Abstract
Impaired regulation of blood pressure upon standing can lead to adverse outcomes, including falls, syncope, and disorientation. Mean arterial pressure typically increases upon standing; however, an insufficient increase or a decline in mean arterial pressure upon standing may result in decreased cerebral perfusion. Orthostatic hypotension has been reported in older people with increased arterial stiffness, whereas the association between orthostatic change in mean arterial pressure and arterial stiffness in young-to-middle aged individuals has not been examined. We analyzed orthostatic blood pressure response and comprehensive hemodynamic data in 3205 participants (1693 [53%] women) in the Framingham Heart Study Third Generation cohort. Participants were predominantly middle-aged (mean age: 46±9 years). Arterial stiffness was assessed using carotid-femoral pulse wave velocity, forward pressure wave amplitude, and characteristic impedance of the aorta. Adjusting for standard cardiovascular disease risk factors, orthostatic change in mean arterial pressure (6.9±7.7 mm Hg) was inversely associated with carotid-femoral pulse wave velocity (partial correlation, rp = −0.084, P<0.0001), forward wave amplitude (rp = −0.129, P<0.0001), and characteristic impedance (rp = −0.094, P<0.0001). The negative relation between forward wave amplitude and change in mean arterial pressure on standing was accentuated in women (P=0.002 for sex interaction). Thus, higher aortic stiffness was associated with a blunted orthostatic increase in mean arterial pressure, even in middle age. The clinical implications of these findings warrant further study.
Keywords: arterial stiffness, orthostatic hypotension, mean arterial pressure, pulse wave velocity, characteristic impedance
Blood pressure (BP) typically falls initially when changing from a supine to standing position, and then stabilizes at a higher mean arterial pressure (MAP) soon after standing when the baroreceptors sense the change in systemic pressure and trigger a compensatory increase in heart rate and peripheral vascular resistance.1 This orthostatic increase in MAP is necessary to maintain sufficient cerebrovascular perfusion given the change in gravitational potential energy when moving from a supine to standing posture. If there is an insufficient increase in MAP, cerebral vasodilation is required in order to maintain cerebral perfusion at a constant level. Prior studies have shown that arterial stiffness is associated with short-term orthostatic pressure variability in older adults.2;3 Increased vascular stiffness may affect BP homeostasis by diminishing cardiovagal baroreflex sensitivity,4–6 which may blunt orthostatic BP response via limited changes in heart rate or peripheral vasoconstriction.7 Arterial stiffness may be assessed via characteristic impedance (Zc) or forward pressure wave amplitude, which takes into account both aortic stiffness and peak aortic flow. Additionally, carotid-femoral pulse wave velocity (CFPWV) has emerged as the reference standard measure of aortic stiffness.8 Previous studies have shown a positive relation between CFPWV and short-term BP variability in older people,2;3;9 but these observations have been largely overlooked in younger adults. The combination of blunted increase in MAP and impaired microvascular reactivity may represent a particularly harmful insult to the brain and may explain recent findings of a relation between stiffness measures and vascular brain injury in young to middle-aged adults10 or cognitive impairment in older adults.11;12 We hypothesized that higher arterial stiffness, as assessed by CFPWV, Zc, or forward pressure wave amplitude, is associated with a diminished compensatory increase in MAP upon standing in middle-aged individuals, and tested this hypothesis in a community-based cohort.
Methods
Study sample
Third Generation participants of the Framingham Heart Study were evaluated during their second examination. Participants were predominantly middle-aged and of European descent. Satisfactory evaluation of hemodynamic and covariate data was obtained in 3205 (94%) of 3411 participants. The Boston University Medical Center Institutional Review Board approved the protocol, and all participants gave written informed consent.
