Yes | No | No | Yes | No | Yes |
| Pneumonia Severity Index risk class | Class III | Class III | Class I | Class II | Class IV | Class V | Class IV | Class IV | Class II | Class V | Class V | Class 11 | Class III |
| Days to clinical stability | 2 | 2 | 7 | 2 | 3 | Unknown | 4 | 6 | 4 | 2 | 6 | 2 | 3 |
| Culture positive | Yes | Yea | Yes | Yes | No | Yes | No | No | No | No | No | Yes | No |
| Length of stay (days) | 3 | 4 | 8 | 3 | 3 | 15 | 0 | 6 | 6 | 6 | 6 | 2 | 9 |
| In-hospital mortality | No | No | No | No | No | Yes | No | No | No | No | No | No | Yes |
3.2 Cytokine profiles
Based on the results of ensemble clustering, Euclidean distance and Ward's D linkage metrics were identified as the most clinically meaningful for our needs. Each heatmap and dendrogram depict some separation of patients' inflammatory response. A heatmap and dendrogram for cytokines in sputum is depicted in Figure 1a. A heatmap and dendrogram for cytokines in plasma is depicted in Figure 1b. The cophenetic correlation coefficient comparing the clustering of patients in plasma and sputum determinations was 0.14, suggesting low correlation between plasma and sputum clusterings. A heatmap and dendrogram for cytokines in plasma/sputum ratio is depicted in Figure 1c. In each figure, a horizontal line was drawn to indicate a suggested separation of inflammatory response patterns based on the cluster dendrograms and visual inspection of the heatmaps.
Fig. 1.
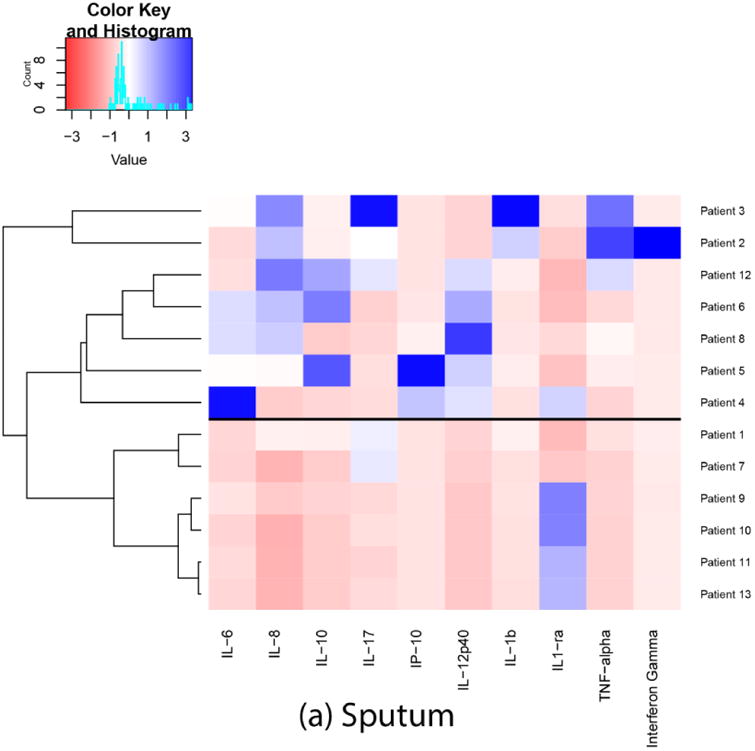
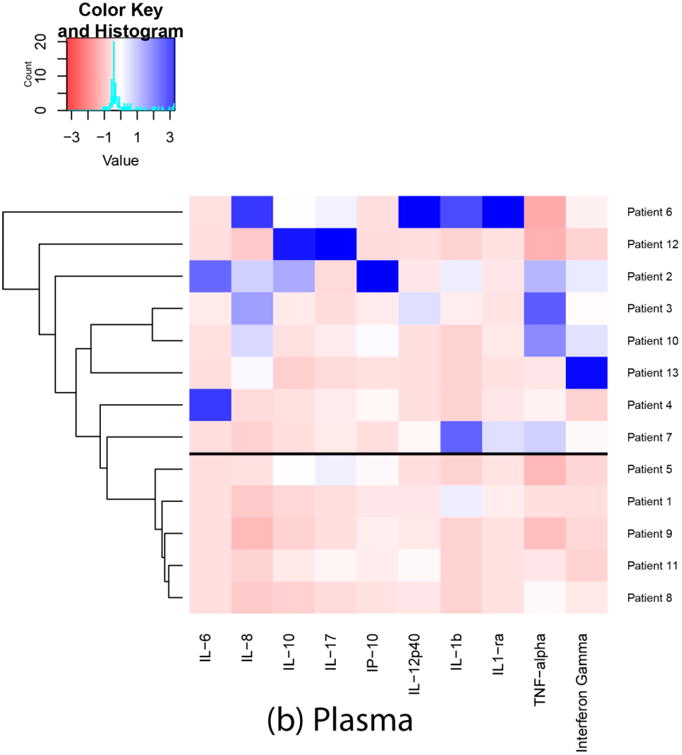
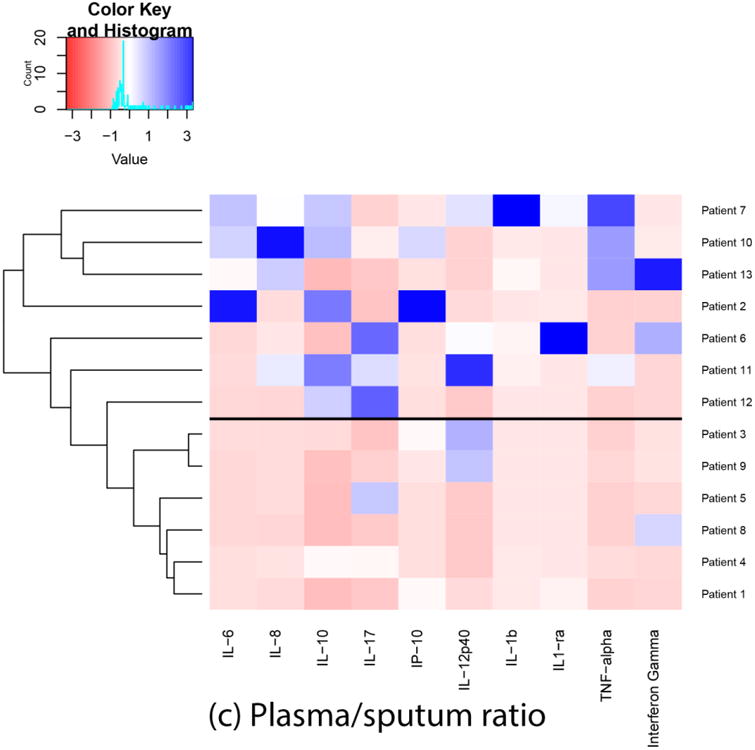
