Abstract
Background and study aims The success of any colonoscopy procedure depends upon the quality of bowel preparation. We evaluated the efficacy and safety of a new tailored dosing (TD) regimen compared with the approved PICOPREP day-before dosing regimen (DBD) in the European Union.
Patient and methods Patients (≥ 18 years) undergoing colonoscopy were randomised (2:1) to TD (Dose 1, 10 – 18 hours; Dose 2, 4 – 6 hours before colonoscopy) or DBD (Dose 1 before 8:00AM on the day before colonoscopy; Dose 2, 6 – 8 hours after Dose 1). The primary endpoint of overall colon cleansing efficacy was based on total Ottawa Scale (OS) scores (0 – 14, excellent-worst). The key secondary endpoint was a binary endpoint based on the ascending colon OS (success 0 or 1, failure [≥ 2]). Convenience and satisfaction were evaluated similar to the primary and key secondary endpoints. Safety and tolerability were also evaluated.
Results Use of the PICOPREP TD regimen resulted in a statistically significant reduction in the mean total Ottawa Scale score compared to the PICOPREP DBD regimen (–3.93, 95 % confidence intervals [CI]: – 4.99, – 2.97; P < 0.0001) in the intent-to-treat analysis set. The PICOPREP TD regimen also resulted in a statistically significant increase in the odds of achieving an ascending colon OS score ≤ 1, compared to the PICOPREP DBD regimen (estimated odds ratio 9.18, 95 % CI: 4.36, 19.32; P < 0.0001). There was no statistically significant difference in the overall rate of treatment-emergent adverse events (12 % (TD) and 5.7 % (DBD), respectively, P = 0.2988). The convenience and satisfaction were comparable in the two groups.
Conclusion The TD regimen was superior to the DBD regimen for overall and ascending colon cleansing efficacy.
ClinicalTrials.gov Identifier: NCT02239692
Introduction
Colonoscopy procedures are now being routinely used for evaluating the colon, mostly for screening and early detection of colorectal cancer, which has a high incidence and mortality 1. Extensive data show that adequate bowel preparation is instrumental in success of any colonoscopy procedure 2. Poor bowel preparation accounts for nearly 20 % of failed colonoscopies, limiting the procedure’s diagnostic/therapeutic value 2. Also, patients may require a repeat colonoscopy, adding to the burden for both patients and colonoscopists, and increased costs 2 3.
Of various factors governing the quality of bowel preparation, concerns such as unpleasant taste of the preparation and/or cumbersome experience of taking large volumes of liquid have been addressed to a great extent 4 5 6. However, guidelines 7 8 and emerging data 9 10 suggest that dosing regimen, and importantly, the time between the last dose of bowel preparation and the colonoscopy procedure are instrumental in achieving a successful colonoscopy. Hence, there have been continued efforts to improve bowel cleansing by optimizing the dosing regimen 9 10 11.
Day-before dosing (DBD) regimens may be an option for (in particular) morning colonoscopies 4, however, they introduce a time interval of at least 12 hours between the last dose and an afternoon colonoscopy procedure. During that time, chyme from the small intestine accumulates and coats the proximal colon, hindering visualization and detection of flat lesions 9 11 12. To address this, split-dosing regimens have been introduced 9 11 13. This reduces the time interval between the last dose and afternoon/evening colonoscopy procedure and is associated with improved quality of bowel preparation 14 15. The split-dosing regimen of PICOPREP has been shown to be efficacious, safe and well-tolerated 13 16 17 18 19 20 21, and has also been studied in children 22 23 24.
The current non-inferiority study evaluated efficacy and safety of a new tailored dosing (TD) regimen, which is customised based on time of colonoscopy, and also offers ease of consumption similar to split-dosing vs. the approved DBD regimen of PICOPREP for bowel preparation prior to colonoscopy in the European Union.
Patients and methods
Patients
Patients aged ≥ 18 years who were scheduled to undergo an elective colonoscopy were enrolled. Patients had to have ≥ 3 spontaneous bowel movements/week for a month prior to inclusion in the study. Furthermore, patients were excluded from the study if they had acute surgical abdominal conditions, active inflammatory bowel disease, colon disease (toxic megacolon, toxic colitis, idiopathic pseudo-obstruction, and hypomotility syndrome), ascites, gastrointestinal disorders (active ulcers, gastric outlet obstruction, retention, gastroparesis, and ileus), uncontrolled angina and/or myocardial infarction (within the past 3 months), congestive heart failure or uncontrolled hypertension, severely reduced renal function (glomerular filtration rate < 30 mL/min/1.73 m2), rhabdomyolysis, nausea, vomiting and hypermagnesaemia, and severe dehydration at screening. Patients who had a previous gastrointestinal surgery (including gastric resection, banding, or bypass), those participating in other studies, and those who were hypersensitive to the study drug were also excluded. Pregnant and lactating women, and patients in any way related to study or site staff were not eligible to participate.
