Abstract
Tumor cells actively produce, release and utilize exosomes to promote tumor growth. Mechanisms through which tumor-derived exosomes subserve the tumor are under intense investigation. These exosomes are information carriers, conveying molecular and genetic messages from tumor cells to normal or other abnormal cells residing at close or distant sites. Tumor-derived exosomes are found in all body fluids. Upon the contact with target cells, they alter phenotypic and functional attributes of recipients, reprogramming them into active contributors to angiogenesis, thrombosis, metastasis and immunosuppression. Exosomes produced by tumors carry cargos that in part mimic contents of parent cells and are of potential interest as non-invasive biomarkers of cancer. Their role in inhibiting the host antitumor responses and in mediating drug resistance is important for cancer therapy. Tumor-derived exosomes may interfere with cancer immunotherapy, but they also could serve as adjuvants and antigenic components of antitumor vaccines. Their biological roles in cancer development or progression as well as cancer therapy suggest that tumor-derived exosomes are critical components of oncogenic transformation.
1. Introduction
Exosomes are small extracellular vesicles (EVs) produced by all cells and present in all body fluids [1]. Cells release several types of EVs which differ in size and include apoptotic bodies (1,000–5,000nm), intermediate-size microvesicles (MVs, 200–1,000nm) and the smallest, exosomes (30–150nm)[2]. Exosomes are similar in size to viruses. In addition to size, EVs also differ from one another by cellular mechanisms used for their secretion, molecular content and functional properties [3, 4]. Apoptotic bodies represent post-apoptotic remnants of parent cells. Microvesicles (MVs) are formed by “blebbing” or “pinching off” of the cellular membrane in the parent cell and contain parts of the cytosol more or less randomly enclosed in vesicular “blebs.” The biogenesis of exosomes which involves the endosomal compartment sets them apart from other EVs, and it suggests a potential role for exosomes in maintaining cellular homeostasis [5]. In fact, when in the 1980s the Stahl’s and Johnstone’s pioneering work [6, 7] showed that 50nm vesicles were released from maturing reticulocytes carrying and externalizing the transferrin receptor, this process was considered a form of disposing of cellular waste rather than the deliberate re-cycling of the receptor for its future use. It took several decades to replace the image of exosomes as “cellular garbage collectors” with one of exosomes as information conveying vesicles. Today, exosomes are emerging as excellently equipped vehicles for information transfer between cells [8]. As such, exosomes play a critical biological role in cellular interactions and influence a broad variety of cellular activities in health and disease.
In cancer, where the tumor develops to become the main driver of cellular interactions, converting the normal physiological processes to the ones benefiting the tumor, exosomes are likely to play a particularly important role. Horizontal information transfer by exosomes from the tumor to local or distant body sites facilitates tumor growth and metastasis [9]. Exosomes have been dubbed “oncosomes,” as they carry oncogenes between tumor and normal cells [10]. They transfer proteins, lipids, and nucleic acids in a functionally-active form and regulate gene expression in recipient cells thus determining their behavior [11]. The exosomal cargo packaged and enclosed inside the exosome membrane is safe from extracellular enzymes and is delivered intact to targeted cells. While it is not yet clear whether exosomes are specifically “addressed” to different recipient cells, and the mechanisms of sorting and packaging of the exosomal cargo are only now emerging [2], the possibility that exosomes are an integral part of a complex, well-organized intercellular system of communication has to be considered. Exosomes are produced by bacteria, plants and animals, including man, and thus this communication system is evolutionarily conserved [12, 13]. Its ubiquitous existence in unicellular and multicellular organisms suggests that it is indispensable for survival. The biology of tumor-derived exosomes is incompletely understood, and much remains to be done in order to define the molecular and genetic underpinnings of their operation in cancer and other human diseases. This chapter reviews the currently available data to illustrate the mechanisms of cellular cross-talk these exosomes utilize and the consequences of information transfer by exosomes for cancer progression.
2. Exosome biogenesis in cancer
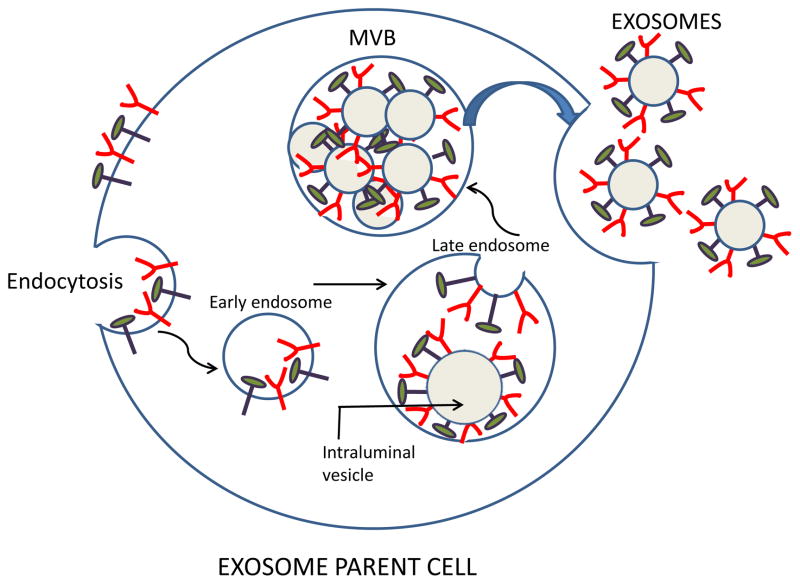
Exosomes are produced by virtually all normal and pathological cells and are found in all body fluids, including plasma, urine, saliva, amniotic fluids, ascites, cerebrospinal fluid, etc. [2]. Exosomes originate from the multivesicular endosome (MVE) and thus have a different biogenesis than other EVs [2, 14]. It begins with the formation of the endosome by the inward invagination of the cellular plasma membrane. As an early endosome matures into a late endosome, its membrane undergoes a series of inward invaginations which then close and form numerous intraluminal vesicles (Figure 1). At this point, the endosome becomes a multivesicular body (MVB) containing multiple vesicles each enclosing a small part of the cytosol, including various proteins and nucleic acids [14]. When MVBs fuse with the plasma membrane, exosomes are released into an extracellular space (Figure 1). Not all MVBs fuse with the plasma membrane to release exosomes; an alternative degradation pathway for the MVB cargo is fusion with the lysosome. In the presence of a high content of ceramides in the membrane-associated lipids, MVBs are spared from lysosomal degradation and tend to fuse with the cell membrane releasing the exosomes [2]. Millions of exosomes are released in this fashion by the tumor parent cell. Each exosome carries an imprint of the parent cell. Its content includes nucleic acids, proteins, enzymes, lipids, cytokines and other soluble factors, which are components of the parent cell [4, 12]. Because of their endosomal origin, exosomes carry components of the endosomal sorting complex required for transport (ESCRT) and various ESCRT-associated molecules [15, 16]. These molecules are often used as exosome markers confirming the endocytic origin of vesicles.
Figure 1.
Exosome biogenesis and their release from a parent cell. The surface membrane undergoes endocytosis enclosing surface-residing molecules into an early endosome. Invagination of the endosomal membrane in late endosomes leads to the formation of intraluminal vesicles, each decorated with molecules originally present on the cell surface. Endosomes form a multivesicular body (MVB) containing pools of intraluminal vesicles. Fusion of the MVB with the cell surface membrane leads to release of free exosomes into the extracellular space. Note that components of the parent cell surface are now present on the membrane surrounding each exosome.
The exosome biogenesis produces exosomes with the molecular profile which partly, but not completely, resembles that of the parental cell and contains components of the endocytic pathway [2, 12, 17]. Exosomes carry the same molecules as MVEs but, as a consequence of intraluminal invagination, with the orientation analogous to that of the parent cell membrane. The membranes of exosomes are enriched in endosome-related membrane transport and fusion proteins, such as Annexins, flotillin or GTPases and lipids such as ceramides, sphingomyelin, and cholesterol [18–20]. The exosome surface is also decorated with endosome-specific tetraspanins (CD9, CD63, CD81, CD82) [21, 22] as well as the accessory ESCRT pathway proteins, ALIX, TS101 and others [15]. Tetraspanins are organized into membrane microdomains which appear to play an important role in exosome biogenesis [21]. The ESCRT complex represents the machinery for exosome sorting, packaging and transport [23]. It consists of four membrane-bound aggregates of ubiquitinated proteins [15]. Two other ESCRT-independent sorting mechanisms have also been described [15]. The vesicular and membrane content of exosomes does not precisely duplicate that of parent cells, although similarities in the molecular and genetic profile are usually present, and this partial similarity has given rise to the concept of exosomes as putative biomarkers of the tumor. Newer data suggest that the profiles of the exosome cargo may differ substantially from those in parent cells. In one study, the relative quantities of proteins and nucleic acids in exosomes were found to be different from those in the parent cells [23]. Such differences may reflect the existence of a sorting process taking place during packaging of the cellular content into exosomes, although the exact way of how this process is regulated is still unknown. The exosome liberation from the parent cell appears to involve several highly regulated steps: trafficking of MVBs to the plasma membrane, docking, fusion and release. These events require participation of the cytoskeleton (actin and microtubules), associated kinesins and myosins acting as molecular motors, Rab GTPases functioning as molecular switches, and a set of molecules driving MVB fusion with the cell membrane, including the SNARE complex [12, 17].
Other EVs, especially MVs formed by membrane “blebbing” and called ectosomes [14], might be equally capable of disseminating information from parental to target cells. To date, the distinction made between exosomes and MVs is not entirely clear [14]. The difference in vesicle size is not a reliable defining criterion, as exosomes might aggregate into larger vesicles or MVs can split into smaller vesicles. It is also not clear at present whether exosomes or MVs carry the molecular cargo that is more representative of the parent cell and thus more useful as a potential cancer biomarker. Perhaps because the biogenesis of exosomes is an active, energy-requiring and molecularly-orchestrated process rather than passive “pinching off” from the surface membrane, the general tendency is to think of exosomes as more faithful “representatives” of the parental cell phenotype and genotype. While exosomes are emerging as carriers of potentially useful information about the genetic, molecular and functional characteristics of the cell that secrets them, it is becoming clear that cells differ greatly not only in the quantity but also the quality of exosomes they secrete.
The exosome biogenesis is enhanced in cancer. Notably, tumor cells produce and secrete many more exosomes than normal proliferating cells [24, 25]. Exosome levels in plasma and other body fluids of patients with cancer are frequently elevated [23]. It has been suggested that stress, including hypoxia prevalent in the tumor microenvironment (TME), accounts for this copious exosome secretion by tumor cells [26, 27]. Exosome production and release by tumor cells was also reported to be regulated by the p53 protein which is often aberrantly stimulated in cancer [28]. Heparanase, an enzyme overexpressed in in many tumor cell lines, was recently reported to regulate exosome secretion [29]. The Rap GTPase proteins, especially Rab27a and Rab27b, controlling the secretory pathways are strongly implicated in exosome release [30, 31]. Knock down of Rab proteins has been shown to decrease exosome secretion by tumor cells [31, 32]. To date, the mechanisms tumor cells employ to regulate exosome secretion remain unknown, although emerging insights suggest that several distinct mechanisms may be involved and may depend on the cancer type and its aggressiveness.