Heatmaps and dendrograms for cytokines in sputum and plasma. Each heatmap and dendrogram depict some separation of patients' inflammatory response. The cophenetic correlation coefficient comparing the clustering of patients in plasma and sputum determinations was 0.14, suggesting low correlation between plasma and sputum clusterings. a horizontal line was drawn to indicate a suggested separation of inflammatory response patterns based on the cluster dendrograms and visual inspection of the heatmaps.
4. Discussion
This pilot study suggests that distinct cytokine patterns in plasma and sputum in hospitalized patients with CAP at the time of hospital admission. We found that approximately half of the patients were in each inflammatory response group across analysis of sputum cytokines, plasma cytokines, and the plasma/sputum cytokine ratio. Several patients were retained in the same group in all 3 analyses. Since this was a pilot study, larger studies may identify more than the two patterns identified in our study. The only clinically relevant difference identified in the patient characteristics comparisons between high vs low inflammatory phenotypes was the need for direct ICU admission when comparing both sputum and plasma cytokines. In both comparisons, patients with lower inflammatory phenotypes necessitated more intensive care on admission compared to those with higher inflammatory phenotypes. With our small sample size it is unclear as to if these differences are real or due to chance however this may be an interesting variable to evaluate in future larger studies. For sputum cytokines, it is possible that patients with a lower inflammatory response on admission have a poor local immune response and may end with a more severe disease leading to ICU admission.