Noninvasive hemodynamic data acquisition and analysis
Participants were studied in the supine position after resting for approximately 5 minutes. Auscultatory BP was assessed at the level of the brachial artery by using a computer-controlled device that automatically inflated the cuff to a user preset maximum pressure (160 mm Hg) and then precisely controlled deflation at 2 mm Hg/sec. If Korotkoff sounds were detected immediately after cuff inflation, the acquisition was aborted and repeated with maximum cuff pressure increased by 40 mm Hg, up to a maximum of 240 mm Hg. The device digitized and recorded mean cuff pressure and a cuff microphone channel (12 kHz) throughout the inflation and deflation sequence so that auscultatory BP measurements could be over-read at the core laboratory (Cardiovascular Engineering, Inc.) by analysts who were blinded to participant characteristics. Arterial tonometry was obtained from the brachial, radial, femoral and carotid arteries using a custom transducer (Cardiovascular Engineering, Inc., Norwood, MA). Next, 2-dimensional echocardiographic images of the left ventricular outflow tract were obtained from a parasternal long-axis view followed by pulsed Doppler of the left ventricular outflow tract from an apical 5-chamber view. Body surface measurements from suprasternal notch to pulse recording sites were obtained by using a fiberglass tape measure for carotid, brachial and radial sites and a caliper for the femoral site.
At the end of the hemodynamic data acquisition, participants were asked to sit up momentarily and then stand. A second BP measurement was taken starting the cuff inflation approximately 2 minutes after standing, meaning that the blood pressure measurement corresponded to a time interval of approximately 2–3 minutes after standing. A prior study using continuous blood pressure monitoring in a community-based cohort has shown that blood pressure stabilizes at a new equilibrium value within approximately 20–30 seconds following standing and remains stable out to 2 minutes post-standing in the age range of our cohort.13 Therefore, change in blood pressure 2–3 minutes after standing should provide a robust estimate of change in equilibrium blood pressures between supine and standing. Changes in blood pressure components were defined as the standing value minus the supine value. Systolic and diastolic BP often have opposite directional responses to standing, whereas, MAP, which is the primary contributing factor to mean cerebrovascular perfusion, should increase upon standing from a supine position. Thus, we focused our analyses on continuous orthostatic change in MAP as the primary outcome variable.
Tonometry waveforms were signal-averaged using the electrocardiographic R-wave as a fiducial point. Average supine systolic and diastolic cuff pressures were used to calibrate peak and trough of the signal-averaged brachial waveform. Diastolic and integrated mean brachial pressures were used to calibrate carotid waveforms.14 MAP was calculated from the integrated brachial pressure waveform based on tonometry or the oscillometric cuff waveform. CFPWV was calculated, accounting for parallel transmission in the aortic arch and carotid artery, as previously described.15 Left ventricular outflow tract diameter was measured on echocardiographic images by finding the largest early systolic diameter at a point just proximal to the aortic leaflets. The left ventricular outflow tract Doppler flow velocity waveform was multiplied by outflow tract area to compute aortic volumetric flow. Zc of the aorta was computed in the time domain by dividing the increase in pressure during early systole by the corresponding increase in volumetric flow.16 Central pressure and flow waves were separated into their forward and backward components, as previously described.17
Statistical analyses
Demographic and hemodynamic characteristics of the sample were tabulated. Given the low prevalence (2.3%) of OH by the standard definition in this relatively young cohort, we have focused statistical analyses on continuous BP change variables rather than dichotomous variables. We estimated partial correlations of arterial stiffness measures (CFPWV, Zc, and forward wave amplitude) with orthostatic change in BP variables (systolic, diastolic, mean and pulse pressure) and heart rate. We adjusted for age, sex, height, BMI, total cholesterol, high density lipoprotein cholesterol, triglycerides, fasting glucose, hemoglobin A1c, current smoking, use of medication to treat hypertension or dyslipidemia, prevalent diabetes, and prevalent cardiovascular disease. In order to evaluate effect modification by age, sex and presence of diabetes in the MAP change on standing, we examined interactions of median age, sex and presence of diabetes with aortic stiffness measures in linear regression models that adjusted for risk factors note above. In a secondary analysis, we included an indicator variable for hypertension (blood pressure ≥140/90 or treated) in the model. For all analyses, a two-tailed alpha value of <0.05 indicated statistical significance.