3. Morphology and molecular content of tumor-derived exosomes
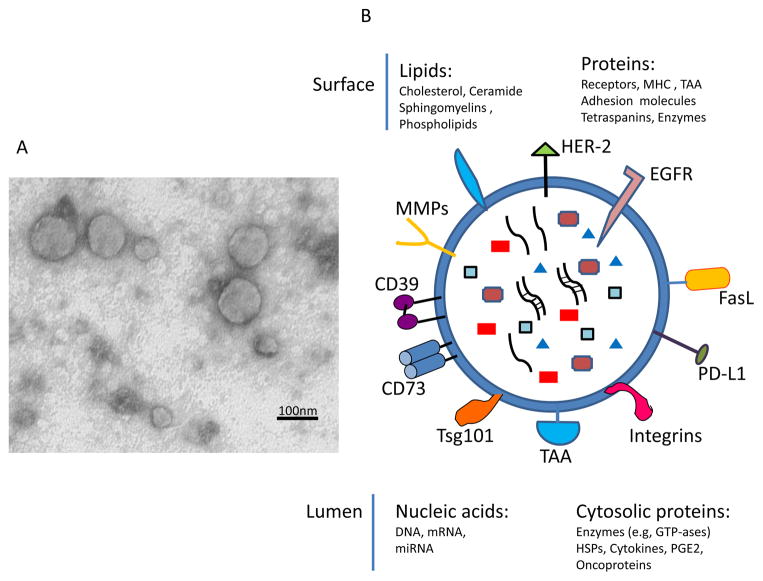
Morphology of tumor-derived exosomes can only be determined by electron microscopy (EM), and it is similar to that of all other exosomes. They are spherical membrane-bound vesicles, which often measure less than 50nm in diameter and form aggregates of various sizes. Preparation of tumor-derived exosomes for EM may result in artifacts, such as the frequently-reported doughnut-shaped appearance or smaller than expected sizes which may be due to shrinking during sample processing. Scanning EM or epon-embedded exosome sections provide a more realistic view of the exosomes tumors secrete, as illustrated in Figure 2A. Immuno-EM has confirmed the presence on the surface of tumor-derived exosomes of molecules known to be present on the surface of parent tumor cells [33].
Figure 2.
Exosome morphology and the molecular content. In A, transmission electron microscopy (TEM) of tumor-derived exosomes isolated from a tumor cell supernatant, pelleted by ultracentrifugation, embedded in Epon, sectioned and viewed by TEM. Note different sizes of membranous vesicles (courtesy of Dr. Simon Watkins, University of Pittsburgh). In B, the cartoon of an exosome illustrating the presence of a broad variety of molecules on its surface (some of the most common are indicated) and in the vesicle lumen.
The molecular cargo tumor-derived exosomes carry is in part derived from the surface of parent tumor cells (Figure 1). This molecular signature discriminates among exosomes produced by different tumor cells, and it also distinguishes tumor-derived exosomes from exosomes released by non-malignant cells [34]. Western blots of exosomes isolated from tumor-cell supernatants and exosome fractions obtained from cancer patients’ plasma confirm the presence of a broad variety of molecular species associated with different cellular activities. As cancer cells tend to express and utilize various immunosuppressive molecules to blunt anti-tumor immune responses [35], tumor-derived exosomes are enriched in immunosuppressive proteins, including death receptor ligands such as FasL or TRAIL, check point receptor ligands such as PD-L1, inhibitory cytokines, e.g., IL10 and TGF-β1, as well as prostaglandin E2 (PGE2) and ectoenzymes engaged in the adenosine pathway, CD39 and D73 [36, 37] (Figure 2B). However, in addition to this immunosuppressive cargo, tumor-derived exosomes also carry tumor-associated antigens (TAA), co-stimulatory molecules and the MHC components, which enable them to stimulate immune cells and promote anti-tumor responses [38–40]. This type of molecular profile endows tumor-derived exosomes with a dual capability of mediating either immune suppression or immune stimulation, presumably depending on the microenvironment environment into which they are released by parent cells. This example of exosome capabilities to interact in opposing ways with immune cells and mediate either a loss or a gain of anti-tumor immune responses [40, 41] illustrates the plasticity of exosomal interactions that is likely orchestrated by the tumor and that has led to a major controversy concerning the role of tumor-derived exosomes in cancer. Although the current evidence suggests that the immunostimulatory or immunoinhibitory functions of exosomes released by tumor cells might be variable, depending on the cargo they transport and the functional status of immune cells in the TME, it has been difficult to reconcile these two opposing aspects of exosome functionality. The reasonable conclusion is that tumor-derived exosomes can simultaneously impact a large number of functions in recipient cells and that the composition of their cargo and the ability of recipient cells to accept or reject the transported signals determines outcome.
4. Communication of tumor-derived exosomes with their cell targets
Before tumor-derived exosomes can transfer information they carry from tumor to other malignant or normal cells, they have to ensure its delivery [8, 11]. In fact, exosomes are admirably equipped to serve as communication vehicles. Their surface is decorated by the parent cell-derived signaling molecules and their intra-vesicular content includes DNA, mRNA, micro RNA as well as enzymes and soluble factors, all biologically active and capable of executing functional responses in target cells and re-programing activities of these cells [19]. Exosomes interact with target cells utilizing one or more of the following mechanisms: (a) direct signaling via surface molecules to activate intracellular signaling pathways; (b) fusion with the target cell membrane followed by transfer of proteins or genes the cell lumen; (c) phagocytosis of opsonized exosomes and their internalization; (d) receptor-mediated endocytosis [42]. The exosome-delivered cargo taken up by phagocytosis or endocytosis may be either directed to the lysosomes for degradation and clearance or directly incorporated into the cellular machinery to initiate the re-programming. Figure 3 summarizes the various pathways of exosome uptake by recipient cells and cellular consequences of such uptake.
Figure 3.
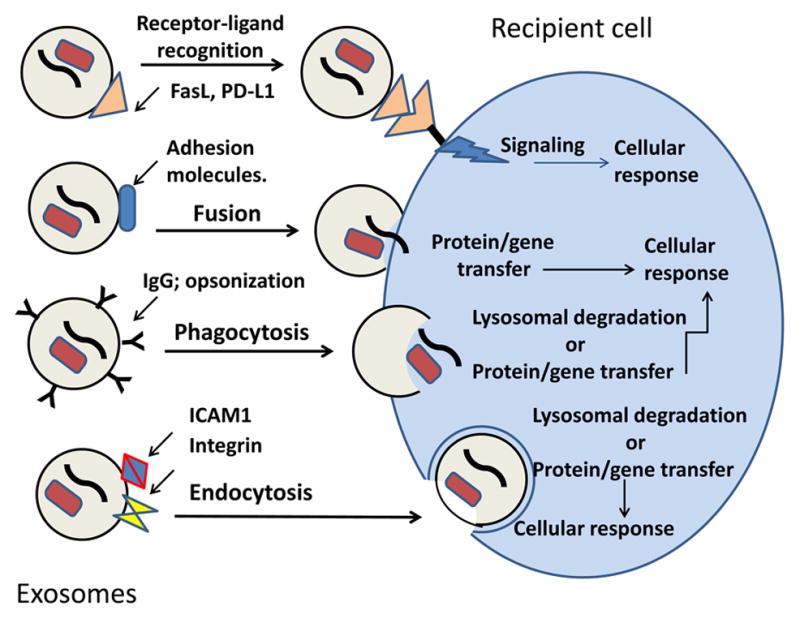
Exosome uptake by cells. Exosomes may interact with target cells by one of several pathways, which depend on the cargo components they carry and on the presence of the recognizable “address” on the target cell membrane. Following the surface contact or uptake, exosomes transfer proteins and genes to the target cell and re-program its activities.
5. Biological functions of tumor-derived exosomes
Similar to other exosomes, those produced by tumor cells are involved in a broad variety of cellular functions and participate in physiological as well as pathological events [12]. Exosomes transfer information locally within the TME as well as systemically to distant tissue sites. The latter requires that exosomes migrate or be delivered from the tumor to distant tissues, but since they freely distribute throughout all body fluids they readily access all sites creating a communication network. Cancer cells secrete millions of exosomes in order to re-program their surroundings to tumor-promoting microenvironment. The communication network exosomes create is entirely tumor-driven and designed to promote tumor progression and metastasis, in part by silencing anti-tumor immune responses but also by altering stromal cell responses, supporting new vessel growth or promoting survival of tumor cells (Table 1). The ability of tumor-derived exosomes produced by mouse melanoma cells to educate and transform the bone marrow environment into a melanoma-promoting milieu has been elegantly demonstrated by Peinado et al [32] and exosome-mediated alterations of the bone marrow have been shown to interfere with the hematopoietic cell development, differentiation and functions [8, 63]. The role of exosomes in blood coagulation has been extensively investigated [64, 65]. Exosomes can deliver functioning surface receptors or ectoenzymes from one cell to another and thus restore missing functions. Various mRNAs and microRNAs carried by exosomes can be transferred to recipient cells and translated into functional proteins [66]. Most importantly, tumor derived exosomes are called “oncosomes,” because of evidence for the presence in their cargo of oncogenes and factors promoting tumor growth [10]. The ability of oncosomes to carry and deliver oncogenic signals to target cells, sustain autocrine growth promoting pathways in parent tumor cells and modify functions of stromal cells in the TME supports the conclusion that tumor-derived exosomes play a critical role in oncogenic transformation [10]. Table 1 illustrates functional imprints of tumor-derived exosomes on recipient cells.
Table 1.
Functions of tumor-derived exosomes in cancer1
| Cancer | Exosome component | Targeted cell | Function | Reference |
|---|---|---|---|---|
| AML | TGF-β | NK Cells | ↓ cytotoxicity | [24] |
| Breast CA | miR-10b | Mammary epithelial cell | ↑ migration | [43] |
| Breast CA | miR-122 | Lung fibroblasts neurons | ↑ metastasis | [44] |
| CML | miR-210 | EC | ↑ angiogenic activity | [45] |
| Colon CA | Hsp 70 | MDSC | ↑ Immune suppression | [46] |
| Colon CA MUT | KRAS | Colon CA cells (wild-type KRAS) | ↑ tumor cell growth | [47] |
| Colon CA | TF | EC | ↑ coagulation | [48] |
| Cervical CA | Survivin | Cervical CA cells | ↑ survival | [49], [50] |
| Cervical CA | MICA | NK cells | ↓ cytotoxicity | [51] |
| GIST | KIT | Progenitor muscle cells | ↑ Invasiveness | [52] |
| Glioblastoma | EGFR vIII | Glioblastoma cells | ↑ tumor growth | [53] |
| HCC | miR-584, 517c, 378 | HCC cells | ↑ tumor growth ↑ metastasis |
[54] |
| HNC | FasL | Activated CD8+ T cells | Induces apoptosis | [33] |
| Melanoma | MET | BM progenitor cells | ↑ tumor growth ↑ metastasis |
[32] |
| Multiple myeloma (BM-MSC) | IL-6, fibronectin | MM cells | ↑ tumor growth | [55] |
| Multiple myeloma | miR-135b | EC | ↑ migration ↑ tube formation |
[56] |
| Mesothelioma | TGF-β | Fibroblasts | ↑ angiogenesis ↑ differentiation |
[57] |
| NPC | HIF1α | NPC cells | ↑ migration ↑ invasion |
[58] |
| Ovarian CA | FasL | Activated T cells | ↑ immune suppression | [59] |
| Pancreatic CA | MIF | Liver Kupfer cells | ↑ metastatic niche formation | [60] |
| Prostate CA | αvβ6 Integrin | Prostate CA cells | ↑ migration | [61] |
| Various tumors | CD39/CD73 | T cells, Treg tumor cells | ↑ adenosine production | [62] |
A selected list of functional changes induced by tumor derived exosomes to illustrate the involvement of different components of the exosome cargo in promoting tumor progression and metastasis.