Concomitant use of lithium, laxatives, constipating drugs, antidiarrheal agents, or oral iron preparations were not permitted in the study, and it was ensured that their use was suspended before administration of the bowel preparation.
Study design and treatment
This was a Phase 3, randomized, assessor-blinded, multicenter study comparing efficacy and safety of the PICOPREP TD regimen to the DBD regimen for bowel preparation prior to colonoscopy. Randomization was stratified by time of colonoscopy procedure, i. e. morning or afternoon, in a 2:1 ratio to either the PICOPREP TD regimen or to the PICOPREP DBD regimen. Patients were followed up until 7 days after the procedure.
PICOPREP (sodium picosulfate 10 mg; magnesium oxide 3.5 g, and citric acid 12 g) powder for oral solution was provided in sachets and was administered in 2 divided doses. PICOPREP was reconstituted by mixing the contents of the sachet in a cup with approximately 150 mL of cold water. Patients were instructed to consume at least 5 250-mL glasses of clear liquids, spread over several hours, after the first administration of PICOPREP and at least 3 250-mL glasses of clear liquids, spread over several hours, after the second administration. For the TD regimen, the first dose was administered 10 to 18 hours before the colonoscopy, and the second dose was administered 4 to 6 hours before the colonoscopy. For the DBD regimen, the first dose was taken the day before colonoscopy before 8 am, and the second dose was taken 6 to 8 hours later, also on the day before colonoscopy, in accordance with the approved EU label.
Patient compliance was monitored by recording the date and quantity of PICOPREP dispensed and used by each patient, volume of liquid intake, time between intake of each dose and actual start time of the colonoscopy corresponding to the 2 PICOPREP dosing regimens. Patients were contacted by phone on the last working day before bowel preparation administration and were reminded of the time of the administrations.
The study is registered at ClinicalTrials.gov (NCT02239692). The patients were recruited from 11 sites across Germany, France and the Netherlands between November 2014 and June 2015. The study was approved by an independent ethics committee for all participating sites, and was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, and applicable regulatory requirements. All patients provided written informed consent.
Study endpoints and assessments
The primary endpoint was the total Ottawa Scale score, derived from a validated 5-grade Ottawa Scale (Table 1). The total Ottawa Scale score is calculated by adding the ratings (0 to 4) for each of the 3 colon segments and the overall fluid quantity rating (0 to 2) and ranges from 0 (best) to 14 (worst) for the overall assessment of colon cleansing. The key secondary endpoint was a binary endpoint based on whether the ascending colon Ottawa Scale score ≤ 1. The other efficacy endpoints were mid (transverse, descending) and recto-sigmoid colon cleansing, assessed depending on whether the Ottawa Scale score ≤ 1 for the corresponding colon segment. The proportion of repeat colonoscopy procedures was also evaluated. Convenience and satisfaction of both dosing regimens, absence from work and impact on daily activities were assessed by separate patient questionnaires (Appendix), on the day of colonoscopy, prior to sedation (if needed). Safety was assessed throughout the study by recording adverse events (AEs), findings on physical examinations, orthostatic vital sign measurements, laboratory test results (haematology, coagulation, blood chemistry, and urinalysis), and 12-lead electrocardiograms (ECGs). The AEs were coded using Medical Dictionary for Regulatory Activities, version 17.0.
Table 1. Ottawa Scale.
| Score | Description |
| 0 | Excellent: Mucosal detail clearly visible. If fluid is present, it is clear. Almost no stool residue. |
| 1 | Good: Some turbid fluid or stool residue but mucosal detail still visible. Washing and suction not necessary. |
| 2 | Fair: Turbid fluid or stool residue obscuring mucosal detail. However, mucosal detail becomes visible with suctioning. Washing not necessary. |
| 3 | Poor: Presence of stool obscuring mucosal detail and contour. However, with suctioning and washing, a reasonable view is obtained. |
| 4 | Inadequate: Solid stool obscuring mucosal detail and contour despite aggressive washing and suctioning. |
Statistical assumptions and analysis
The sample size calculation was based on the assumptions of an overall significance level α of 0.05, a power (1-β) of 0.85, a non-inferiority margin of 1.5 units, a standard deviation (SD) of 3.2 for the total Ottawa Scale score, and a dropout rate of 5 % for randomized patients. With these assumptions, a total of 198 patients were needed to be randomized in a 2:1 ratio to either the TD regimen or to the DBD regimen.