Results
Characteristics of the sample are summarized in Table 1. The cohort was comprised of young to middle-aged adults who were, on average, overweight. Hemodynamic variables are summarized for the full cohort in Table 2 and separately by sex in Supplemental Table S1. Average supine BP fell within the normotensive range. Standing was associated with substantial increases in diastolic BP, MAP, and heart rate. In contrast, systolic BP increased only modestly, and pulse pressure fell substantially (Table 2). Consistent with the observed fall in pulse pressure, which, at any given level of MAP will tend to reduce systolic BP and increase diastolic BP, the increase in diastolic BP exceeded the increase in MAP.
Table 1.
Characteristics of the sample (N=3205)
| Variable | Value |
|---|---|
| Age, years | 46 ± 9 |
| Women, N (%) | 1693 (53) |
| Height, in. | 67 ± 4 |
| Weight, lbs | 177 ± 41 |
| Body mass index, kg/m2 | 27.7 ± 5.4 |
| Fasting glucose, mg/dL | 96 ± 18 |
| Triglycerides, mg/dL | 111 ± 73 |
| Total/HDL cholesterol ratio | 3.4 ± 1.2 |
| Hemoglobin A1c, % | 5.5 ± 0.5 |
| Diabetes, N (%) | 154 (5) |
| Prevalent cardiovascular disease, N (%) | 69 (2) |
| Treatment for hypertension, N (%) | 524 (16) |
| Treatment for lipid disorder, N (%) | 512 (16) |
| Current smoking, N (%) | 370 (12) |
Values are mean ± SD except as noted. HDL, high density lipoprotein.
Table 2.
Hemodynamic variables (N = 3205)
| Variable | Value |
|---|---|
| Carotid-femoral pulse wave velocity, m/s | 7.1 ± 1.4 |
| −1000/Carotid-femoral pulse wave velocity, ms/m | −146 ± 25 |
| Characteristic impedance, dyne*s*cm−5 | 194 ± 59 |
| Forward pressure wave, mm Hg | 45 ± 11 |
| Supine hemodynamics | |
| Systolic blood pressure, mm Hg | 121 ± 15 |
| Diastolic blood pressure, mm Hg | 63 ± 9 |
| Mean arterial pressure, mm Hg | 87 ± 11 |
| Pulse pressure, mm Hg | 57 ± 12 |
| Heart rate, min−1 | 65 ± 10 |
| Systolic ejection period, ms | 311 ± 24 |
| Change upon standing, median (25th, 75th percentile) | |
| Systolic blood pressure, mm Hg | 2 (−4, 8) |
| Diastolic blood pressure, mm Hg | 10 (4, 14) |
| Mean arterial pressure, mm Hg | 7 (2, 12) |
| Pulse pressure, mm Hg | −8 (−15, −1) |
| Heart rate, min−1 | 14 (9, 20) |
| Systolic ejection period, ms | −68 (−81, −55) |
Standing blood pressure measurements were taken 2 minutes after standing.
Partial correlations of change in BP components and heart rate with CFPWV, Zc, and forward wave amplitude, adjusted for standard cardiovascular risk factors, are shown in Table 3. Changes in MAP and systolic pressure were inversely related to CFPWV, Zc, and forward wave amplitude, indicating that individuals with stiffer arteries exhibited a smaller increase in MAP upon standing. Change in pulse pressure was also negatively related to Zc and forward wave amplitude. In contrast, change in diastolic pressure had a positive relation with Zc and forward wave amplitude. Relations between change in heart rate and stiffness measures were modest (forward wave amplitude) or absent. When we further adjusted models for change in MAP for initial supine MAP, relations between CFPWV and change in MAP on standing became positive (rp = 0.101, P<0.001) whereas relations of Zc or forward wave amplitude with change in MAP were no longer significant (P>0.9).
Table 3.
Partial correlations among stiffness and orthostatic BP change variables
| Change Variables | Carotid-Femoral Pulse Wave Velocity |
Characteristic Impedance |
Forward Wave Amplitude |
|---|---|---|---|
| Systolic blood pressure | −0.061 | −0.194 | −0.247 |
| P-value | <0.001 | <0.001 | <0.001 |
| Diastolic blood pressure | −0.029 | 0.103 | 0.116 |
| P-value | 0.10 | <0.001 | <0.001 |
| Mean arterial pressure | −0.084 | −0.094 | −0.129 |
| P-value | <0.001 | <0.001 | <0.001 |
| Pulse pressure | −0.034 | −0.256 | −0.315 |
| P-value | 0.053 | <0.001 | <0.001 |
| Heart rate | −0.005 | −0.004 | −0.062 |
| P-value | 0.79 | 0.83 | <0.001 |
Orthostatic change variables represent standing value minus supine value. Correlations were adjusted for age, sex, height, body mass index, total cholesterol, high density lipoprotein cholesterol, triglycerides, fasting glucose, hemoglobin A1c, current smoking, use of medication to treat hypertension or dyslipidemia, and prevalent diabetes or cardiovascular disease.
