Abstract
Spinal muscular atrophy (SMA) is one of the leading genetic diseases of children and infants. SMA is caused by deletions or mutations of Survival Motor Neuron 1 (SMN1) gene. SMN2, a nearly identical copy of SMN1, cannot compensate for the loss of SMN1 due to predominant skipping of exon 7. While various regulatory elements that modulate SMN2 exon 7 splicing have been proposed, intronic splicing silencer N1 (ISS-N1) has emerged as the most promising target thus far for antisense oligonucleotide-mediated splicing correction in SMA. Upon procuring exclusive license from the University of Massachussets Medical School in 2010, Ionis Pharmaceuticals (formerly ISIS Pharamaceuticals) began clinical development of Spinraza™ (synonyms: Nusinersen, IONIS-SMNRX, ISIS-SMNRX), an antisense drug based on ISS-N1 target. Spinraza™ showed very promising results at all steps of the clinical development and was approved by US Food and Drug Administration (FDA) on December 23, 2016. Spinraza™ is the first FDA-approved treatment for SMA and the first antisense drug to restore expression of a fully functional protein via splicing correction. The success of Spinraza™ underscores the potential of intronic sequences as promising therapeutic targets and sets the stage for further improvement of antisense drugs based on advanced oligonucleotide chemistries and delivery protocols.
Keywords: spinal muscular atrophy, antisense oligonucleotides, survival motor neuron protein, SMN, Spinraza, ISIS-SMNRX, IONIS-SMNRX, nusinersen, ISS-N1, splicing regulation
Introduction
Spinal muscular atrophy (SMA) is the leading genetic cause of infant mortality [1]. SMA is characterized by the degeneration of α-motor neurons in the spinal cord, leading to progressive muscle weakness followed by respiratory insufficiency [1, 2]. SMA is caused by low levels of Survival Motor Neuron (SMN) protein due to homozygous deletion or mutation of the SMN1 gene [3, 4]. SMN is involved in several critical functions including but not limited to snRNP assembly, snoRNP assembly, telomerase biogenesis, transcription, translation, DNA repair, RNA trafficking, selenoprotein synthesis, stress granule formation, and various signaling pathways [5]. SMA is unique among genetic disorders in that humans carry a second copy of the SMN gene, SMN2 [3, 6]. However, due to a translationally silent C-to-T mutation (C6U in RNA) at the 6th position of exon 7, SMN2 exon 7 is inefficiently spliced producing a truncated protein SMNΔ7, which is unstable and only partially functional [6, 7, 8]. While several additional splice isoforms are generated by alternative splicing of both SMN1 and SMN2 [9–12], transcripts lacking exon 7 appear to be the major isoform produced by SMN2 in all tissues except in testis [10, 13]. Therefore, mechanism of SMN2 exon 7 splicing has been intensively studied [14–21]. Due to the potential for SMN2 to produce full-length SMN protein, it remains the principal target for therapies designed to increase production of functional SMN protein in conditions of SMA [22, 23, 24].
Discovery of ISS-N1 as potential therapeutic target
Multiple approaches have been explored as potential methods to increase production of SMN protein from SMN2, including increasing transcription [22, 24, 25, 26], modulating SMN2 exon 7 splicing [27–30], inducing translational read through of SMNΔ7 transcript [31], and increasing stability of SMN protein [32,33]. One of the most promising methods is the redirection of SMN2 splicing of exon 7 through antisense oligonucleotides (ASOs), short oligonucleotides designed to anneal to complementary sequences within a gene of interest [30, 34]. ASOs can exert their influence on SMN2 exon 7 splicing through multiple ways, including but not limited to blocking binding of trans-acting protein factors by steric hindrance [35, 36], causing structural rearrangements within the target RNA molecule [37, 38, 39], or recruiting additional trans-acting protein factors to the target molecule, in the case of bifunctional ASOs [40, 41, 42].
As the most promising target for an ASO-based therapy of SMA, Intronic Splicing Silencer N1(ISS-N1) was discovered in the Singh laboratory in 2004 at University of Massachusetts Medical School, Worcester, MA (US patent 7838657) [43]. ISS-N1 confers a very strong inhibitory effect on inclusion of SMN2 exon 7 and sequestration of ISS-N1 by an ASO leads to full splicing correction in SMA patient cells [44]. Because of the strong inhibitory effect, ISS-N1 is also referred to as the master checkpoint of splicing regulation of SMN2 exon 7 [45]. Discovery of ISS-N1 was possible thanks to the in vivo selection that revealed that the 5’ splice site of SMN exon 7 is very weak [17, 19, 46, 47]. Subsequent studies revealed that ISS-N1 is a complex regulatory element being affected by the presence of other regulatory elements upstream and downstream of ISS-N1 [20, 36, 38, 48]. ASOs targeting ISS-N1 are predicted to enhance SMN2 exon 7 inclusion by at least two mechanisms; first, by blocking binding of hnRNP A1 to two target motifs in the region [35], second, by causing secondary structural rearrangements and preventing an inhibitory long-distance interaction with downstream sequences deep within intron 7 [37, 38, 39]. Numerous studies have demonstrated the efficacy of ASOs targeting ISS-N1 in both SMA patient cells and mouse models of SMA and using multiple ASO chemistries [28, 34, 35, 44, 49–59). Based on the number of the independent studies performed, ISS-N1 would easily rank as the most studied antisense target for splicing correction for human disease. ISS-N1 targeting ASOs remain the most potent drugs for SMA therapy in independent pre-clinical studies [28, 53].