Abbreviations in Table 1: AML, acute myeloid leukemia; BM, bone marrow; CA, carcinoma; CML, chronic myelogenous leukemia; EC, endothelial cells; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; MDSC, myeloid-derived suppressor cells; MIF, migration inhibitory factor; NK, natural killer cells; TF, transfer factor; TGF-β, transforming growth factor β
6. Methods available for studies of tumor-derived exosomes
Most of the molecular studies performed to date used exosomes isolated from supernatants of cultured tumor cells. The methodology for exosome isolation generally involved a series of differential centrifugations of supernatants to remove cell fragments and large EVs followed by ultracentrifugation at 100,000xg for 2–3 h and further purification of exosomes by floating them on a continuous sucrose density gradient [67]. In these supernatants, tumor cells were the only source of exosomes. To study tumor-derived exosomes present in cancer patients’ body fluids, it is necessary to separate them from larger EVs and also from exosomes derived from non-malignant cells. This requires the development of methods for the capture of tumor-derived exosomes and their quantitative recovery. Both ultracentrifugation, which tends to aggregate exosomes and sucrose density gradients, which lead to a substantial loss of aggregated vesicles have been recently replaced by size-exclusion chromatography on small Sepharose 2A columns [33, 68, 69]. This isolation method allows for recovery of exosomes in the void volume fractions and the removal of “contaminating” plasma proteins in late-eluting fractions. The total isolated exosomal fraction can then be used for immunocapture of tumor-derived exosomes and their separation from exosomes produced by non-malignant cells. Because tumor-derived exosomes carry membrane-embedded molecules which mimic those in parent tumor cells [70], antibodies recognizing tumor-associated antigens (TAA) can be coated on beads and used for exosome capture [71]. Immunocapture of tumor-derived exosomes from plasma of AML patients with CD34+ blasts has been successful in our hands, and it serves as a proof of principle for immune capture of tumor-derived exosomes from body fluids. The captured blast-derived exosomes were morphologically intact by TEM and functionally active, as they were able to down-regulate NKG2D expression in activated normal human natural killer (NK) cells [72]. Methods for immunocapture of tumor-derived exosomes from plasma of patients with solid tumors are being developed. If successful, this strategy will make it possible to study tumor-derived exosomes in parallel with exosomes produced by non-malignant cells and determine which of the two fractions induces changes in target cells that favor tumor progression. Numerous commercially-developed isolation methods for exosomes are available on the market; however, caution has to be exercised in ascertaining that these methods distinguish exosomes from larger EVs and do not compromise exosome integrity or functions.
7. Exosomes in the tumor microenvironment
Tumors are known to be able to re-program their milieu into one favoring tumor progression. The mechanisms responsible for this re-programming have not been well understood, although direct transfer of proteins or genes from tumor cells to their neighbors has been considered [73]. Exosomes fit this role well, providing an explanation for information transfer between the tumor and neighboring tissues. Figure 4 summarizes exosome interactions with cells present in the TME.
Figure 4.
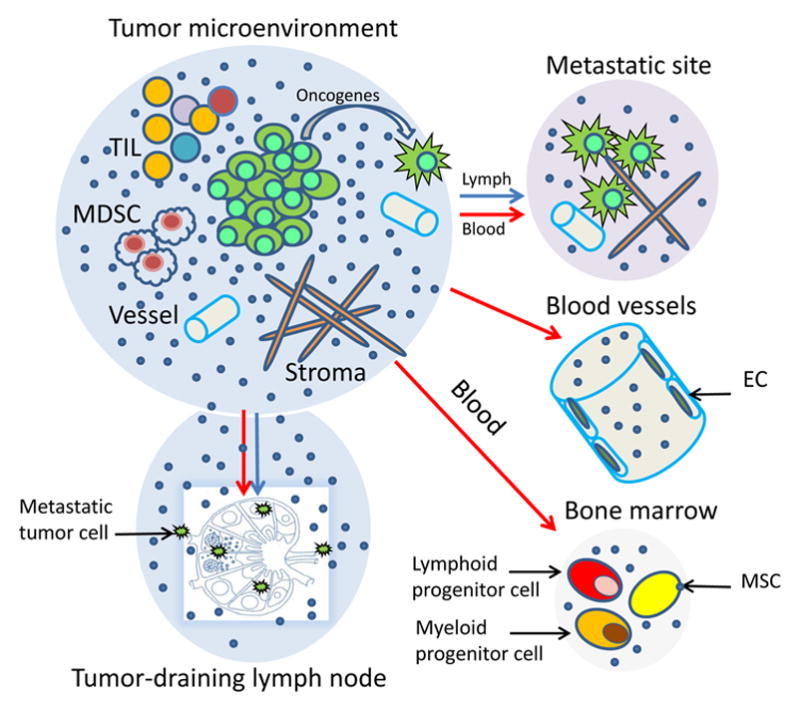
Tumor-derived exosomes and their local and systemic effects. Exosomes alter the tumor microenvironment (TME) and prepare distant tissue sites for metastasis. In the TME, exosomes interact with all cellular components and impact on the stromal, immune as well as vascular compartments. They access tumor-draining lymph nodes, facilitating entry of metastatic cells. They also carry and transfer oncogenes and oncogenic proteins to metastasizing tumor cells. Systemic effects of exosomes at distant tumor sites require that exosomes migrate via the blood or lymph. Once in place, they prepare tissue sites for metastasis or alter/educate the bone marrow (BM) environment, creating a pre-metastatic niche to promote tumor invasion and growth. They re-program mesenchymal stem cells (MSC) as well as progenitor hematopoietic cells. Tumor-derived exosomes interact with vascular endothelial cells (EC) favoring their proliferation, sprouting and the angiotube formation.
7.1. Tumor stroma
Tumors depend on the stroma for support of their growth. A close relationship is established between the developing tumor and stromal fibroblasts. Cancer-associated fibroblasts (CAFs) have long been known to positively or negatively influence tumor growth, depending on conditions prevailing in the TME [74]. Fibroblasts undergo changes in morphology, protein expression, patterns of growth and the cytokine profile during the tumor progression [74, 75]. Specifically, CAF acquire the characteristics of activated fibroblasts and increase the production of collagen and hyaluronic acid [76]. Cancer-derived exosomes have been shown to induce such changes in fibroblasts through the TGFβ/Smad pathway [57, 77]. Further, CAFs co-incubated with tumor-derived exosomes acquire the capability to transform normal epithelial cells to the pre-malignant phenotype [78]. Changes induced in epithelial cells by these exosomes included immature pleomorphic appearance, aberrant mitosis, loss of polarity altered cell cycle protein expression and increased proliferation. These changes were consistent with the signature of oncogenic transformation in fibroblasts.
7.2. Angiogenesis
Tumor growth depends on an access to the host vasculature, so that the tumor can tap into the blood stream. To do so, tumors create their own vascular system by changing the TME from anti-angiogenic to pro-angiogenic milieu (an “angiogenic switch”) [79]. Tumor-derived exosomes have been shown to play a role in promoting angiogenesis [79]. Hypoxic conditions in the growing tumor induce malignant cells to increase production of exosomes [26, 27]. The hypoxia-induced exosomes are taken up by normal endothelial cells, where the exosome cargo stimulates new angiotube formation, eventually establishing a network of new blood vessels [80]. When tumor-derived exosomes were fluorescently labeled and co-incubated with endothelial cells (ECs), they were shown to move within nanotubular structures connecting the ECs, were internalized and stimulated proliferation as well as angiotube formation [81]. The exosomes induced a loss of E-cadherin and β-catenin from the EC surface and promoted EC motility [81]. Upon their addition to EC-containing Matrigel plugs, which were implanted in vivo, exosomes induced EC proliferation and rapid vascularization of the plugs. These alterations were associated with Src phosphorylation and activation of the downstream Src pathway in HUVEC. Further, pro-angiogenic effects of exosomes were abrogated by the use of a tyrosine kinase inhibitor [81]. It has been reported that upon uptake of tumor-derived exosomes, ECs begin secreting cytokines and growth factors which stimulate pericytes via the PI3 kinase/AKT pathway [59]. The molecular mechanisms that are involved in EC re-programming may be also driven by the EGFR delivered to EC on the exosome surface, which activates ECs to produce VEGF and up-regulate VEGFR2 signaling [82]. Exosomes carrying the tetraspanin 8 induce transcriptional changes in EC, resulting in increased expression of pro-angiogenic genes and increased proliferation of ECs [83]. Some of these exosome-mediated post-transcriptional alterations in ECs were shown to be associated with horizontal transfer of mi-RNAs from tumor to ECs [84, 85]. Exosomes were shown to deliver components of the Notch pathway to ECs, promoting sprouting and branching of the new vessels [85]. These and numerous other experiments convincingly demonstrate that exosomes released by tumor cells directly affect molecular and genetic programs in ECs and thus promote the process of neovascularization.
7.3. Effects on immune cells present in the TME
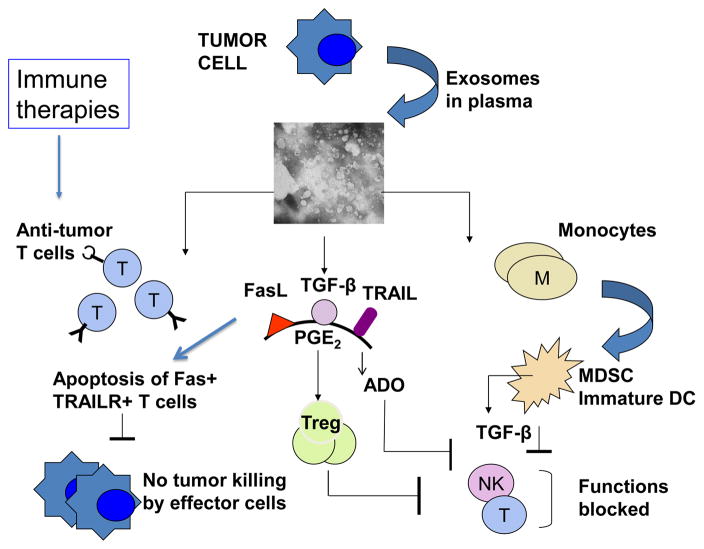
Tumor-infiltrating immune cells are considered to be important anti-tumor effector cells, and accumulations of activated immune cells at tumor sites is taken as a positive prognostic sign in such human solid tumors as, for example, colon carcinoma [86, 87]. Exosomes produced in the TME may stimulate functions of immune cells or suppress their anti-tumor activities. This dual capacity of exosomes to modulate stimulatory or inhibitory immune cell functions is dependent on their cargo and their origin. In early phases of tumor growth, when the host immune system mounts a strong anti-tumor response, activated immune cells are a main source of exosomes in the TME. These exosomes could promote anti-tumor functions of immune cells, especially of dendritic cells (DC) and lymphocytes. On the other hand, in more advanced cancers, expanding tumor cells, which have escaped immune surveillance, produce exosomes which carry an immunosuppressive cargo and become active participants in the tumor escape from the host immune system. Exosome-mediated suppression leads to: (a) inhibition of T-cell proliferation and cytolytic activity of CD8+ effector T cells; (b) inhibition of natural killer (NK) cell anti-tumor functions; and (c) skewing of the differentiation of myeloid progenitor cells and of T lymphocytes toward suppressive phenotypes, including myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg), respectively.