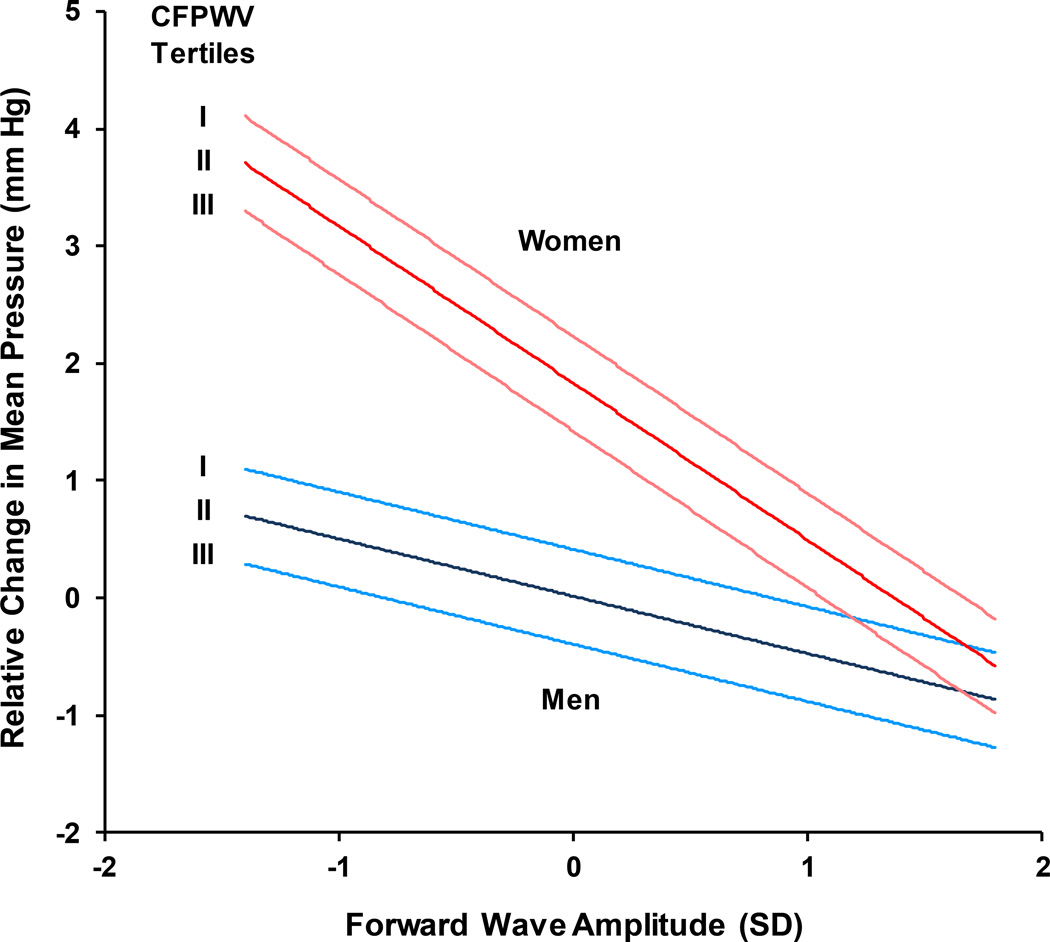
A linear regression model of change in MAP on standing, adjusted for age and cardiovascular disease risk factors, revealed a significant interaction between sex and forward wave amplitude (P=0.002). To illustrate the sex interaction, the relation between change in MAP on standing and forward wave amplitude was plotted separately by sex (Figure). In addition, estimates for change in MAP in men and women according to sex-specific tertiles of CFPWV are presented. Values represent differences in change in MAP when other covariates in the model were fixed at their mean values. Change in MAP was higher in women (P<0.0001) but the increase was progressively blunted in women with higher forward wave amplitude (P=0.002 for interaction between sex and forward wave). In addition, the increase in MAP was lower in men or women with higher CFPWV (P<0.001).