A timeline of the inflammatory response in patients with pneumonia can be generalized as follows (Figure 2): At time zero, during the initial invasion of organisms into the alveolar space, there is an interaction of the invading pathogen with cells of the innate immune system. The identification of the pathogen is made by receptors called pattern recognition receptors (PRR). Streptococcus pneumoniae, as well as other extracellular pathogens may be recognized by a family of membrane-bound PRPs called Toll-like receptors. Influenza and other intracellular pathogens may be recognized by a family of cytoplasmic PRPs called NOD-like receptors.
Fig. 2. A timeline of the inflammatory response in patients with pneumonia.
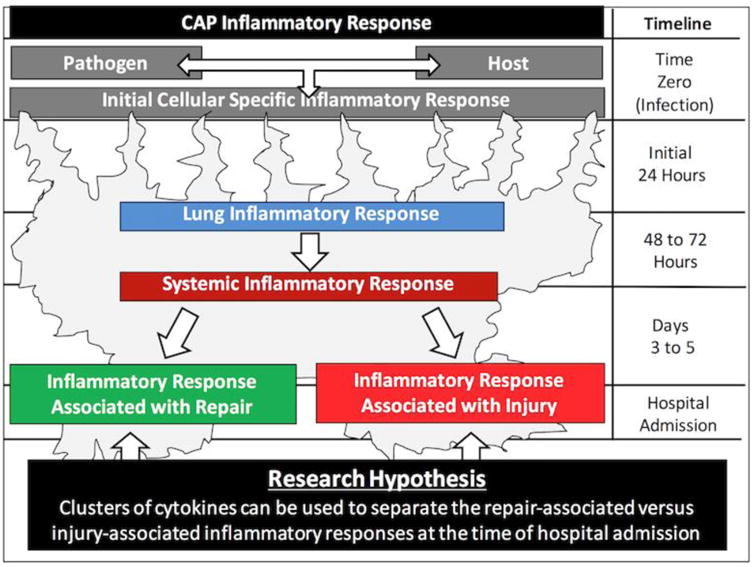
During the initial 24 hours after infection, activation of theses receptors will trigger synthesis and secretion of cytokines. Since different pathogens are recognized by different receptors with different signaling mechanisms, it is predictable that the initial inflammatory response may be pathogen specific. On the other hand, the inflammatory response will also be host specific, as the initial cytokine response will be different in patients with different underlying medical conditions, who have chronic inflammation, or who are taking medications that may modulate the inflammatory response. Due to the multiple pathogen and host factors that may influence the inflammatory response, the initial cellular response and cytokine pattern is likely to be pathogen and host specific.
On days two and three post infection, a well-developed lung inflammatory response should be present. The initially specific inflammatory response may begin to evolve into non-specific inflammatory pathways. At this time, the local lung inflammatory response may spill over into the circulatory system, generating a systemic inflammatory response.
Patients with pneumonia are hospitalized with an average of 3 to 5 days of signs and symptoms. By this time, we hypothesized that patients present with two patterns of inflammatory response, one associated with repair, and another associated with injury, as depicted in Figure 2. We further hypothesized that these patterns of response can be identified by performing a cluster analysis of cytokines at the time of hospital admission. In the current pilot study, we were able to characterize patterns of local (sputum) and systemic (plasma) inflammatory response in hospitalized patients with pneumonia.
We speculate that patients with an exaggerated inflammatory pattern only in the sputum may not be ideal candidates for clinical trials of immunomodulatory therapies, since a local immune response is an appropriate physiologic response to pneumonia. Reducing a non-exaggerated immune response may not be therapeutic, could bias a trial's results to the null, and could even lead to poor outcomes. However, patients with an exaggerated inflammatory response in the circulation may be excellent candidates for these trials due to their systemic cytokine storm. As depicted in Figure 2, 8 of the 13 CAP patients in our study appeared to have an exaggerated inflammatory response in plasma. These patients would be appropriate candidates for immunomodulation.
Since this was a pilot study, we enrolled a limited number of patients. Evaluating only these 13 patients limits the generalizability. Furthermore, this small sample size allows for potentially biased cluster analyses. Another limitation of our study is that the line separating our definition of the exaggerated inflammatory response was partially based on visual inspection of the heatmap. This may lead to misclassification of some patients due to the partially subjective nature of this classification. Not all aspects of inflammation and anti-inflammation were evaluated in this study. Other makers may be important for determining an exaggerated inflammatory response to infection. Another limitation is that the bacterial burden was not measured. This can influence inflammatory response and bias the clustering results.