The effects of tumor-derived exosomes on immune cells can be direct (when signals or cargo delivered by exosomes block the targeted cell functions) or indirect (when exosomes re-program differentiation program of targeted cells, which then suppress functions of other cells). Together, these effects create an immunoinhibitory microenvironment favoring tumor immune escape. Exosome-mediated dysregulation of lymphocytes results in a partial or complete inhibition of their functions, including signaling via the key receptors such as the T-cell receptor (TCR) or IL-2R, differentiation, cytokine production, proliferation, migration and cytotoxicity [39]. Tumor-derived exosomes induce apoptosis of activated CD8+ effector T cells in vitro and in the circulation of patients with cancer [88–90]. NK cells are another subset of lymphocytes engaged in elimination of abnormal targets such as tumor or virally-infected cells. NK cells are highly vulnerable to inhibition by exosomes carrying NKG2D ligands (MICA, MICB and ULBP), as binding of these membrane-bound ligands to the inhibitory receptor, NKG2D, on the surface of NK cells delivers inhibitory signals and blocks anti-tumor cytotoxicity [91, 92]. The inhibitory effects of tumor-derived exosomes are also attributed to the presence in their cargo of transforming growth factor beta 1 (TGFβ1), a cytokine known to suppress NK- cell cytotoxicity [24]. Monocytes interacting with tumor-derived exosomes carrying TGF-β and PGE2 fail to differentiate into antigen-presenting DC and instead are directed to a differentiation pathway generating MDSC [93]. The newly-minted MDSC accumulate and produce numerous immunosuppressive inhibitory factors, including nitric oxide (NO) and reactive oxygen species (ROS), which cause nitration of TcRs or T-cell apoptosis [94, 95]. MDSC consume and cause deprivation of arginine and cysteine, which are required for T-cell activities [94, 95]. With the paucity of DC to present antigens to T cells and the presence of MDSC, the TME becomes strongly immunoinhibitory. Tumor-derived exosomes also induce skewing of CD4+T lymphocyte differentiation converting conventional CD4+CD25neg T cells to CD4+CD25highFOXP3+ regulatory T cells (Treg). TGFβ1 and IL-10 signaling is critical for this conversion[96]. Treg co-incubated with tumor-derived exosomes up-regulate expression levels of FasL, TGFβ, IL-10, CTLA-4, granzyme B (GrB), perforin and suppressor functions [96, 97]. Immunosuppressive potential of human tumors is maintained by many different mechanisms, and exosomes released by tumor cells play a key role in disseminating immunoinhibitory signals throughout the TME and beyond.
The TME is dominated by the tumor which exerts inhibitory effects on the immune cells accumulating locally and shifts the balance toward immune suppression. Therefore, exosome-mediated immune stimulation presumably represents a relatively small part of the overall anti-tumor immune response mounted by the host. Nevertheless, since the cargo of tumor-derived exosomes contains all of the components necessary for generation for immune responses to TAA [39, 40, 70], it is expected that these exosomes upon reaching tissue sites in which the microenvironment is favorable can activate immune cells and drive anti-tumor immunity. In effect, immunostimulatory effects of tumor-derived exosomes are likely to be systemic and optimally effective away from the local TME, as discussed below.
7.4. Effects on tumor cells
Exosomes represent an effective way of autocrine or paracrine communication between tumor cells. It has been reported that the presence of previously secreted exosomes in the microenvironment inhibits further exosome release, indicating that a negative feedback loop controls the secretory pathway [98]. Tumors also readily take up exosomes [99, 100], which suggests that a positive feedback loop also exists and may be important for tumor cell functions, as suggested, for example, by the study examining expression and functions of soluble CD171 (L1, an adhesion molecule on the surface of ovarian carcinoma cells) in exosomes [101]. The paracrine interactions involve communication among tumor cells or those between tumor and normal cells. Exosome-mediated transfer of oncogenes and oncogenic signals from one tumor cell to another or from tumor cells to normal cells is well documented in the literature [10, 102]. For example, colon carcinoma cells expressing mutant KRAS release exosomes which carry mutant KRAS and such growth-promoting proteins as EGFR, SRC family kinases and integrins [47]. Exosomes derived from glioblastoma expressing activating EGFRvIII mutation transfer this receptor to glioblastoma cells lacking this mutation and convert the recipient cells to a more malignant EGFRvIII-dependent phenotype [53]. Intercellular transfer of molecules containing oncogenic mutations to normal or malignant recipient cells includes activated oncoproteins, their transcripts, oncogenic DNA sequences and oncogenic micro-RNAs and leads to re-programming of cellular pathways, especially those responsible for growth factor production [53, 80, 102].
8. Systemic effects of tumor-derived exosomes
Communication between adjacent cells in multicellular organisms usually consists of exchanges or sharing of cellular fragments via the formation of intercellular junctions, synapses or by trogocytosis, all of which require cellular contact and operate at short distances. In contrast, exosomes represent a unique form of information delivery that operates at short and long distances. Tumor-derived exosomes can transfer signals and convey information from tumors to distant tissues and organs. They are present in the circulation and have ready access to all parts of the body (Figure 5). They carry surface components that enable contact with endothelial cells and facilitate exosome entry into vessels and tissues [80, 82]. But tumor-derived exosomes represent only a fraction of total exosomes present in plasma of patients (Figure 5). This fraction may change in volume depending on tumor progression.
Figure 5.
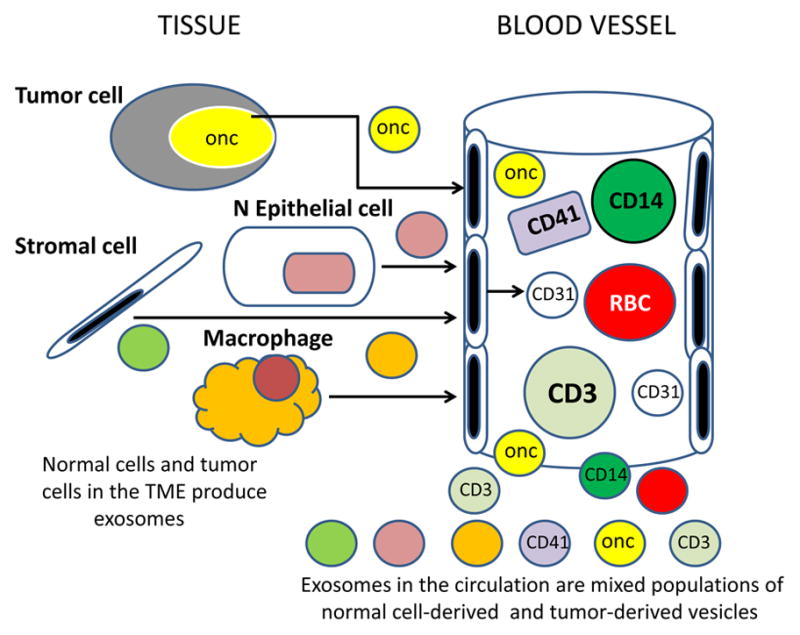
Exosomes present in the circulation or in body fluids of cancer patients are derived from many different normal and abnormal cells. Exosomes released into the extracellular space are distributed via the blood and lymph to distant body sites. In plasma, for example, tumor-derived exosomes represent only a small fraction of total exosome population which derives from normal circulating as well as tissue cells.
8.1 Metastasis
The process of metastasis begins with tumor cells undergoing an epithelial-to-mesenchymal transition (EMT). Tumor cells gain the ability to migrate, to access the blood or lymphatic routes, circulate and establish metastases. These tumor cells now produce exosomes with a distinct molecular profile, which is characterized by the appearance of EMT-associated proteins and molecules needed for migration and invasion, including αvβ6 integrin in exosomes released by prostate cancer [103], the Wnt pathway components in leukemia- or breast cancer-derived exosomes [46, 63], and KIT in exosomes produced by gastrointestinal stromal tumors (GIST) [104]. The content of HIFs is increased in these exosomes, as is the content of pro-inflammatory factors [90]. The exosomes produced by tumor cells ready to migrate are equipped to interact with blood vessels, stromal elements and immune cells in order to establish a pre-metastatic niche [105] (Figure 6). Melanoma-derived exosomes were shown to accumulate in sentinel lymph nodes, stimulate angiogenesis, re-model extracellular matrix and induce melanoma cells recruitment to the lymph nodes [106]. Peinado and colleagues demonstrated that exosomes derived from highly metastatic murine melanoma cells were able to re-program the bone marrow to a pre-metastatic niche that now supported development of highly invasive melanoma cells. The metastatic burden increased three folds in mice injected with melanoma cells and progenitor cells previously treated with these exosomes compared to untreated controls [32]. The changes that occurred in the bone marrow were associated with the transfer by exosomes of the oncoprotein MET. In numerous recent studies with murine and human tumor-derived exosomes, it has been demonstrated that these exosomes also carry micro-RNA species, transfer them to normal cells and induce changes in genetic and protein profiles which favor metastasis formation [95, 107, 108]. Tumor-derived exosomes have been shown to carry CD39 and CD73, the ectonucleotidases catalyzing adenosine production [37]. Adenosine is a well-known factor that mediates immunosuppression, promotes angiogenesis and drives stromal remodeling [62], all of which facilitate migration of tumor cells and their entry into lymph nodes (Figure 7). This ability of tumor-derived exosomes to support metastasis may be accomplished by engaging different molecular pathways, and adenosine is but one example of how tumors co-opt a factor normally involved in the regulation of inflammation to facilitate metastasis [62].
Figure 6.
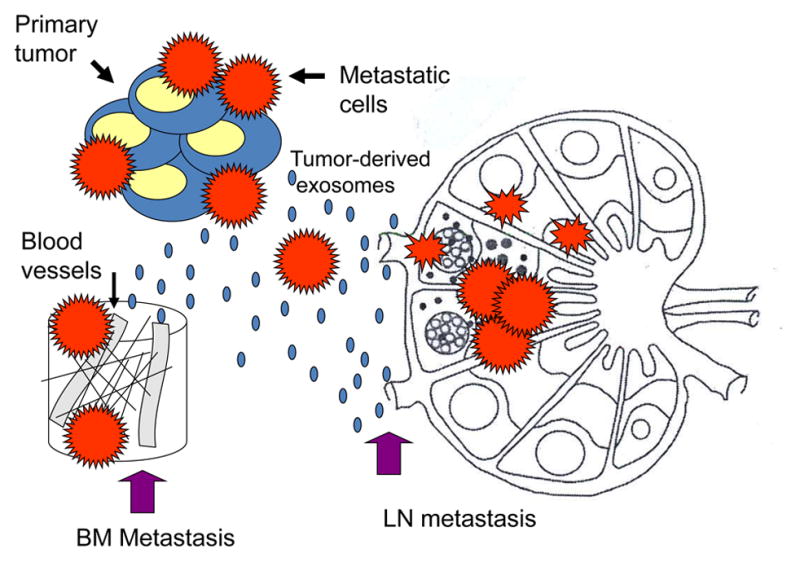
Pre-conditioning of lymph nodes (LN) or bone marrow (BM) niches by tumor-derived exosomes. These exosomes reach LNs or the BM prior to metastasizing tumor cells and re-program the local environment to facilitate the entry and “prepare the soil” for tumor cells.
Figure 7.