Figure 1.
Orthostatic change in mean arterial pressure as a function of forward wave amplitude in groups defined by sex-specific tertiles of carotid-femoral pulse wave velocity (CFPWV). Values represent estimated differences in change in mean arterial pressure as a function of forward wave amplitude in a model that adjusted for age, sex, height, BMI, total cholesterol, high density lipoprotein cholesterol, triglycerides, fasting glucose, hemoglobin A1c, current smoking, use of medication to treat hypertension or dyslipidemia, and prevalent diabetes or cardiovascular disease. Values for covariates were fixed at the mean value for the cohort and the range of values plotted correspond to the 5th and 95th percentiles of forward wave amplitude. Women had a greater increase in mean arterial pressure than men. However, higher stiffness by either measure was associated with a lower increase in mean arterial pressure upon standing; the relation between forward wave amplitude and change in mean arterial pressure was particularly strong in women. P<0.0001 for sex, P=0.01 for forward wave, P=0.002 for interaction between sex and forward wave, and P<0.001 for CFPWV.
In light of the propensity for individuals with diabetes to develop aortic stiffness and orthostatic hypotension, we also assessed for effect modification by the presence of diabetes. We found a modest accentuation of the negative relations of change in MAP with Zc (interaction p=0.02) and forward wave amplitude (interaction p=0.05). Similarly, we found accentuated negative relations of change in DBP with Zc (interaction p=0.005) and forward wave amplitude (interaction p<0.001).
Discussion
We assessed relations of aortic stiffness with the compensatory orthostatic increase in MAP in response to standing in a community-based cohort of middle-aged adults. We report that higher aortic stiffness, assessed as CFPWV, Zc or forward wave amplitude, was associated with a blunted increase in MAP upon standing from a supine position. The relation between higher forward wave amplitude and lower increase in MAP on standing was accentuated in women and should be interpreted in light of the marked increase in forward wave amplitude that is known to occur after midlife, particularly in women.18;19 Relations between aortic stiffness measures and change in heart rate on standing were modest or absent. The observed blunted increase in MAP on standing has important implications for mean perfusion pressure at the level of the brain in an upright individual. If a blunted increase in MAP on standing is combined with blunted cerebral microvascular reactivity in individuals with a stiffened aorta, the combination may contribute to enhanced susceptibility of the brain to adverse effects of aortic stiffening.10;20;21
The negative relation between arterial stiffness measures and orthostatic change in MAP may indicate that the response of strain-sensitive baroreceptors is attenuated in individuals with stiff arteries.3–6 For example, in older participants of the Rotterdam Study, arterial stiffness was shown to be associated with impaired baroreflex sensitivity and orthostatic BP changes.6 Furthermore, Okada et al. showed that baroreflex sensitivity was inversely correlated with arterial stiffness among elderly individuals, particularly in older women.4 However, limited relations between aortic stiffness measures and change in heart rate on standing in the present study suggests that blunted vasoconstriction or accentuated reduction in stroke volume, rather than blunted heart rate response, may contribute more to relations between stiffness and MAP response in our cohort.
In order to maintain cerebrovascular perfusion pressure upon standing from a supine position, MAP must increase in proportion to the change in gravitational potential energy at the level of the brain, which is 30 cm or more above the heart (and brachial artery, where BP is measured) when standing. In light of the foregoing, in the absence of a change in MAP at the level of the brachial artery, change in posture will produce a hydrostatic reduction in perfusion pressure at the level of the brain of approximately 23 mm Hg (30 cm H2O) or more, depending on height. Individuals who have either a decrease or minimal increase in MAP at the level of the brachial artery upon standing may experience a drop in cerebrovascular perfusion, particularly if cerebrovascular vasodilation is impaired and insufficient to counteract the drop in perfusion pressure at the level of the brain. Previous studies have demonstrated inverse relations between aortic stiffness and microvascular reactivity.22 Recently, Rucka et al. showed that arterial stiffness contributes to impaired cerebrovascular reactivity in individuals with coronary artery disease.23 Given the association between arterial stiffness and cerebrovascular structural damage24;25 and impaired cerebrovascular reactivity,23;24;26 individuals with stiff arteries may have compounded risk for hypoperfusion due to the combined effects of insufficient increase in MAP and potentially impaired cerebral microvascular vasodilatory response. Thus, a blunted increase in MAP on standing could be an important contributor to relations between arterial stiffness and cognitive impairment, which is a premise that warrants further investigation.