These interesting results suggest a prospective study with a larger number of patients is warranted, with the goal to characterize patterns of inflammatory response in blood and sputum in patients with CAP at the time of hospital admission. If cytokine values were to be determined immediately upon admission each value could be automatically uploaded to and analysed by a cloud-based application using supervised machine learning algorithms. Results of the analysis could be reported back to a mobile device in real-time as a yes/no decision rule. This would allow for rapid determination of the level of inflammation to define candidacy into clinical trials for immunomodulatory therapies.
In conclusion, we were able to define patterns of inflammatory response using cytokine data in this pilot study. Our results suggest that airway and systemic inflammation in CAP is heterogeneous, providing proof of principle that stratification based on the inflammatory patterns may be possible for future trials of immunomodulation.
Acknowledgments
Funding source: This study was unfunded. Dr. Restrepo's time is partially protected by Award Number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health nor the Department of Veterans Affairs.
Footnotes
Author contributions: All authors met ICMJE authorship criteria. TLW, RRK, RFB, WAM, FWA, PP, JB, and JAR conceived and designed the research plan. RFB conducted the laboratory research. TLW, RRK, WAM, RFB, and JAR conducted the data analysis. All authors were involved in data interpretation. TLW and JAR wrote the first draft of the manuscript. All authors critically reviewed the manuscript for important intellectual content. All authors agree with the manuscript results and conclusions.
Competing interests: All authors declare no competing interests
References
- 1.Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, Winzeler B, Bingisser R, Elsaesser H, Drozdov D, Arici B, Urwyler SA, Refardt J, Tarr P, Wirz S, Thomann R, Baumgartner C, Duplain H, Burki D, Zimmerli W, Rodondi N, Mueller B, Christ-Crain M. Adjunct prednisone therapy for patients with communityacquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. The Lancet. 2015 doi: 10.1016/S0140-6736(14)62447-8. [DOI] [PubMed] [Google Scholar]
- 2.Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F, Umberger R, Meduri GU. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171(3):242–8. doi: 10.1164/rccm.200406-808OC. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/15557131. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Serrano S, Dorca J, Garcia-Vidal C, Fernandez-Sabe N, Carratala J, Fernandez-Aguera A, Corominas M, Padrones S, Gudiol F, Manresa F. Effect of corticosteroids on the clinical course of communityacquired pneumonia: a randomized controlled trial. Crit Care. 2011;15(2):R96. doi: 10.1186/cc10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marik P, Kraus P, Sribante J, Havlik I, Lipman J, Johnson DW. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia. a randomized controlled study. Chest. 1993;104(2):389–92. doi: 10.1378/chest.104.2.389. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/8339624. [DOI] [PubMed] [Google Scholar]
- 5.McHardy VU, Schonell ME. Ampicillin dosage and use of prednisolone in treatment of pneumonia: co-operative controlled trial. Br Med J. 1972;4(5840):569–73. doi: 10.1136/bmj.4.5840.569. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/4404939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, Voorn GP, van de Garde EM, Endeman H, Grutters JC, Bos WJ, Biesma DH. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9782):2023–30. doi: 10.1016/S0140-6736(11)60607-7. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/21636122. [DOI] [PubMed] [Google Scholar]
- 7.Mikami K, Suzuki M, Kitagawa H, Kawakami M, Hirota N, Yamaguchi H, Narumoto O, Kichikawa Y, Kawai M, Tashimo H, Arai H, Horiuchi T, Sakamoto Y. Efficacy of corticosteroids in the treatment of communityacquired pneumonia requiring hospitalization. Lung. 2007;185(5):249–55. doi: 10.1007/s00408-007-9020-3. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/17710485. [DOI] [PubMed] [Google Scholar]
- 8.Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community acquired pneumonia - a randomized double blinded clinical trial. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.200905-0808OC. [DOI] [PubMed] [Google Scholar]