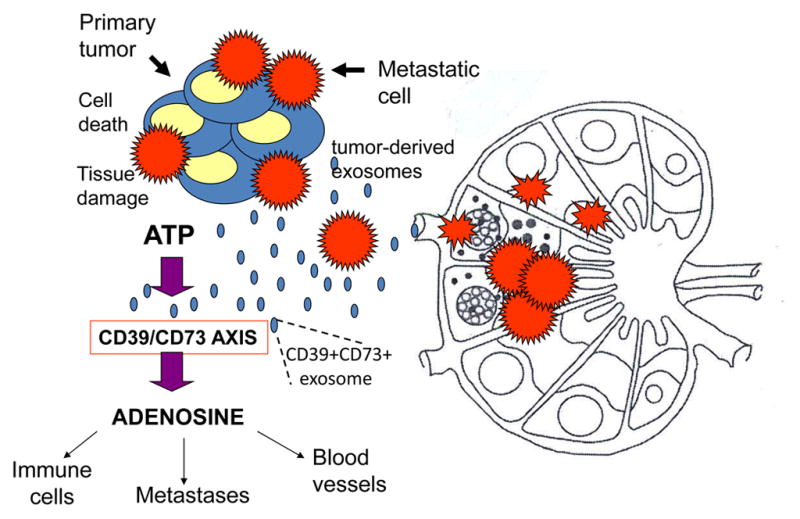
Adenosine as a known metastasis-promoting factor. Tumor-derived exosomes carry CD39 and CD73, the ectonucleotidases which catalyze adenosine production in the presence of ATP. The TME is rich in ATP, and the activated CD39/CD73 axis promotes adenosine production. Adenosine induces inhibition of antitumor immune cell functions, promotes angiogenesis and favors metastasis, adenosine is only one of numerous other pro-tumorigenic factors that are regulated by tumor-derived exosomes.
8.2. Exosomes and drug resistance
Resistance of tumors to radio- and chemotherapy is a serious and so far unsolved problem. One of the clinically undesirable effects of tumor-derived exosomes that merits attention is their ability to horizontally transfer drug resistance [109, 110]. Tumor-derived exosomes contribute to the development of drug resistance by several mechanisms. The initially described functions of exosomes as “garbage shuttles” may be a correct one in respect to drug resistance (Figure 8). Chemotherapeutic compounds such as cisplatin may be concentrated and removed from the cytosol by exosomes [109]. Alternatively, tumor cells could simply package chemotherapy drugs into exosomes to protect themselves from cytotoxic effects. Drug-resistant tumor cells could transmit resistance to sensitive cells via exosomes creating new pools of resistant tumor cells. It has been shown, for example, that certain micro-RNAs (miR-100, miR-222, miR-30a and miR-17) were when transferred in exosomes from adriamycin- and docetaxel-resistant breast cancer resistant cell lines to sensitive cell lines conferred resistance [97]. In breast carcinoma, exosomes carrying HER2 and produced by HER2+ cell lines or obtained from plasma pf patients with HER2+ tumors scavenge trastuzumab, thus reducing its availability in the circulation [111, 112]. Docetaxel resistance has been studied in prostate cancer, where it was found to be conferred by transfer by exosomes of multidrug resistance protein-1 (MDR-1/P-gp), a P-glycoprotein transporter that is commonly overexpressed in drug-resistant tumors [110]. Cisplatin-resistant ovarian carcinomas produce exosomes which are enriched in other transporter proteins such as MDR-2, ATP-7A, and ATP-7B [109]. More recent studies indicate that drug resistance is, in part, attributable to intercellular transfer by exosomes of miRNA species from drug-resistant to sensitive cancer cells [97]. In vivo studies in melanoma animal models indicated that resistance to cisplatin attributed to exosomes could be effectively reduced by the combined use of proton pump inhibitors (PPI) and low pH [113]. While exosomes are clearly implicated in transferring drug resistance in cancer, a more detailed molecular and genetic profiling will be necessary to corroborate the early studies mentioned above and to identify mechanisms underlying this process.
Figure 8.
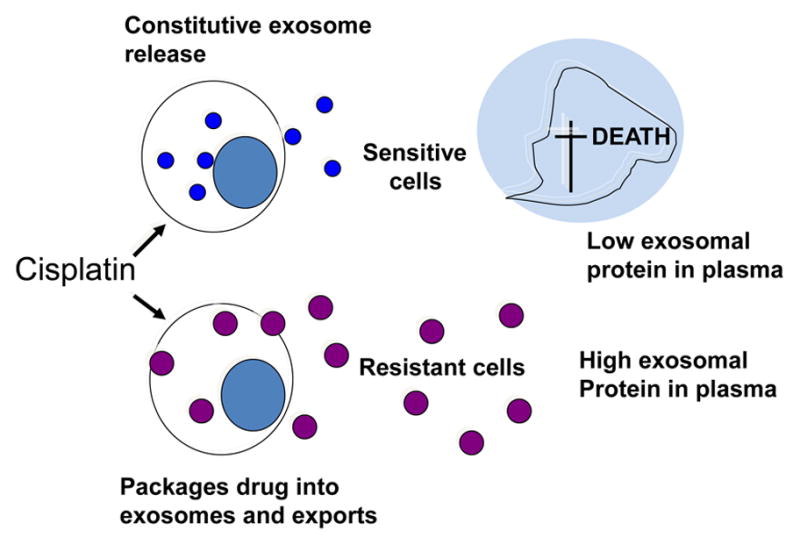
Tumor-derived exosomes in drug resistance. Resistance of tumor cells to chemotherapy may be mediated by exosomes. Cisplatin-sensitive tumor cells die and, as a result, levels of tumor-derived exosomes decrease in plasma. In contrast, cisplatin-resistant tumor cells package the drug into exosomes and export large quantities of exosomes out of the cell. Thus, the exosomal content of plasma increases.
8.3 Exosomes and thrombosis
Patients with an advanced malignancy may suffer from life-threatening hypercoagulation. Tumor-derived exosomes which carry transfer factor (Tf) are strongly implicated in inducing cancer-associated thrombotic events [65]. Overexpression of Tf, also known as clotting factor, is closely associated with tumor progression and metastasis [114]. When cancer cells undergo the EMT, they begin to release exosomes containing elevated levels of Tf. These TF-rich exosomes can be internalized by endothelial cells and induce their rapid conversion to a pro-coagulant phenotype. The presence of large numbers of pro-coagulant vesicles in cancer patients’ plasma is a bad prognostic factor [65]. It is not yet clear, however, how transfer of Tf by exosomes and their pro-thrombotic effects contribute to cancer progression and metastasis formation.
8.4 Effects on the host immune system
Immunosuppressive or immunostimulatory effects of tumor-derived exosomes are not limited to the TME. Immune cells in the circulation and those in various lymphoid organs are exposed to these exosomes, and in leukemia, where leukemic blasts circulate, blast-derived exosomes are enriched in plasma and directly interact with immune cells [24]. In the presence of tumor, interactions of exosomes with immune cells in the periphery result in immune suppression [39] as illustrated in Figure 9. Functions of immune cells in the periphery are altered upon injection of tumor-derived exosomes, as demonstrated in many in vivo studies in experimental mouse models [115, 116]. These alterations translate into an enhancement of tumor growth and shorter survival [117]. Co-incubation of ex vivo separated human T cells, B cells or NK cells with tumor-derived exosomes leads to a partial or complete loss of anti-tumor functions mediated by the mechanisms identical to those described above for the exosomes in the TME. Accumulations of Treg and MDSC in the blood and/or lymphoid organs of cancer patients are commonly seen and seem to correlate with levels of exosomes in the plasma. The molecular and genetic profiles to circulating tumor-derived exosomes in part reflects those found in tumor cells, and these profiles are being intensively examined as potential approaches to the identification of noninvasive biomarkers of cancer progression [118].
Figure 9.
Systemic effects of tumor-derived exosomes on the host immune system. These effects may be direct, such as apoptosis of activated CD8+ T cells in the presence of FasL+ exosomes and blocking of monocyte differentiation into dendritic cells (DC), or indirect, such as exosome-driven expansion of regulatory T cells (Treg) or myeloid derived suppressor cells (MDSC) which suppress activities of other immune cells. Anti-tumor effector T cells generated in response to immune therapies are especially sensitive to exosomes carrying immunosuppressive factors. Natural killer (NK) cell activities are inhibited by transforming growth factor β (TGF-β) carried by exosomes or produced by MDSC. Prostaglandin2 (PGE2) and adenosine (ADO) are factors that are delivered by exosomes (PGE2) or produced by exosomes equipped with CD39/CD73 in the presence of ATP (ADO). The figure is reproduced with permission from ref (#39).
One of many immunoinhibitory mechanisms representing systemic effects of tumor-derived exosomes is apoptosis of activated CD8+ T effector cells in the circulation of patients with cancer. Nearly all CD8+ T lymphocytes in the circulation of cancer patients, express surface CD95 [90] and many express PD-1[51]. They are, therefore, susceptible to apoptosis by exosomes carrying the membrane form (42kDa) of FasL [33, 89] or by exosomes carrying PD-L1, respectively. Expression levels of these apoptosis-inducing molecules in exosomes was shown to correlate with the frequency of activated CD8+ T cells sensitive to apoptosis in the circulation of cancer patients. Further, there was a significant correlation between “spontaneous apoptosis” of circulating CD8+ T cells and the disease stage and prognosis [33, 89, 119]. TEX-mediated signals leading to apoptosis of activated CD8+ T cells were associated with early membrane changes (i.e., Annexin V binding) in target cells, caspase 3 cleavage, cytochrome C release from mitochondria, loss of the mitochondrial membrane potential (MMP) and, finally, DNA fragmentation[88]. The PI3K/AKT pathway emerges as the key target of tumor-derived exosomes in activated CD8+ T cells [88, 120]. Recently, PTEN, which regulates PI3K-AKT signaling, was found to be a component of the exosome cargo and to mediate phosphatase activity in recipient cells [121]. Co-incubation of activated CD8+ T cells with tumor-derived exosomes caused dramatic time-dependent AKT de-phosphorylation and concomitant down-regulation of the expression levels of the anti-apoptotic proteins, Bcl-2, Bcl-xL and MCl-1. At the same time, the pro-apoptotic protein Bax was up-regulated by tumor-derived exosomes [59, 120]. These studies showed that tumor-derived exosomes induce apoptosis of activated CD8+ T cells by engaging the extrinsic and intrinsic apoptosis pathways [120]. The in vitro data are consistent with reports of similar changes in expression of the pro- or anti-apoptotic family members in circulating T cells of patients with cancer [90, 119].
Systemic effects of tumor-derived exosomes on the host immune system may be stimulatory (Figure 7). Their immunostimulatory effects of tumor-derived exosomes have been examined in a great detail because of the discovery that tumor-associated antigens (TAA), MHC molecules, chaperones, such as heat shock proteins HSP-70 and HSP-90, were present in the exosome cargo [39, 122]. In view of a ready up-take of these exosomes by DC, it was expected that processing of their cargo by the antigen processing machinery (APM) and presentation to T cells would lead to generation of anti-tumor responses [41]. In fact, exosomes released by tumors and internalized by DC were found to be good a source of TAA in the development of anti-tumor vaccines [123, 124]. Recent vaccination studies in murine tumor models confirmed that effective vaccination using tumor-derived exosomes can lead to the development of strong anti-tumor immunity and tumor rejection [125]]. Based on these reports, tumor-derived exosomes are being considered as both immune activators and contributors of immunogenic antigens in the design of vaccine adjuvants and therapeutic vaccines, respectively, in human clinical trials [125].