The observation that relations between aortic stiffness measures and change in MAP on standing were reversed (CFPWV) or no longer significant (Zc, forward wave amplitude) after adjusting for supine MAP may suggest that abnormalities in resting MAP rather than aortic stiffness contributed to the blunted increase in MAP on standing. However, we have previously shown that higher initial aortic stiffness is associated with blood pressure progression and incident hypertension during 7 years of prospective follow-up. In contrast, initial blood pressure was not associated with progression of CFPWV.27 Therefore, adjusting a model examining relations of CFPWV with change in MAP for supine MAP may represent adjusting for a downstream effect of CFPWV. In addition, regardless of whether aortic stiffness contributes to blunted increase in MAP on standing, it is important to note that individuals with stiffer arteries had a smaller increase (or a decrease) in MAP on standing. As noted above, MAP must increase and the cerebral microvasculature must vasodilate in order to offset the substantial hydrostatic reduction in mean perfusion pressure in the brain when moving from a supine to seated or standing posture. Regardless of mechanism, individuals with a stiffer aorta had a blunted increase in MAP on standing. In light of the known impairment of microvascular reactivity in individuals with a stiffer aorta,23;24;26 both mechanism that contribute to maintenance of cerebral perfusion on standing are impaired, rendering the brain susceptible to episodic relative ischemia in individuals with a stiffened aorta.
Whereas MAP increased, pulse pressure fell substantially upon standing in our cohort. Pulse pressure is associated with arterial stiffness and cerebrovascular damage25 and is a known risk factor for adverse cardiovascular events.28–30 Our study has shown that moving from a supine to standing position resulted in a lower pulse pressure overall and that the reduction was enhanced in individuals with elevated arterial stiffness. The reduction in pulse pressure upon standing may contribute to previously described beneficial effects of increasing the percentage of the workday that is spent standing.31;32 However, beneficial effects of standing on pulse pressure may be attenuated in those with stiffer arteries because of the associated blunted increase in MAP on standing. Importantly, differing patterns of change in pulse pressure and MAP on standing produced variable relations of stiffness measures with various BP components. For example, relations of stiffness measures with change in systolic as compared to diastolic BP were comparably robust but opposite in sign (Table 3). As a result, studies of relations between aortic stiffness and blood pressure variability or baroreceptor sensitivity may arrive at discrepant conclusions depending on whether SBP or DBP is examined. The foregoing points require further elucidation.
Our study has limitations that must be considered. Participants rested for 5 minutes prior to baseline BP measurements; however, it may take more than 5 minutes of rest for BP to completely stabilize at a new, lower plateau after lying down, depending on level of activity prior to the rest period. In addition, we waited only for two minutes prior to initiating the acquisition of standing blood pressure; however, the acquisition takes approximately 90–120 sec, meaning that the recording corresponds to waiting approximately 3 minutes between standing and assessment of BP. As a result, we may have under- or overestimated the magnitude of orthostatic change in BP in some individuals. However, we followed a standardized protocol that included use of a digital timer to confirm the time delay between standing and taking the upright BP. Additionally, given that we focused on changes in MAP at a fixed interval after standing, early transient changes in BP after standing may have been missed. Finally, the cohort consisted primarily of young-to-middle-aged adult participants of European descent, so results may not be generalizable to other ages, races or ethnicities.
Supplementary Material
Perspectives.