- 9.J Wagner HN, J Bennett IL, Lasagna L, Cluff LE, Rosenthal MB, Mirick GS. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98(3):197–215. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/13304518. [PubMed] [Google Scholar]
- 10.Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, Gabarrus A, Sellares J, Restrepo MI, Anzueto A, Niederman MS, Agusti C. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313(7):677–86. doi: 10.1001/jama.2015.88. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/25688779. [DOI] [PubMed] [Google Scholar]
- 11.Corrales-Medina VF, Musher DM. Immunomodulatory agents in the treatment of community-acquired pneumonia: a systematic review. J Infect. 2011;63(3):187–99. doi: 10.1016/j.jinf.2011.06.009. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/21763343. [DOI] [PubMed] [Google Scholar]
- 12.Horita N, Otsuka T, Haranaga S, Namkoong H, Miki M, Miyashita N, Higa F, Takahashi H, Yoshida M, Kohno S, Kaneko T. Adjunctive systemic corticosteroids for hospitalized community-acquired pneumonia: Systematic review and meta-analysis 2015 update. Sci Rep. 2015;5:14061. doi: 10.1038/srep14061. [Online]. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4571641/pdf/srep14061.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aspa J, Rajas O, Rodriguez de Castro F, Huertas MC, Borderias L, Cabello FJ, Tabara J, Hernandez-Flix S, Martinez-Sanchis A, Torres A G. Pneumococcal Pneumonia in Spain Study. Impact of initial antibiotic choice on mortality from pneumococcal pneumonia. Eur Respir J. 2006;27(5):1010–9. doi: 10.1183/09031936.06.00126004. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/16455824. [DOI] [PubMed] [Google Scholar]
- 14.Baddour LM, Yu VL, Klugman KP, Feldman C, Ortqvist A, Rello J, Morris AJ, Luna CM, Snydman DR, Ko WC, Chedid MB, Hui DS, Andremont A, Chiou CC G. International Pneumococcal Study. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440–4. doi: 10.1164/rccm.200311-1578OC. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/15184200. [DOI] [PubMed] [Google Scholar]
- 15.Dwyer R, Ortqvist A, Aufwerber E, Henriques Normark B, Marrie TJ, Mufson MA, Torres A, Woodhead MA, Alenius M, Kalin M. Addition of a macrolide to a ss-lactam in bacteremic pneumococcal pneumonia. Eur J Clin Microbiol Infect Dis. 2006;25(8):518–21. doi: 10.1007/s10096-006-0183-2. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/16896822. [DOI] [PubMed] [Google Scholar]
- 16.Martinez JA, Horcajada JP, Almela M, Marco F, Soriano A, Garcia E, Marco MA, Torres A, Mensa J. Addition of a macrolide to a beta-lactam-based empirical antibiotic regimen is associated with lower inhospital mortality for patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2003;36(4):389–95. doi: 10.1086/367541. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/12567294. [DOI] [PubMed] [Google Scholar]
- 17.Mufson MA, Stanek RJ. Revisiting combination antibiotic therapy for community-acquired invasive streptococcus pneumoniae pneumonia. Clin Infect Dis. 2006;42(2):304–6. doi: 10.1086/499110. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/16355349. [DOI] [PubMed] [Google Scholar]
- 18.Weiss K, Low DE, Cortes L, Beaupre A, Gauthier R, Gregoire P, Legare M, Nepveu F, Thibert D, Tremblay C, Tremblay J. Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic streptococcus pneumoniae pneumonia in adults. Can Respir J. 2004;11(8):589–93. doi: 10.1155/2004/461392. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/15611810. [DOI] [PubMed] [Google Scholar]
- 19.Chalmers JD, Singanayagam A, Murray MP, Hill AT. Prior statin use is associated with improved outcomes in community-acquired pneumonia. Am J Med. 2008;121(11):1002–1007 e1. doi: 10.1016/j.amjmed.2008.06.030. [Online]. Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18954848. [DOI] [PubMed] [Google Scholar]
- 20.Douglas I, Evans S, Smeeth L. Effect of statin treatment on short term mortality after pneumonia episode: cohort study. BMJ. 2011;342:d1642. doi: 10.1136/bmj.d1642. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/21471172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frost FJ, Petersen H, Tollestrup K, Skipper B. Influenza and copd mortality protection as pleiotropic, dose-dependent effects of statins. Chest. 2007;131(4):1006–12. doi: 10.1378/chest.06-1997. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/17426203. [DOI] [PubMed] [Google Scholar]