9.0 Tumor-derived exosomes as cancer biomarkers
Tumor-derived exosomes bearing the molecular and genetic cargo, which, at least in part, reflects the composition of tumor cells, are abundant in the blood and other body fluids, including urine [1, 126]. The idea that these exosomes can serve as a “liquid biopsy” allowing for non-invasive analyses of the tumor in real time has generated much attention [127, 128]. The properties of tumor-derived exosomes place them in an ideal position to serve as especially promising surrogates of tumor-derived proteins and transcripts. Various biologically-active molecules they carry reflect the content of parent cells are encapsulated by a membrane which protects the vesicle content from degradation during transit. They represent an enriched source of carefully packaged biomarkers, proteins, lipids, nucleic acids, which would ordinarily constitute a fraction of total proteome or transcriptome of tumor cells. These attributes of tumor-derived exosomes are likely to facilitate cancer diagnosis, prognosis and monitoring of therapeutic responses.
9.1 Exosomal protein levels
It appears that even a simple measurement of the protein content of plasma exosomes from cancer patients has a predictive value and has been reported to correlate to the disease stage and outcome [32, 129]. Protein values in exosome fractions are higher in cancer than in normal donors and they appear to increase in patients with advanced disease [24, 32]. Further, not only the total protein levels but also the content of individual proteins in exosomes can provide prognostic information. For example, levels of TGF-β1, a factor known to inhibit activation and functions of natural killer (NK) cells, was found to be significantly elevated in exosomes isolated from plasma of patients with AML [24, 130] and these exosomes were enriched in the biologically-active, mature form of TGF-β1 [130]. AML exosomes isolated before, during or after chemotherapy were shown to contain different levels of active TGF-β1 which effectively down-regulated cytotoxicity mediated by NK cells [130]. These data suggested that TGF-β1 expression levels as well as the maturation state of TGF-β1 carried by exosomes can be directly linked to immune cell suppression commonly seen in patients with AML and to the disease progression. Based on this type of correlative data, interest in molecular profiling of the entire proteome of tumor-derived exosomes by mass spectrometry has led to the accumulation of a large volume of proteomics data now available in the database called Vesiclepedia [84]. Although largely based on analyses of all EVs present in supernatants of tumor cell lines, the data indicate that tumor-derived vesicles have a distinct protein signature which is tissue/cell-type specific and can be of potential use in identifying cancer biomarkers for future validation in prospective studies.
9.2 Nucleic acids in exosomes
Tumor-derived exosomes carry mRNA and microRNAs (miRNAs) and deliver them to recipient cells [66]. In 2008, Skog and colleagues reported that exosomes derived from clinical glioblastoma samples contained 4700 distinct mRNA species which were selectively packaged for delivery [80]. Hong et al detected over 11,000 distinct mRNAs in exosomes derived from supernatants of SW 480, a colorectal cell line [131]. mRNAs carried by exosomes are known to be involved in in critical cellular activities such as cell cycle regulation, chromosome segregation, proliferation and migration. Skog detected mRNA coding the mutant epidermal growth factor receptor (EGFRvIII) in EVs derived from plasma of patients with glioblastoma [80]. Baran et al identified transcripts for MAGE-1 and HER-2/neu in EVs obtained from plasma of patients with gastric carcinoma [132]. The known prostate cancer mRNA biomarkers PCA-3 and the chimeric fusion oncoprotein TMPRSS2-ERG were detected in urine exosomes [126]. These data suggested that transcripts carried in exosomes from cancer patients’ plasma could serve as biomarkers for non-invasive detection of malignant cells. We have studied mRNAs expression levels for 24 immunoregulatory genes in exosomes isolated from paired pre- and post-vaccination plasma samples of glioma patients who received a DC-based anti-tumor vaccine [133]. We observed significant post-vaccine down-regulation in expression levels of four genes, IL-8, TGF-β, TIMP-1 and ZAP-70, all four known to be related in one way or another to clinical outcome in gliomas [133]. The observed changes in levels of these transcripts correlated with immunological and clinical responses to vaccination therapy, suggesting that measurements of mRNA expression levels in plasma exosomes may be useful as surrogates of responses to immune therapies in patients with cancer.
Exosomal miRNAs have recently emerged as especially promising biomarker candidates. miRNAs delivered to recipient cells regulate gene expression by either repressing the translation or causing degradation of multiple mRNAs, depending on the cellular content. The horizontal transfer of miRNA from tumor to recipient cells results in re-programming of their transcriptome and induces functional alterations that impact on tumor progression and metastasis [134]. Accumulating data indicate that miRNA signatures for EVs derived from plasma of patients with different cancer are distinct [135]. However, these signatures do not correspond to those present in the parent tumor cell [136]. This suggests that selection, sorting and packaging of miRNAs into exosomes takes place and that tumor cell determines the content of miRNAs species encapsulated in exosomes. Taylor et al reported that ovarian cancer cells can be reliably distinguished from benign cells by the signature consisting of eight specific miRNAs (miR-21, -141, -200a, -200b, -200c, -203, -205 and -214) [137, 138]. Similarly, exosomes in plasma of patients with colorectal cancer carried a distinctive miRNA signature [139]. Multiple other studies have shown that exosomes isolated from plasma of patients with different cancers, including glioblastoma [140], lung cancer [141], breast carcinoma [142], have distinct miRNA profiles. Certain miRNA species, e.g., miR-21, were found to be highly expresses in exosomes from plasma of patients with solid tumors such as glioblastoma, ovarian, breast and prostate carcinomas [118], and miR-21 expression levels correlated with the disease presence, progression and response to therapy [138, 143]. In non-small cell lung cancer, exosomal mi-RNA correlated with disease free survival and overall survival [144]. The accumulating evidence for the unique miRNAs signatures of tumor-derived exosomes in different cancers is promising, but much work is still needed to validate the use of these signatures as cancer biomarkers.
10.0. Tumor-derived exosomes and cancer immunotherapy
Immunotherapy of cancer has recently become one of the main anti-cancer therapeutic strategies, especially for patients unresponsive to conventional therapies [145]. Clinical efficacy of immune therapies may be influenced by tumor-derived exosomes. The molecular cargo carried by these exosomes can be either immunostimulatory or immunoinhibitory, and the end result of exosome interactions with the host immune system could lead to a better or worse anti-tumor immune response, respectively. As it is beneficial for the tumor to suppress anti-tumor immunity, the TME is likely to promote immune suppression. To this end, tumor-derived exosomes not only participate in blunting anti-tumor immune responses mounted by the host using various mechanisms described above, but they also interfere with and inhibit anti-tumor immune therapies.
10.1 Interference with antibody-based therapies
Because tumor-derived exosomes carry TAA, they can efficiently bind and sequester tumor-reactive antibodies (Abs) used for immunotherapy and dramatically reduce binding of these Abs to tumor cells. This has been shown for Trastuzumab in breast cancer therapy [111]. HER-2+ exosomes isolated from plasma of patients with breast cancer bound Trastuzumab and were shown to inhibit Trastuzumab-mediated effects on SKBR3 cell proliferation [111]. The Ab sequestration also reduces antibody-dependent cytotoxicity (ADCC) by immune effector cells, one of the major mechanisms of therapeutic activity of anti-cancer Abs [112]. In a model of an aggressive B-cell lymphoma, tumor-derived exosomes were shown to bind and consume complement, thus preventing tumor cells from mediating complement-dependent cytolysis [95]. It can also be surmised that TAA+ exosomes lymphocytes (CTL) generated as a result of vaccination therapies or adoptively transferred immune cells to patients with cancer. The available insights into the molecular cargo of tumor-derived exosomes suggest that they are likely to play a critical role not only in regulating anti-tumor activities of immune effector cells but also in protecting tumor cells from immune therapies.
10.2. Exosomes and the coagulation cascade in cancer
In body fluids of cancer patients, exosomes are increased in numbers relative to normal individuals, although the majority of these vesicles likely originate not from the tumor but from normal cells, especially platelets, erythrocytes and immune cells. Regardless of their cellular origin, the increased content of exosomes in plasma has been linked to venous thrombotic events (VTEs) which commonly occur in patients with advanced malignancies [65]. Since exosomes carry tissue factor (Tf) and can interact with components of the coagulation cascade [146], they are prime suspects in contributing to VTEs. Thus, therapeutic removal or reduction of such vesicles is one strategy that is currently being evaluated in clinical trials [44, 147].
10.3. Strategies for relieving exosome-mediated effects
The negative effects of tumor-derived exosomes in cancer call for the development of novel therapies capable of silencing the troublesome exosomes and protecting endogenous or therapy-induced immune cells from suppression/inhibition mediated by these exosomes. There are also good reasons for selective silencing of tumor-derived exosomes which deliver oncogenes promoting cancer progression. Currently, few strategies are available for relieving exosome-mediated immune suppression or eliminating exosome-mediated transfer of messages promoting tumor growth. Some of those strategies are listed below, and they are largely at the pre-clinical stage of development: (a) reducing the yield of extracellular exosomes by interfering with their biogenesis and release; (b) selectively blocking uptake of exosomes by target cells; and (c) interfering with exosome-driven signaling in recipient cells [51, 120]. Exosome biogenesis could be attenuated by e.g., small-molecule inhibitors of enzymes or proteins such as ALIX involved in exosome formation [148]. Exosome secretion can be regulated by controlling intracellular Ca2+ levels in K562 CML cells [13] or the use of drugs such as dimethyl amilioride (DMA), an inhibitor of Na+/Ca2+exchange. DMA was shown to be effective in vivo by inhibiting growth of mouse and human tumors due to its interference with the secretion of immunosuppressive tumor-derived exosomes carrying HSP70 [116]. Also, exosome secretion could potentially be modulated by interfering with functions of the endo-lysosomal compartments in cells via proton pump inhibitors [70]. Uptake of exosomes could also be targeted and potentially attenuated by blocking surface adhesion molecules, for example, phosphatidylserine, ICAM1, cell surface heparin sulfate proteoglycans or of other receptors participating in exosome internalization [31, 105]. Targeting of intracellular signaling pathways activated by exosomes or oncogenes delivered to recipient cells by the use of inhibitory RNAs (RNAi) is being also explored [32]. These and other therapeutic options for ameliorating pathological effects of exosomes are now being considered, with a caveat that many of these strategies may lack specificity required for selective targeting of exosomes without interference with normal cellular functions.
10.4. Exosomes can act as adjuvants for anti-tumor immune responses
Tumor or normal cell-derived exosomes can also stimulate anti-tumor immune responses and act as antigen-presenting vesicles capable of delivering TAA to dendritic cells (DCs) [21, 40, 122, 149]. TAA carried by exosomes, which are readily taken up by DCs, could thus be delivered to the antigen-processing machinery (APM), leading to effective presentation of the antigens on the MHC molecules to cognate T cells. The ability of exosomes released by antigen-presenting cells, such as B cells or DC to induce and sustain anti-tumor immune responses has been well documented [124]. However, there is a concern that tumor-derived exosomes might induce immune suppression rather than immune activation by blocking the antigen-processing or -presentation pathways in DCs. Zoller and her colleagues asked whether vaccination with exosomes derived from a mouse myeloid leukemia cell line, WEHI3B, or renal cell carcinoma cell line, RENCA, could lead to the generation of anti-tumor responses in vaccinated mice. The results showed that DC loaded with TAA from tumor-derived exosomes were superior to DC loaded with tumor lysates in inducing tumor-specific T cells [125]. Tumor-derived exosomes were efficiently taken up by DC, supported expression of CD11b, MHC class II molecules on DC and up-regulated IL-12 production. Further, vaccination with these DC significantly prolonged survival of the mice [125]. In these experiments, tumor-derived exosomes did not induce the suppressive phenotype in DC and served as a better TAA source than tumor lysates due to a better efficacy of antigen processing and presentation by the APM pathway in DC. These and other pre-clinical experiments suggest that tumor-derived exosomes recovered from plasma of cancer patients’ might serve as a source of individual (personalized) antigens for DC loading or vaccine production. It has been suggested that immune stimulatory exosomes might serve as adjuvants for anti-tumor vaccines in the future [44, 125].