Impaired regulation of BP upon standing can result in adverse effects such as dizziness, confusion, or fainting due to insufficient cerebral perfusion. MAP assessed at the level of the heart must rise upon standing in order to maintain cerebral perfusion in response to a change in gravitational potential energy and hydrostatic perfusion pressure at the level of the brain. In our young-to-middle-aged cohort, those with greater arterial stiffness experienced a blunted increase in MAP upon standing. Interventions that reduce arterial stiffness may improve regulation of MAP, and in turn minimize potential adverse effects on cerebral perfusion. Future studies are needed that examine relations between arterial stiffness, cerebrovascular reactivity, and brain structure and function and that assess effects of interventions that reduce stiffness on orthostatic change in MAP.
Novelty and Significance.
1) What is new?
The association of arterial stiffness and the change in mean arterial pressure upon standing has not been examined previously in a large community-based cohort of young to middle-aged adults. Individual with a stiffer aorta exhibited a blunted increase in mean arterial pressure upon standing.
2) What is relevant?
Upon standing, the combination of an increase in mean arterial pressure and dilation of intracerebral arterioles contributes to maintain sufficient cerebrovascular perfusion. Aortic stiffness is associated with the blunting of both responses, potentially leading to episodic reductions in cerebral perfusion in individuals with a stiff aorta.
Summary
Higher aortic stiffness, assessed by various measures, is associated with a blunted orthostatic increase in mean arterial pressure that may be associated with a reduction in cerebrovascular perfusion pressure. In light of prior studies demonstrating blunted reactivity of small vessels in individuals with higher aortic stiffness, blunted orthostatic increase in mean arterial pressure in association with higher aortic stiffness may contribute to known associations between aortic stiffness and cerebrovascular disease.
Acknowledgments
Sources of Funding
This work was supported by the NHLBI, Framingham Heart Study, (NHLBI/NIH Contract #N01-HC-25195 and HHSN268201500001I) and the Boston University School of Medicine and by HL076784, G028321, HL070100, HL060040, HL080124, HL071039, HL077447, HL107385, HL126136 and 2-K24-HL04334. Dr. Cooper is also supported by the UNCF/Merck Science Initiative.
Footnotes
Disclosures
Dr. Mitchell is owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness, serves as a consultant to and receives honoraria from Novartis, Merck, Servier and Philips Healthcare and is funded by research grants from the National Institutes of Health. Ms. Torjesen is an employee of Cardiovascular Engineering, Inc. The remaining authors have no ownership interest in Cardiovascular Engineering, Inc. and no additional relevant disclosures.
Reference List
- 1.Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34:375–386. doi: 10.1002/j.1552-4604.1994.tb04977.x. [DOI] [PubMed] [Google Scholar]
- 2.Boddaert J, Tamim H, Verny M, Belmin J. Arterial stiffness is associated with orthostatic hypotension in elderly subjects with history of falls. J Am Geriatr Soc. 2004;52:568–572. doi: 10.1111/j.1532-5415.2004.52163.x. [DOI] [PubMed] [Google Scholar]
- 3.Mattace-Raso FU, van der Cammen TJ, Knetsch AM, van den Meiracker AH, Schalekamp MA, Hofman A, Witteman JC. Arterial stiffness as the candidate underlying mechanism for postural blood pressure changes and orthostatic hypotension in older adults: the Rotterdam Study. J Hypertens. 2006;24:339–344. doi: 10.1097/01.hjh.0000202816.25706.64. [DOI] [PubMed] [Google Scholar]
- 4.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59:98–104. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol. 2001;281:H284–H289. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 6.Mattace-Raso FU, van den Meiracker AH, Bos WJ, van der Cammen TJ, Westerhof BE, Elias-Smale S, Reneman RS, Hoeks AP, Hofman A, Witteman JC. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam Study. J Hypertens. 2007;25:1421–1426. doi: 10.1097/HJH.0b013e32811d6a07. [DOI] [PubMed] [Google Scholar]
- 7.Lu DY, Sung SH, Yu WC, Cheng HM, Chuang SY, Chen CH. Wave reflections, arterial stiffness, heart rate variability and orthostatic hypotension. Hypertens Res. 2014;37:1056–1061. doi: 10.1038/hr.2014.127. [DOI] [PubMed] [Google Scholar]