The intention-to-treat (ITT) population consisted of all randomized patients. For patients in the ITT analysis set with partially or completely missing Ottawa Scale scores, the missing values were imputed using the worst possible score. The per-protocol (PP) population consisted of all patients in the ITT population with no major protocol deviations deemed to impact the efficacy of the PICOPREP dosing regimens. The completer analysis set consisted of all patients in the ITT population who underwent the colonoscopy and for whom, complete Ottawa Scale scores were available. The completer analysis set was used to perform sensitivity analyses assessing the impact of the imputation algorithm on the primary analysis.
In order to control the family wise error rate for the primary and key secondary endpoints, the following hierarchical testing procedure was pre-specified in the trial protocol:
Test non-inferiority of the PICOPREP TD regimen compared to the DBD regimen with respect to the primary endpoint at a 5 % significance level.
Test the superiority of the PICOPREP TD regimen vs. the DBD regimen with respect to the key secondary endpoint at a 5 % significance level.
Test the superiority of the PICOPREP TD regimen vs. DBD regimen with respect to the primary endpoint at a 5 % significance level.
Regulatory guidelines require non-inferiority to be established in the both the ITT and PP populations 25. This requirement is driven by the fact that analyses based on the ITT principle are not necessarily deemed to be conservative in the non-inferiority setting. Therefore, the primary analysis was performed for both the ITT and PP populations while other efficacy analyses were performed only in the ITT population.
The primary analysis compared the difference in the mean total Ottawa Scale score between the PICOPREP TD and DBD regimens based on an analysis of covariance (ANCOVA) controlling for time of colonoscopy (AM/PM) and country. The analysis of the secondary efficacy endpoints was based on logistic regression, controlling for time of colonoscopy (AM/PM) and country.
Convenience, satisfaction, absence from work (duration) and impact on daily activities were evaluated similar to the primary and key secondary endpoints.
Results
Patient demographics and baseline characteristics
A total of 204 patients were randomized in the study and 195 patients (95.6 %) received the study drug. Patient disposition and analysis populations are presented in Fig. 1. Baseline patient characteristics are presented in Table 2.
Fig. 1.
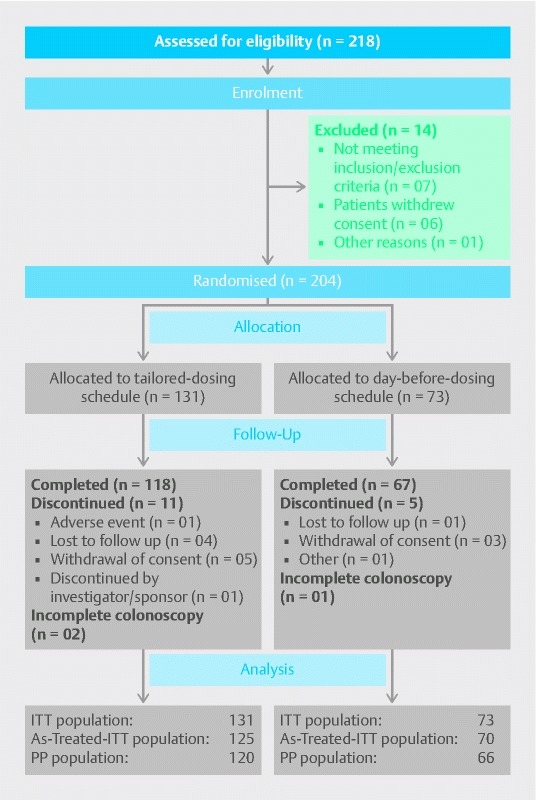
CONSORT flow diagram.As-Treated-ITT analysis set comprised of patients in the ITT analysis set who received the planned treatment, and excluded were the patients who were not treated.ITT, intention-to-treat; PP, per-protocol
Table 2. Demographic and baseline characteristics.