The development of exosome-targeted inhibitory or stimulatory therapies is dependent the progress being made in understanding of the exosome biology and their clinical impact. Currently this progress is hampered by insufficient information about most aspects of exosome pathologic, as opposed to homeostatic, activities in disease and health, respectively. Methods for separating normal-cell derived from pathological exosomes and for selective regulation of exosome activities are not yet available. While new data emerge almost daily, it may be premature to contemplate translation of these new insights to the clinic. Nevertheless, in instances when tumor antigen-carrying exosomes are known to bind to therapeutic antibodies and interfere with therapy, as is the case, for example with CD20+ exosomes produced by B-cell lymphoma cells which bind to rituximab [45], removal of exosomes from plasma using a well-known plasmapheresis platform might be justified [44]. There is a concern, however, that indiscriminate removal of all exosomes may be a double-edged sword. This introduces a second reason for caution in advancing exosome-targeted therapies; namely, the existence of “good” exosomes which carry information necessary for the host well-being and are critical for physiologically normal responses. Indiscriminate therapeutic interference with these “good” exosomes might have unexpected and harmful consequences.
11. Conclusions
In the last 10 years, exosomes have emerged as previously unrecognized vehicles for transfer of information between cells. While information shuttling may be the major biological role of exosomes, this mechanism of vesicular communication appears to transcend almost all cellular functions and regulate molecular and genetic signatures of all normal and abnormal cells. Tumor-derived exosomes have been of special interest due to the perception that they not only deliver messages from the tumor to near or distant normal cells but also alter the phenotype and functions of these target cells to promote tumor progression. In the TME, these exosomes directly or indirectly contribute to the tumor welfare. In cancer, the load and the repertoire of circulating exosomes differ from those in healthy donors. Tumor-derived exosomes represent a substantial part of the plasma vesicular content, and their molecular and genetic profiles change in the course of disease or therapy. Their molecular and genetic profiles reflect those found in the parent tumor cells. This provides an opportunity for linking profiles of tumor-derived exosomes with the disease presence, progression and even outcome. Further, through autocrine or paracrine signaling, tumor-derived exosomes regulate all aspects of tumor growth from re-programming of the bone marrow environment and of the tumor stroma, driving neovascularization, cell differentiation migration and survival to orchestrating metastatic tumor spread. It appears that tumor-derived exosomes play a role in the entire process of carcinogenesis and are programmed by tumor cells to promote it. They also inhibit anti-tumor immune responses. Moreover, they horizontally transfer oncogenes and oncogenic proteins or their transcripts from tumor to normal cells. Interestingly, exosomes produced by normal hematopoietic or tissue cells mediate anti-tumor responses and maintain homeostasis. This introduces a need to discriminate between “good’ and “bad” exosomes and represents a major challenge for future therapies aiming at silencing tumor-derived exosomes. Such future targeting will have to be selective and specific, and much basic and clinical work will be needed before tumor-derived exosomes can enter the field as therapeutic targets or cancer biomarkers.
Acknowledgments
This study was supported in part by the NIH grant R01 CA16862 to TLW and Cancer Center Support Grant P30 CA047904.
Funding support, grant #R21 CA205644
Footnotes
Conflict of Interest
The author has no conflict of interest.
References
- 1.Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86. doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinton LT, Sloane HS, Kester M, Kelly KA. Formation and role of exosomes in cancer. Cell Mol Life Sci. 2015;72:659–71. doi: 10.1007/s00018-014-1764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, et al. The Simoa HD-1 Analyzer: A Novel Fully Automated Digital Immunoassay Analyzer with Single-Molecule Sensitivity and Multiplexing. J Lab Autom. 2015 doi: 10.1177/2211068215589580. [DOI] [PubMed] [Google Scholar]
- 6.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–39. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–78. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 8.Boyiadzis M, Whiteside TL. Information transfer by exosomes: A new frontier in hematologic malignancies. Blood Rev. 2015;29:281–90. doi: 10.1016/j.blre.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Atay S, Godwin AK. Tumor-derived exosomes: A message delivery system for tumor progression. Commun Integr Biol. 2014;7:e28231. doi: 10.4161/cib.28231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rak J, Guha A. Extracellular vesicles--vehicles that spread cancer genes. Bioessays. 2012;34:489–97. doi: 10.1002/bies.201100169. [DOI] [PubMed] [Google Scholar]
- 11.Martins VR, Dias MS, Hainaut P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr Opin Oncol. 2013;25:66–75. doi: 10.1097/CCO.0b013e32835b7c81. [DOI] [PubMed] [Google Scholar]
- 12.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berleman J, Auer M. The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environ Microbiol. 2013;15:347–54. doi: 10.1111/1462-2920.12048. [DOI] [PubMed] [Google Scholar]
- 14.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–72. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–65. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 16.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–25. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Marks MS, Heijnen HF, Raposo G. Lysosome-related organelles: unusual compartments become mainstream. Curr Opin Cell Biol. 2013;25:495–505. doi: 10.1016/j.ceb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breen EC, Reynolds SM, Cox C, Jacobson LP, Magpantay L, Mulder CB, et al. Multisite comparison of high-sensitivity multiplex cytokine assays. Clin Vaccine Immunol. 2011;18:1229–42. doi: 10.1128/CVI.05032-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–82. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M, et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010;51:2105–20. doi: 10.1194/jlr.M003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rana S, Zoller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011;39:559–62. doi: 10.1042/BST0390559. [DOI] [PubMed] [Google Scholar]
- 23.Keustermans GC, Hoeks SB, Meerding JM, Prakken BJ, de Jager W. Cytokine assays: an assessment of the preparation and treatment of blood and tissue samples. Methods. 2013;61:10–7. doi: 10.1016/j.ymeth.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96:1302–9. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dabitao D, Margolick JB, Lopez J, Bream JH. Multiplex measurement of proinflammatory cytokines in human serum: comparison of the Meso Scale Discovery electrochemiluminescence assay and the Cytometric Bead Array. J Immunol Methods. 2011;372:71–7. doi: 10.1016/j.jim.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–99. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 29.Tighe P, Negm O, Todd I, Fairclough L. Utility, reliability and reproducibility of immunoassay multiplex kits. Methods. 2013;61:23–9. doi: 10.1016/j.ymeth.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. sup pp 1–13. [DOI] [PubMed] [Google Scholar]
- 31.Bobrie A, Thery C. Unraveling the physiological functions of exosome secretion by tumors. Oncoimmunology. 2013;2:e22565. doi: 10.4161/onci.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–20. [PubMed] [Google Scholar]
- 34.Wieckowski E, Whiteside TL. Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol Res. 2006;36:247–54. doi: 10.1385/IR:36:1:247. [DOI] [PubMed] [Google Scholar]
- 35.Whiteside TL. Immune responses to malignancies. J Allergy Clin Immunol. 2010;125:S272–83. doi: 10.1016/j.jaci.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183:3720–30. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuler PJ, Saze Z, Hong CS, Muller L, Gillespie DG, Cheng D, et al. Human CD4(+) CD39(+) regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73(+) exosomes or CD73(+) cells. Clin Exp Immunol. 2014;177:531–43. doi: 10.1111/cei.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bobrie A, Thery C. Exosomes and communication between tumours and the immune system: are all exosomes equal? Biochem Soc Trans. 2013;41:263–7. doi: 10.1042/BST20120245. [DOI] [PubMed] [Google Scholar]
- 39.Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes) Biochem Soc Trans. 2013;41:245–51. doi: 10.1042/BST20120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabrielsson S, Scheynius A. Exosomes in immunity and cancer--friends or foes? Semin Cancer Biol. 2014;28:1–2. doi: 10.1016/j.semcancer.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh R, Pochampally R, Watabe K, Lu Z, Mo YY. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13:256. doi: 10.1186/1476-4598-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183–94. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32:2747–55. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 46.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–71. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12:343–55. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garnier D, Magnus N, Lee TH, Bentley V, Meehan B, Milsom C, et al. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem. 2012;287:43565–72. doi: 10.1074/jbc.M112.401760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan S, Jutzy JM, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis : an international journal on programmed cell death. 2011;16:1–12. doi: 10.1007/s10495-010-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan S, Aspe JR, Asumen MG, Almaguel F, Odumosu O, Acevedo-Martinez S, et al. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br J Cancer. 2009;100:1073–86. doi: 10.1038/sj.bjc.6604978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuler PJ, Schilling B, Harasymczuk M, Hoffmann TK, Johnson J, Lang S, et al. Phenotypic and functional characteristics of CD4+ CD39+ FOXP3+ and CD4+ CD39+ FOXP3neg T-cell subsets in cancer patients. Eur J Immunol. 2012;42:1876–85. doi: 10.1002/eji.201142347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atay S, Banskota S, Crow J, Sethi G, Rink L, Godwin AK. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci U S A. 2014;111:711–6. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 54.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237–48. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–55. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–57. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–30. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 58.Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, et al. Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33:4613–22. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor DD, Gercel-Taylor C, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9:5113–9. [PubMed] [Google Scholar]
- 60.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–26. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lazaro-Ibanez E, Sanz-Garcia A, Visakorpi T, Escobedo-Lucea C, Siljander P, Ayuso-Sacido A, et al. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: apoptotic bodies, microvesicles, and exosomes. Prostate. 2014;74:1379–90. doi: 10.1002/pros.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller-Haegele S, Muller L, Whiteside TL. Immunoregulatory activity of adenosine and its role in human cancer progression. Expert Rev Clin Immunol. 2014;10:897–914. doi: 10.1586/1744666X.2014.915739. [DOI] [PubMed] [Google Scholar]
- 63.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–45. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 64.Tekin HC, Gijs MA. Ultrasensitive protein detection: a case for microfluidic magnetic bead-based assays. Lab Chip. 2013;13:4711–39. doi: 10.1039/c3lc50477h. [DOI] [PubMed] [Google Scholar]
- 65.Chowdhury F, Williams A, Johnson P. Validation and comparison of two multiplex technologies, Luminex and Mesoscale Discovery, for human cytokine profiling. J Immunol Methods. 2009;340:55–64. doi: 10.1016/j.jim.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 67.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter. 2006;3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 68.Taylor DD, Chou IN, Black PH. Isolation of plasma membrane fragments from cultured murine melanoma cells. Biochem Biophys Res Commun. 1983;113:470–6. doi: 10.1016/0006-291x(83)91749-7. [DOI] [PubMed] [Google Scholar]