- 8.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P, Battista F, Settimi L, Desamericq G, Dolbeau G, Faini A, Salvi P, Mannarino E, Parati G. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension. 2012;60:369–377. doi: 10.1161/HYPERTENSIONAHA.112.197491. [DOI] [PubMed] [Google Scholar]
- 10.Maillard P, Mitchell GF, Himali JJ, Beiser A, Tsao CW, Pase MP, Satizabal CL, Vasan RS, Seshadri S, DeCarli C. Effects of Arterial Stiffness on Brain Integrity in Young Adults From the Framingham Heart Study. Stroke. 2016;47:1030–1036. doi: 10.1161/STROKEAHA.116.012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic Stiffness and the Risk of Incident Mild Cognitive Impairment and Dementia. Stroke. 2016;47:2256–2261. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geijselaers SL, Sep SJ, Schram MT, van Boxtel MP, van Sloten TT, Henry RM, Reesink KD, Kroon AA, Koster A, Schaper NC, Dagnelie PC, van der Kallen CJ, Biessels GJ, Stehouwer CD. Carotid stiffness is associated with impairment of cognitive performance in individuals with and without type 2 diabetes. The Maastricht Study. Atherosclerosis. 2016;253:186–193. doi: 10.1016/j.atherosclerosis.2016.07.912. [DOI] [PubMed] [Google Scholar]
- 13.Finucane C, O'Connell MD, Fan CW, Savva GM, Soraghan CJ, Nolan H, Cronin H, Kenny RA. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA) Circulation. 2014;130:1780–1789. doi: 10.1161/CIRCULATIONAHA.114.009831. [DOI] [PubMed] [Google Scholar]
- 14.Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell GF, Izzo JL, Jr, Lacourciere Y, Ouellet JP, Neutel J, Qian C, Kerwin LJ, Block AJ, Pfeffer MA. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002;105:2955–2961. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]
- 16.Lucas CL, Wilcox BR, Ha B, Henry GW. Comparison of time domain algorithms for estimating aortic characteristic impedance in humans. IEEE Trans Biomed Eng. 1988;35:62–68. doi: 10.1109/10.1337. [DOI] [PubMed] [Google Scholar]
- 17.Westerhof N, Sipkema P, van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res. 1972;6:648–656. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension. 2008;51:1123–1128. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsao CW, Seshadri S, Beiser AS, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S. Association of Aortic Stiffness With Cognition and Brain Aging in Young and Middle-Aged Adults: The Framingham Third Generation Cohort Study. Hypertension. 2016;67:513–519. doi: 10.1161/HYPERTENSIONAHA.115.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 23.Rucka D, Marek J, Rucklova Z, Lubanda JC, Havranek S, Skvaril J, Varejka P, Chochola M, Karetova D, Korinek J, Linhart A. Arterial stiffening contributes to impairment of cerebrovascular reactivity in patients with coronary artery disease without carotid stenosis. Physiol Res. 2015;64:335–343. doi: 10.33549/physiolres.932837. [DOI] [PubMed] [Google Scholar]
- 24.Cooper LL, Woodard T, Sigurdsson S, van Buchem MA, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Harris TB, Gudnason V, Launer LJ, Mitchell GF. Cerebrovascular Damage Mediates Relations Between Aortic Stiffness and Memory. Hypertension. 2016;67:176–182. doi: 10.1161/HYPERTENSIONAHA.115.06398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zavoreo I, Demarin V. Breath holding index and arterial stiffness as markers of vascular aging. Curr Aging Sci. 2010;3:67–70. doi: 10.2174/1874609811003010067. [DOI] [PubMed] [Google Scholar]
- 27.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 29.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell GF, Moye LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, Flaker GC, Pfeffer MA. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997;96:4254–4260. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- 31.Henson J, Dunstan DW, Davies MJ, Yates T. Sedentary behaviour as a new behavioural target in the prevention and treatment of type 2 diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):213–220. doi: 10.1002/dmrr.2759. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama T, Wijndaele K, Koohsari MJ, Tanamas SK, Dunstan DW, Owen N. Adverse associations of car time with markers of cardio-metabolic risk. Prev Med. 2016;83:26–30. doi: 10.1016/j.ypmed.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.