|
Tailored dosing
(N = 131) |
Day-before dosing
(N = 73) |
|
|
58.4 (13.3) | 56.6 (15.1) |
|
54 (41.2): 77 (58.8) | 30 (41.1): 43 (58.9) |
| Ethnicity, n (%) | ||
|
3 (2.3) | 2 (2.7) |
|
128 (97.7) | 71 (97.3) |
| Race, n (%) | ||
|
1 (0.8) | – |
|
130 (99.2) | 73 (100.0) |
| BMI (kg/m2) | ||
|
26.5 (4.71) | 25.6 (4.33) |
Overall colon cleansing efficacy based on Ottawa Scale scores
Estimated differences in mean total Ottawa Scale score between the TD and DBD regimens for the ITT and PP populations were – 3.93 (95 % CI: [–4.99, – 2.87]) and – 4.38 (95 % CI: [–5.34, – 3.41]). This clearly establishes non-inferiority, as well as superiority of the TD regimen vs. DBD regimen, with respect to overall colon cleansing efficacy as illustrated in Fig. 2.
Fig. 2.
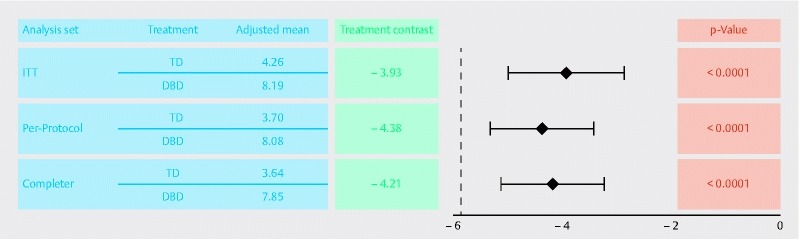
Difference in total Ottawa Scale scores between the tailored dosing and the day-before dosing regimens (ITT, PP and completer analysis set).Lower Ottawa Scale scores correspond to better colon cleansing efficacy.DBD, day-before dosing; ITT, intention-to-treat; PP, per-protocol; TD, tailored dosing
A sensitivity analysis, based on the completer analysis set was performed to assess the impact of the imputation methods on the primary analysis. The results of this completer analysis are also presented in Fig. 2. The consistency of these results suggest that the imputation method did not impact the conclusions of the study.
The observed distribution of Ottawa Scale scores, summarized by colon segment and dosing schedule, are provided in Table 3. The observed proportion of patients reporting Ottawa Scale segment scores of either 0 (excellent) or 1 (good), in Table 3, further demonstrates the improved efficacy of the TD regimen vs. the DBD regimen.
Table 3. Observed total Ottawa Scale score distribution by colon segment and dosing schedule (ITT population).
| Tailored dosing (N = 131) | Day-before dosing (N = 73) | |
| Ascending colon (numerical), Mean (SD) | 1.4 (1.14) | 2.8 (1.08) |
| Ascending colon (categorical), % (r/n) | ||
|
23.7 ( 31/131) | 1.4 ( 1/ 73) |
|
37.4 ( 49/131) | 13.7 ( 10/ 73) |
|
25.2 ( 33/131) | 19.2 ( 14/ 73) |
|
6.1 ( 8/131) | 32.9 ( 24/ 73) |
|
7.6 ( 10/131) | 32.9 ( 24/ 73) |
| Mid colon (numerical), Mean (SD) | 1.2 (1.20) | 2.4 (1.25) |
| Mid colon (categorical), % (r/n) | ||
|
37.4 ( 49/131) | 9.6 ( 7/ 73) |
|
27.5 ( 36/131) | 12.3 ( 9/ 73) |
|
23.7 ( 31/131) | 32.9 ( 24/ 73) |
|
3.8 ( 5/131) | 20.5 ( 15/ 73) |
|
7.6 ( 10/131) | 24.7 ( 18/ 73) |
| Recto-sigmoid colon (numerical), Mean (SD) | 1.0 (1.15) | 2.1 (1.27) |
| Recto-sigmoid colon (categorical), % (r/n) | ||
|
42.0 ( 55/131) | 13.7 ( 10/ 73) |
|
32.1 ( 42/131) | 16.4 ( 12/ 73) |
|
13.7 ( 18/131) | 30.1 ( 22/ 73) |
|
6.9 ( 9/131) | 23.3 ( 17/ 73) |
|
5.3 ( 7/131) | 16.4 ( 12/ 73) |
| The global fluid quantity rating (numerical), Mean (SD) | 0.7 (0.73) | 0.9 (0.73) |
| The global fluid quantity rating (Categorical), % (r/n) | ||
|
47.3 ( 62/131) | 31.5 ( 23/ 73) |
|
36.6 ( 48/131) | 46.6 ( 34/ 73) |
|
16.0 ( 21/131) | 21.9 ( 16/ 73) |
ITT, intention-to-treat; N, number of patients; n, number of patients with available information; r, number of patients responding; SD, standard deviation; %, r/n × 100
Ascending, mid and recto-sigmoid colon cleansing efficacy
The estimated odds of the Ottawa Scale score being ≤ 1 for the ascending, mid and recto-sigmoid colon segments are presented in Fig. 3, along with the associated odds ratios comparing the cleansing efficacy of the TD and DBD regimens in each colon segment. The estimated odds ratios, 9.18 (95 % CI: 4.36, 19.32; P < 0.0001), 6.85 (95 % CI: 3.48, 13.48; P < 0.0001) and 6.73 (95 % CI: 3.53, 12.85; P < 0.0001) indicate a statistically significant improvement in colon cleansing efficacy of the TD regimen compared to the DBD regimen in the ascending, mid and recto-sigmoid colon segments, respectively.