- 22.Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006;333(7576):999. doi: 10.1136/bmj.38992.565972.7C. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/17060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortensen EM, Pugh MJ, Copeland LA, Restrepo MI, Cornell JE, Anzueto A, Pugh JA. Impact of statins and angiotensin-converting enzyme inhibitors on mortality of subjects hospitalised with pneumonia. Eur Respir J. 2008;31(3):611–7. doi: 10.1183/09031936.00162006. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/17959631. [DOI] [PubMed] [Google Scholar]
- 24.Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The effect of prior statin use on 30-day mortality for patients hospitalized with community-acquired pneumonia. Respir Res. 2005;6:82. doi: 10.1186/1465-9921-6-82. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/16042797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myles PR, Hubbard RB, Gibson JE, Pogson Z, Smith CJ, McKeever TM. The impact of statins, ace inhibitors and gastric acid suppressants on pneumonia mortality in a uk general practice population cohort. Pharmacoepidemiol Drug Saf. 2009;18(8):697–703. doi: 10.1002/pds.1769. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/19455553. [DOI] [PubMed] [Google Scholar]
- 26.Polgreen LA, Cook EA, Brooks JM, Tang Y, Polgreen PM. Increased statin prescribing does not lower pneumonia risk. Clin Infect Dis. 2015;60(12):1760–6. doi: 10.1093/cid/civ190. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/25759433. [DOI] [PubMed] [Google Scholar]
- 27.Schlienger RG, Fedson DS, Jick SS, Jick H, Meier CR. Statins and the risk of pneumonia: a populationbased, nested case-control study. Pharmacotherapy. 2007;27(3):325–32. doi: 10.1592/phco.27.3.325. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/17316144. [DOI] [PubMed] [Google Scholar]
- 28.Thomsen RW, Riis A, Kornum JB, Christensen S, Johnsen SP, Sorensen HT. Preadmission use of statins and outcomes after hospitalization with pneumonia: population-based cohort study of 29,900 patients. Arch Intern Med. 2008;168(19):2081–7. doi: 10.1001/archinte.168.19.2081. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/18955636. [DOI] [PubMed] [Google Scholar]
- 29.Yende S, Milbrandt EB, Kellum JA, Kong L, Delude RL, Weissfeld LA, Angus DC. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med. 2011;39(8):1871–8. doi: 10.1097/CCM.0b013e31821b8290. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/21516038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wunderink RG, Laterre PF, Francois B, Perrotin D, Artigas A, Vidal LO, Lobo SM, Juan JS, Hwang SC, Dugernier T, LaRosa S, Wittebole X, Dhainaut JF, Doig C, Mendelson MH, Zwingelstein C, Su G, Opal S. Recombinant tissue factor pathway inhibitor in severe community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2011;183(11):1561–8. doi: 10.1164/rccm.201007-1167OC. [Online]. Available: http://www.atsjournals.org/doi/pdf/10.1164/rccm.201007-1167OC. [DOI] [PubMed] [Google Scholar]
- 31.2015. [Online]. Available: https://clinicaltrials.gov/ct2/show/NCT01283009
- 32.Wunderink RG. Corticosteroids for severe community acquired pneumonia: not for everyone. JAMA. 2015;313(7):673–4. doi: 10.1001/jama.2015.115. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/25688777. [DOI] [PubMed] [Google Scholar]
- 33.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J, Group CS. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–24. doi: 10.1056/NEJMoa071366. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/18184957. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Botran R, Uriarte SM, Arnold FW, Rodriguez-Hernandez L, Rane MJ, Peyrani P, Wiemken T, Kelley R, Uppatla S, Cavallazzi R, Blasi F, Morlacchi L, Aliberti S, Jonsson C, Ramirez JA, Bordon J. Contrasting inflammatory responses in severe and non-severe community-acquired pneumonia. Inflammation. 2014;37:1158–1166. doi: 10.1007/s10753-014-9840-2. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/24557760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, Gleich GJ, Dolovich J, Hargreave FE. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154(2 Pt 1):308–17. doi: 10.1164/ajrccm.154.2.8756799. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/8756799. [DOI] [PubMed] [Google Scholar]
- 36.2015. [Online]. Available: http://CRAN.R-project.org/package=clue
- 37.2015. [Online]. Available: http://CRAN.R-project.org/package=gplots
- 38.2015. [Online]. Available: http://CRAN.R-project.org/package=dendextend
- 2015. [Online]. Available: http://CRAN.R-project.org/package=dendextendRcpp
- 40.2014. [Online]. Available: http://CRAN.R-project.org/package=RColorBrewer