- 69.Muller L, Hong CS, Stolz DB, Watkins SC, Whiteside TL. Isolation of biologically-active exosomes from human plasma. J Immunol Methods. 2014 doi: 10.1016/j.jim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–8. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 71.Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015 doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 72.Hong CS, Muller L, Boyiadzis M, Whiteside TL. Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS One. 2014;9:e103310. doi: 10.1371/journal.pone.0103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freer G, Rindi L. Intracellular cytokine detection by fluorescence-activated flow cytometry: basic principles and recent advances. Methods. 2013;61:30–8. doi: 10.1016/j.ymeth.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 74.Donaldson MM, Kao SF, Eslamizar L, Gee C, Koopman G, Lifton M, et al. Optimization and qualification of an 8-color intracellular cytokine staining assay for quantifying T cell responses in rhesus macaques for pre-clinical vaccine studies. J Immunol Methods. 2012;386:10–21. doi: 10.1016/j.jim.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dai S, Feng C, Li W, Jiang W, Wang L. Quantitative detection of tumor necrosis factor-alpha by single molecule counting based on a hybridization chain reaction. Biosens Bioelectron. 2014;60:180–4. doi: 10.1016/j.bios.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 76.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–28. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 77.Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34:290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 78.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Visconti L, Nelissen K, Deckx L, van den Akker M, Adriaensen W, Daniels L, et al. Prognostic value of circulating cytokines on overall survival and disease-free survival in cancer patients. Biomark Med. 2014;8:297–306. doi: 10.2217/bmm.13.122. [DOI] [PubMed] [Google Scholar]
- 80.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neuzillet C, de Gramont A, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, et al. Perspectives of TGF-beta inhibition in pancreatic and hepatocellular carcinomas. Oncotarget. 2014;5:78–94. doi: 10.18632/oncotarget.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–9. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–78. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 84.Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Capone F, Guerriero E, Colonna G, Maio P, Mangia A, Castello G, et al. Cytokinome profile evaluation in patients with hepatitis C virus infection. World J Gastroenterol. 2014;20:9261–9. doi: 10.3748/wjg.v20.i28.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ng CT, Oldstone MB. IL-10: achieving balance during persistent viral infection. Curr Top Microbiol Immunol. 2014;380:129–44. doi: 10.1007/978-3-662-43492-5_6. [DOI] [PubMed] [Google Scholar]
- 87.van Montfoort N, Olagnier D, Hiscott J. Unmasking immune sensing of retroviruses: interplay between innate sensors and host effectors. Cytokine Growth Factor Rev. 2014;25:657–68. doi: 10.1016/j.cytogfr.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Czystowska M, Han J, Szczepanski MJ, Szajnik M, Quadrini K, Brandwein H, et al. IRX-2, a novel immunotherapeutic, protects human T cells from tumor-induced cell death. Cell Death Differ. 2009;16:708–18. doi: 10.1038/cdd.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bergmann C, Strauss L, Wieckowski E, Czystowska M, Albers A, Wang Y, et al. Tumor-derived microvesicles in sera of patients with head and neck cancer and their role in tumor progression. Head Neck. 2009;31:371–80. doi: 10.1002/hed.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–62. [PubMed] [Google Scholar]
- 91.Walsh GM. Therapeutic potential of targeting interleukin 5 in asthma. Biodrugs. 2013;27:559–63. doi: 10.1007/s40259-013-0047-0. [DOI] [PubMed] [Google Scholar]
- 92.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249–58. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 93.Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–33. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rabenhorst A, Hartmann K. Interleukin-31: a novel diagnostic marker of allergic diseases. Curr Allergy Asthma Rep. 2014;14:423. doi: 10.1007/s11882-014-0423-y. [DOI] [PubMed] [Google Scholar]
- 95.Filipazzi P, Burdek M, Villa A, Rivoltini L, Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol. 2012;22:342–9. doi: 10.1016/j.semcancer.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 96.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS One. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mrizak D, Martin N, Barjon C, Jimenez-Pailhes AS, Mustapha R, Niki T, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2015;107:363. doi: 10.1093/jnci/dju363. [DOI] [PubMed] [Google Scholar]
- 98.Boyiadzis M, Whiteside TL. Information transfer by exosomes: A new frontier in hematologic malignancies. Blood Rev. 2015 doi: 10.1016/j.blre.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 99.Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 100.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–72. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 101.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375–85. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 102.Pucci F, Pittet MJ. Molecular pathways: tumor-derived microvesicles and their interactions with immune cells in vivo. Clin Cancer Res. 2013;19:2598–604. doi: 10.1158/1078-0432.CCR-12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288:36691–702. doi: 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang C, Kim SH, Bianco NR, Robbins PD. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One. 2011;6:e22517. doi: 10.1371/journal.pone.0022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28–41. doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 107.Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–75. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 108.Altevogt P, Bretz NP, Ridinger J, Utikal J, Umansky V. Novel insights into exosome-induced, tumor-associated inflammation and immunomodulation. Semin Cancer Biol. 2014;28:51–7. doi: 10.1016/j.semcancer.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 109.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595–604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 110.Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227:658–67. doi: 10.1002/jcp.22773. [DOI] [PubMed] [Google Scholar]
- 112.Amorim M, Fernandes G, Oliveira P, Martins-de-Souza D, Dias-Neto E, Nunes D. The overexpression of a single oncogene (ERBB2/HER2) alters the proteomic landscape of extracellular vesicles. Proteomics. 2014;14:1472–9. doi: 10.1002/pmic.201300485. [DOI] [PubMed] [Google Scholar]
- 113.Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One. 2014;9:e88193. doi: 10.1371/journal.pone.0088193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dai R, Ahmed SA. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl Res. 2011;157:163–79. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–85. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 116.Carissimi C, Carucci N, Colombo T, Piconese S, Azzalin G, Cipolletta E, et al. miR-21 is a negative modulator of T-cell activation. Biochimie. 2014;107(Pt B):319–26. doi: 10.1016/j.biochi.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 117.Curtale G, Citarella F, Carissimi C, Goldoni M, Carucci N, Fulci V, et al. An emerging player in the adaptive immune response: microRNA-146a is a modulator of IL-2 expression and activation-induced cell death in T lymphocytes. Blood. 2010;115:265–73. doi: 10.1182/blood-2009-06-225987. [DOI] [PubMed] [Google Scholar]
- 118.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–52. [PubMed] [Google Scholar]
- 119.Kim JW, Tsukishiro T, Johnson JT, Whiteside TL. Expression of pro- and antiapoptotic proteins in circulating CD8+ T cells of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:5101–10. doi: 10.1158/1078-0432.CCR-04-0309. [DOI] [PubMed] [Google Scholar]
- 120.Czystowska M, Szczepanski MJ, Szajnik M, Quadrini K, Brandwein H, Hadden JW, et al. Mechanisms of T-cell protection from death by IRX-2: a new immunotherapeutic. Cancer Immunol Immunother. 2011;60:495–506. doi: 10.1007/s00262-010-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J, et al. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal. 2012;5:ra70. doi: 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- 122.Dunand-Sauthier I, Santiago-Raber ML, Capponi L, Vejnar CE, Schaad O, Irla M, et al. Silencing of c-Fos expression by microRNA-155 is critical for dendritic cell maturation and function. Blood. 2011;117:4490–500. doi: 10.1182/blood-2010-09-308064. [DOI] [PubMed] [Google Scholar]
- 123.Li W, Kong LB, Li JT, Guo ZY, Xue Q, Yang T, et al. MiR-568 inhibits the activation and function of CD4(+) T cells and Treg cells by targeting NFAT5. Int Immunol. 2014;26:269–81. doi: 10.1093/intimm/dxt065. [DOI] [PubMed] [Google Scholar]
- 124.Gracias DT, Katsikis PD. MicroRNAs: key components of immune regulation. Adv Exp Med Biol. 2011;780:15–26. doi: 10.1007/978-1-4419-5632-3_2. [DOI] [PubMed] [Google Scholar]
- 125.Baxevanis CN, Anastasopoulou EA, Voutsas IF, Papamichail M, Perez SA. Immune biomarkers: how well do they serve prognosis in human cancers? Expert review of molecular diagnostics. 2015;15:49–59. doi: 10.1586/14737159.2015.965684. [DOI] [PubMed] [Google Scholar]
- 126.Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100:1603–7. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rolfo C, Castiglia M, Hong D, Alessandro R, Mertens I, Baggerman G, et al. Liquid biopsies in lung cancer: the new ambrosia of researchers. Biochim Biophys Acta. 2014;1846:539–46. doi: 10.1016/j.bbcan.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 128.Santiago-Dieppa DR, Steinberg J, Gonda D, Cheung VJ, Carter BS, Chen CC. Extracellular vesicles as a platform for ‘liquid biopsy’ in glioblastoma patients. Expert review of molecular diagnostics. 2014;14:819–25. doi: 10.1586/14737159.2014.943193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Szajnik M, Derbis M, Lach M, Patalas P, Michalak M, Drzewiecka H, et al. Exosomes in Plasma of Patients with Ovarian Carcinoma: Potential Biomarkers of Tumor Progression and Response to Therapy. Gynecol Obstet (Sunnyvale) Suppl. 2013;4:3. doi: 10.4172/2161-0932.S4-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hong CS, Muller L, Whiteside TL, Boyiadzis M. Plasma exosomes as markers of therapeutic response in patients with acute myeloid leukemia. Front Immunol. 2014;5:160. doi: 10.3389/fimmu.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hong BS, Cho JH, Kim H, Choi EJ, Rho S, Kim J, et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Whiteside TL. The potential of tumor-derived exosomes for noninvasive cancer monitoring. Expert review of molecular diagnostics. 2015:1–18. doi: 10.1586/14737159.2015.1071666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 135.Henderson MC, Azorsa DO. The genomic and proteomic content of cancer cell-derived exosomes. Front Oncol. 2012;2:38. doi: 10.3389/fonc.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nolte-‘t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, Hoen PAt. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272–85. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roberson CD, Atay S, Gercel-Taylor C, Taylor DD. Tumor-derived exosomes as mediators of disease and potential diagnostic biomarkers. Cancer Biomark. 2010;8:281–91. doi: 10.3233/CBM-2011-0211. [DOI] [PubMed] [Google Scholar]
- 138.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 139.Ogata-Kawata H, Izumiya M, Kurioka D, Honma Y, Yamada Y, Furuta K, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One. 2014;9:e92921. doi: 10.1371/journal.pone.0092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Roth P, Wischhusen J, Happold C, Chandran PA, Hofer S, Eisele G, et al. A specific miRNA signature in the peripheral blood of glioblastoma patients. J Neurochem. 2011;118:449–57. doi: 10.1111/j.1471-4159.2011.07307.x. [DOI] [PubMed] [Google Scholar]
- 141.Cazzoli R, Buttitta F, Di Nicola M, Malatesta S, Marchetti A, Rom WN, et al. microRNAs derived from circulating exosomes as noninvasive biomarkers for screening and diagnosing lung cancer. J Thorac Oncol. 2013;8:1156–62. doi: 10.1097/JTO.0b013e318299ac32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Corcoran C, Friel AM, Duffy MJ, Crown J, O’Driscoll L. Intracellular and extracellular microRNAs in breast cancer. Clin Chem. 2011;57:18–32. doi: 10.1373/clinchem.2010.150730. [DOI] [PubMed] [Google Scholar]
- 143.Liu J, Sun H, Wang X, Yu Q, Li S, Yu X, et al. Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int J Mol Sci. 2014;15:758–73. doi: 10.3390/ijms15010758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Silva J, Garcia V, Zaballos A, Provencio M, Lombardia L, Almonacid L, et al. Vesicle-related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival. Eur Respir J. 2011;37:617–23. doi: 10.1183/09031936.00029610. [DOI] [PubMed] [Google Scholar]
- 145.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gutierrez-Vazquez C, Villarroya-Beltri C, Mittelbrunn M, Sanchez-Madrid F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev. 2013;251:125–42. doi: 10.1111/imr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Muller L, Muller-Haegele S, Mitsuhashi M, Gooding W, Okada H, Whiteside TL. Exosomes isolated from plasma of glioma patients enrolled in a vaccination trial reflect antitumor immune activity and might predict survival. Oncoimmunology. 2015;4:e1008347. doi: 10.1080/2162402X.2015.1008347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]