Fig. 3.
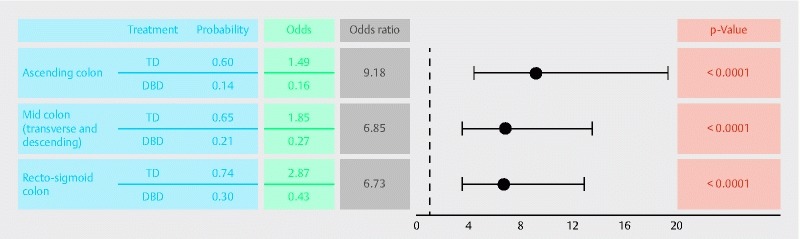
Responder status of tailored dosing and day-before dosing regimens (ITT population).DBD, day-before dosing; ITT, intention-to-treat; TD, tailored dosing
Convenience, satisfaction and overall experience
Results of the Convenience and Satisfaction Questionnaire are presented in Table 4. Ease of consuming the study drug was similar for both dosing regimens and was rated as easy to very easy by 93.6 % and 95.6 % of subjects in the TD and DBD arms, respectively. Convenience and satisfaction were rated as good or excellent by 88 % and 85.7 % of the patients following the TD and DBD regimen, respectively. Of the patients who were randomized to the TD regimen and responded to the questionnaire, 97.6 % stated they would request PICOPREP, if they required a colonoscopy in the future. The difference in the adjusted mean number of hours missed from work due to the colon cleansing procedure was negligible between the dosing regimens (–0.87 (95 % CI: – 3.47, 1.72; P = 0.5056). The adjusted difference in the mean score for the impact question (Did the colon cleansing treatment affect your regular daily activities, other than work at a job?) was significantly lower in the TD regimen vs. DBD regimen (–0.84, 95 % CI: – 1.52, – 0.17; P = 0.0147).
Table 4. Summary of the reported subject convenience and satisfaction – intention-to-treat analysis set.
| Tailored dosing (N = 131) | Day-before dosing (N = 73) | |
| % (r/n) | % (r/n) | |
| 1: How easy or difficult was it to consume the PICOPREP medication? | ||
| Very easy | 66.4 (83/125) | 72.9 (51/70) |
| Easy | 27.2 (34/125) | 22.9 (16/70) |
| Tolerable | 6.4 ( 8/125) | 4.3 ( 3/70) |
| Difficult | ||
| Very difficult | ||
| 2: Were you able to consume the entire PICOPREP medication as instructed? | ||
| Yes | 100.0 (125/125) | 100.0 (70/70) |
| No | ||
| 3: Please describe your overall experience with the PICOPREP medication. | ||
| Excellent | 28.0 (35/125) | 17.1 (12/70) |
| Good | 60.0 (75/125) | 68.6 (48/70) |
| Fair | 12.0 (15/125) | 12.9 ( 9/70) |
| Poor | 1.4 ( 1/70) | |
| Bad | ||
| 5: Would you ask your doctor for this colon cleansing medication if you need another colonoscopy in the future? | ||
| Yes | 97.6 (122/125) | 92.9 (65/70) |
| No | 2.4 ( 3/125) | 7.1 ( 5/70) |
| 6: Would you refuse the same colon cleansing medication again if it were to be prescribed to you in the future? | ||
| Yes | 3.2 ( 4/125) | 5.7 ( 4/70) |
| No | 96.8 (121/125) | 94.3 (66/70) |
ITT = intention-to-treat; N = number of patients; n = number of patients with available information; r = number of patients responding; % = r/n x 100
Repeat colonoscopy
A statistically significant difference in the proportion of patients requiring a repeat colonoscopy due to poor colon preparation was observed based on a post-hoc analysis using Fisher’s exact test (P < 0.0001). A total of three (2.4 %) patients following the TD regimen and 15 (21.4 %) patients following the DBD regimen required a repeat colonoscopy due to poor colon preparation.
Safety
Overall incidence of treatment-emergent AEs was 17 events (in 15 patients: 12.0 %) in TD regimen and seven events (in four patients: 5.7 %) in DBD regimen. The difference in the incidence of treatment-emergent AEs between the 2 regimens was not observed to be statistically significant, based on a post-hoc analysis using Fisher’s exact test (P = 0.2988). The most common AEs were gastrointestinal disorders (7.2 %), with abdominal pain being the most common AE (TD 5 [4 %] patients, and none in DBD) (Table 5). Large intestinal obstruction and post-procedural haemorrhage were considered as SAEs observed with TD, both unrelated to PICOPREP. No clinically relevant findings were observed in haematology, clinical chemistry, and coagulation laboratory parameters. A transient, average increase from baseline in serum magnesium (10.5 %) and bilirubin (49.2 %) was observed on the day of colonoscopy which was similar across both treatment regimens and well within the limits of normal range. No deaths were reported in the study.
Table 5. Most frequent adverse events (at least 1 % in any treatment group) by preferred term.
|
Tailored dosing
(N = 125) |
Day-before dosing
(N = 70) |
|
| Adverse events, n (%) | 15 (12.0) | 4 (5.7) |
|
– | 1 (1.4) |
|
5 (4.0) | – |
|
1 (0.8) | 1 (1.4) |
|
1 (0.8) | 1 (1.4) |
|
– | 1 (1.4) |
|
– | 1 (1.4) |
|
– | 1 (1.4) |
|
1 (0.8) | 1 (1.4) |
N, number of patients; n, number of patients with adverse events; %, percentage of patients with adverse events
Discussion
This study demonstrated that the TD regimen was superior to the DBD regimen in overall and ascending colon cleansing efficacy. For mid (transverse and descending) and recto-sigmoid colon cleansing, the odds that the Ottawa Scale score was ≤ 1, were statistically significantly greater for the TD regimen compared to the DBD regimen. The ease of consuming and overall experience were rated as easy to very easy and good to excellent, respectively, and were comparable for both dosing regimens. Furthermore, the incidence of repeat colonoscopy was significantly lower in the TD regimen (3 patients) as compared with the DBD regimen (15 patients).
The TD regimen demonstrated statistically significant improved colon cleansing for all colon segments (ascending, mid and recto-sigmoid colon) compared with the DBD regimen. This benefit can be attributed to the recommended short time period between the last dose of bowel preparation and colonoscopy procedure due to customised timing of the second dose (6.06 and 19.74 hours for TD and DBD regimen, respectively) 8 26.
The TD regimen had a reduced impact on ability to perform daily non-work-related activities in comparison to the DBD regimen. These results are in line with previous SEE CLEAR study with similar acceptability and tolerability profile of PICOPREP 13 17.
Of the 195 patients exposed to study drug, 19 experienced a treatment emergent AE. The most common AEs belonged to the category Gastrointestinal Disorders by System Organ Class (preferred term, abdominal pain). The safety results of this study were in line with the real-life experiences 6 26 and further support the well-established safety and tolerability profile of PICOPREP irrespective of the dosing regimens.
The current study has several strengths. First, randomization was stratified by the time of colonoscopy to ensure balance between the treatment arms with respect to the number of morning and afternoon colonoscopies. The analyses were also adjusted for time of colonoscopy, to account for any potential imbalance between the treatment arms, occurring by chance due to the randomisation. Furthermore, to minimize evaluation bias, the colonoscopist assessing the outcome of the colon cleansing was blinded to the dosing schedules.
Importantly, the efficacy analyses in ITT and PP populations were given equal weight to evaluate the non-inferiority of TD regimen with the DBD regimen. This is in line with recommendations from regulatory bodies as the analysis based on the ITT populations is not necessarily considered conservative for a non-inferiority study as it would be for a superiority study.
Conclusion
The TD regimen was superior compared with the DBD regimen in ascending and overall colon cleansing in preparation for colonoscopy. PICOPREP was safe and tolerable in both dosing regimens. The TD regimen is therefore efficacious in bowel cleansing prior to colonoscopy, regardless of the planned time of colonoscopy.
Appendix.

Patient Questionnaire.
Acknowledgements
The authors take full responsibility for the content of this manuscript. This study was sponsored by Ferring Pharmaceuticals. Medical writing support was provided by Mohit Joshi and Dr. Payal Bhardwaj (Tata Consultancy Services), both funded by Ferring. The authors also thank the following people from Ferring Pharmaceuticals for their contribution to the OPTIMA study: Christine Angelin, Elda Dinaj, Marianne Vinther Ammitzbøll, Johan Masure, Kyle Raymond, Winston Man A Hing.
Footnotes
Competing interests Ralf Kiesslich has received research support and speaker fee from Ferring pharmaceuticals. Stefan Schubert has received grants from Ferring pharmaceuticals for travel and meetings for this study. Michael Klemt-Kropp has received grants from Gilead Siences for an observational study “Retrieval of patients chronically infected with Hep. B and C in Northern Holland”. He has also received fees from AbbVie as a consulting member of international advisory Board hepatitis C. Furthermore, he has received fees for lectures, presentations and for moderating medical events on hepatitis B and C. Imke Behnken has received payments for lectures including service on speaker’s bureaus from MSD, Astra Zeneca and Novartis. Gillaume Bonnaud has received consulting fees/honarium from Ferring pharmaceuticals, Abbvie, Aptacis, MSD, Covidien and Takeda. He has also received travel allowance for the meetings and manuscript preparation and fees for participation in review activities such as data monitoring boards. Michael Blaker has received payments for lectures and chairs at symposia and conventions from Ferring Pharmaceuticals. Thierry Ponchon has received grants from Boston Scientific, Cook and Olympus Co. He has received consulting fees or honorium from Olympus Co, Boston Scientific, Cook Medical and Mayloy Spindler. He has also received fees for participation in review activities from Norgine and payments for lectures including service on speaker bureaus from Olympus Co, Boston Scientific, Cook Medical Fujifilm and Covidien. Michael Mross, Tobias Klugmann, Eric Keulen, Marcel Groenen and Wilfred Landry have no conflict of interests to disclose. Meredin Stoltenberg is an employee of Ferring pharmaceuticals.
References
- 1.Jemal A, Bray F, Center M M et al. Global cancer statistics. CA Cancer J Clin. 2011;612:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Prakash S R, Verma S, McGowan J et al. Improving the quality of colonoscopy bowel preparation using an educational video. Can J Gastroenterol. 2013;27:696–700. doi: 10.1155/2013/292636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagege H, Laugier R, Nahon S et al. Real-life conditions of use of sodium phosphate tablets for colon cleansing before colonoscopy. Endosc Int Open. 2015;3:346–353. doi: 10.1055/s-0034-1391847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah H, Desai D, Samant H et al. Comparison of split-dosing vs non-split (morning) dosing regimen for assessment of quality of bowel preparation for colonoscopy. World J Gastrointest Endosc. 2014;6:606–611. doi: 10.4253/wjge.v6.i12.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo I K, Lee J S, Chun H J et al. A randomized, prospective trial on efficacy and tolerability of low-volume bowel preparation methods for colonoscopy. Dig Liver Dis. 2015;47:131–137. doi: 10.1016/j.dld.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Jeon S R, Kim H G, Lee J S et al. Randomized controlled trial of low-volume bowel preparation agents for colonic bowel preparation: 2-L polyethylene glycol with ascorbic acid versus sodium picosulfate with magnesium citrate. Int J Colorectal Dis. 2015;30:251–258. doi: 10.1007/s00384-014-2066-9. [DOI] [PubMed] [Google Scholar]
- 7.Hassan C, Bretthauer M F, Kaminski M F et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2013;45:142–150. doi: 10.1055/s-0032-1326186. [DOI] [PubMed] [Google Scholar]
- 8.Mathus-Vliegen E, Pellise M, Heresbach D et al. Consensus guidelines for the use of bowel preparation prior to colonic diagnostic procedures: colonoscopy and small bowel video capsule endoscopy. Curr Med Res Opin. 2013;29:931–945. doi: 10.1185/03007995.2013.803055. [DOI] [PubMed] [Google Scholar]
- 9.Johnson D, Barkun A, Cohen L et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US multi-society task force on colorectal cancer. Gastroenterology. 2014;147:903–924. doi: 10.1053/j.gastro.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Rex D. Optimal bowel preparation – a practical guide for clinicians. Nat Rev Gastroenterol Hepatol. 2014;11:419–425. doi: 10.1038/nrgastro.2014.35. [DOI] [PubMed] [Google Scholar]
- 11.Rex D. Bowel preparation for colonoscopy: entering an era of increased expectations for efficacy. Clin Gastroenterol Hepatol. 2014;12:458–462. doi: 10.1016/j.cgh.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Oh C H, Lee C K, Kim J W et al. Suboptimal bowel preparation significantly impairs colonoscopic detection of non-polypoid colorectal neoplasms. Dig Dis Sci. 2015;60:2294–2303. doi: 10.1007/s10620-015-3628-6. [DOI] [PubMed] [Google Scholar]
- 13.Rex D K, Katz P O, Bertiger G et al. Split-dose administration of a dual-action, low-volume bowel cleanser for colonoscopy: the SEE CLEAR I study. Gastrointest Endosc. 2013;78:132–141. doi: 10.1016/j.gie.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Manes G, Repici A, Hassan C;MAGIC-P study groupRandomized controlled trial comparing efficacy and acceptability of split- and standard-dose sodium picosulfate plus magnesium citrate for bowel cleansing prior to colonoscopy Endoscopy 201446662–669. [DOI] [PubMed] [Google Scholar]
- 15.Kim E S, Lee W J, Jeen Y T et al. A randomized, endoscopist-blinded, prospective trial to compare the preference and efficacy of four bowel-cleansing regimens for colonoscopy. Scand J Gastroenterol. 2014;49:871–877. doi: 10.3109/00365521.2014.910543. [DOI] [PubMed] [Google Scholar]
- 16.Song K H, Suh W S, Jeong J S et al. Effectiveness of sodium picosulfate/magnesium citrate (PICO) for colonoscopy preparation. Ann Coloproctol. 2014;30:222–227. doi: 10.3393/ac.2014.30.5.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz P O, Rex D K, Epstein M et al. A dual-action, low-volume bowel cleanser administered the day before colonoscopy: results from the SEE CLEAR II study. Am J Gastroenterol. 2013;108:401–409. doi: 10.1038/ajg.2012.441. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz-Navas M, Calleja J L, Payeras G et al. A randomized trial to compare the efficacy and tolerability of sodium picosulfate-magnesium citrate solution vs. 4 L polyethylene glycol solution as a bowel preparation for colonoscopy. J Colorectal Dis. 2015;30:1407–1416. doi: 10.1007/s00384-015-2307-6. [DOI] [PubMed] [Google Scholar]
- 19.Bertiger G, Jones E, Dahdal D N et al. Serum magnesium concentrations in patients receiving sodium picosulfate and magnesium citrate bowel preparation: an assessment of renal function and electrocardiographic conduction. Clin Exp Gastroenterol. 2015;28:215–224. doi: 10.2147/CEG.S79216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leitao K, Grimstad T, Bretthauer M et al. Polyethylene glycol vs sodium picosulfate/magnesium citrate for colonoscopy preparation. Endosc Int Open. 2014;2:E230–E234. doi: 10.1055/s-0034-1377520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H G, Huh K C, Koo H S et al. Sodium picosulfate with magnesium citrate (SPMC) plus laxative is a good alternative to conventional large volume polyethylene glycol in bowel preparation: a multicenter randomized single-blinded trial. Gut Liver. 2015;9:494–501. doi: 10.5009/gnl14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner D, Benchimol E I, Dunn H et al. Pico-Salax versus polyethylene glycol for bowel cleanout before colonoscopy in children: a randomized controlled trial. Endoscopy. 2009;41:1038–1045. doi: 10.1055/s-0029-1215333. [DOI] [PubMed] [Google Scholar]
- 23.Di Nardo G, Aloi M, Cucchiara S et al. Bowel preparations for colonoscopy: an RCT. Pediatrics. 2014;134:249–256. doi: 10.1542/peds.2014-0131. [DOI] [PubMed] [Google Scholar]
- 24.Vejzovic V, Wennick A, Idvall E et al. Polyethylene glycol or sodium picosulphate based laxatives before colonoscopy in children. J Pediatr Gastroenterol Nutr. 2016;62:414–419. doi: 10.1097/MPG.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 25.Hahn S. Understanding noninferiority trials. Korean J Pediatr. 2012;55:403–407. doi: 10.3345/kjp.2012.55.11.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rex D K, Imperiale T F, Latinovich D R et al. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97:1696–1700. doi: 10.1111/j.1572-0241.2002.05827.x. [DOI] [PubMed] [Google Scholar]