Clinical development of Spinraza™ for the treatment of SMA
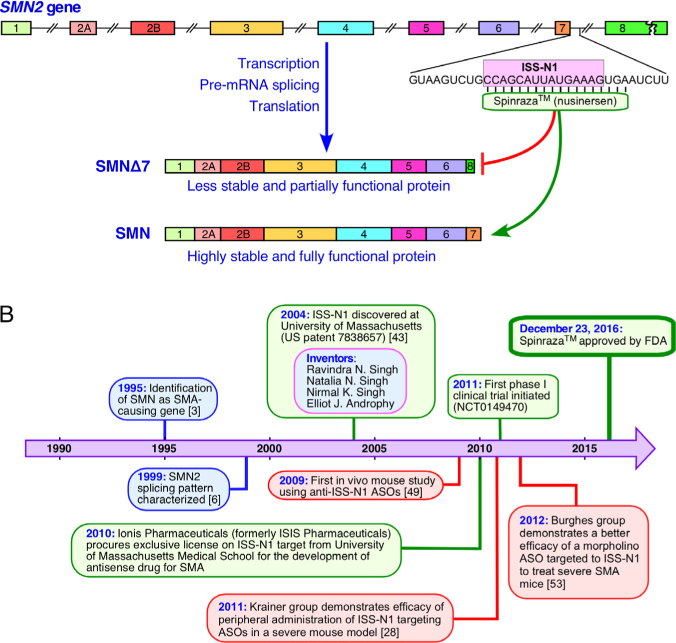
Ionis Pharmaceuticals (formerly ISIS Pharmaceuticals) obtained license for exclusive use of ISS-N1-targeting ASOs from University of Massachusetts Medical School, Worcester, MA in 2010. Spinraza™ (nusinersen), formerly known as IONIS-SMNRx or ISIS-SMNRx, is a 2’-O-methoxyethyl (2’MOE) modified ASO targeting ISS-N1 (Figure 1A). Ionis Pharmaceuticals began phase 1 clinical trials of ISIS-SMNRx in 2011 and results were very encouraging. Subsequently, Nusinersen/Spinraza™ has been the subject of multiple phase 2 and 3 clinical trials by Ionis Pharmaceuticals/Biogen (Figure 1B) [60, 61]. Shown to be both safe and effective in raising SMN protein levels and reducing the disease severity of SMA, Spinraza™ has recently been approved by the FDA for the treatment of both mild and severe SMA (Figure 1B). This represents the first FDA-approved drug for the treatment of SMA, as well as a proof-of-concept for the targeting of an ISS by an ASO for the treatment of a major genetic disease associated with the infant mortality.
Figure 1.
Spinraza™ represents the first FDA-approved drug for the treatment for SMA. (A) Overview of SMN2 genomic sequence and mechanism of Spinraza™ action. Exons are represented by colored boxes. Introns are represented by broken lines. Region of ISS-N1 downstream of exon 7 is shown. ISS-N1 is represented by pink box, annealing location of Spinraza™ is indicated. Protein products of SMN2 are shown below. Spinraza™ acts by redirecting splicing from the dysfunctional SMNΔ7 product to the full-length SMN. (B) Timeline of Spinraza™ target discovery, licensing, and therapeutic development. Purple arrow represents passage of time. Blue, red, and green ovals indicate critical developments in SMA research, landmark studies involving ISS-N1 ASOs, and critical stages in development of Spinraza™, respectively.
Although a promising first step in the treatment of SMA, there is still much progress to be made and many other promising approaches to follow. Most clinical trials of Spinraza™ have focused on treatment of symptomatic infants and children already diagnosed with SMA, by which time many changes have already occurred in motor neurons [62]. One promising approach which is the target of an ongoing clinical trial (NCT02386553) is to treat infants diagnosed with SMA-causing mutations but who have not yet experienced symptoms, thus preventing motor neuron degeneration before it can begin. Other approaches to increase expression of SMN, such as treatment with histone deacetylase (HDAC) inhibitors to increase transcription [25], have not shown sufficient efficacy for treatment of SMA by themselves, but may prove effective in combination with Spinraza™. SMA is not only a disease of motor neurons; low SMN levels can independently impact a number of somatic tissues [57, 58, 63–66] as well as the testis [13].
Currently, it is not known whether lumbar injections can fully ameliorate these peripheral defects. In addition, there are several other promising ASO targets within the SMN2 pre-mRNA, such as ISS-N2 deep within intron 7 [38], Element 1 within intron 6 [27, 67] and a GC-rich sequence that partially overlaps ISS-N1 [68, 69]. A recent report showed utility of an ASO targeting an antisense sequence of SMN2 [70]. In cell-based assays, a dual-masking ASO has shown a better efficacy than an ISS-N1 targeting ASO [71]. However, ISS-N1 still needs to be targeted to maintain the high efficacy of the dual-masking ASO [71]. Currently, it is not known how a variety of targets may be affected by different ASO chemistries or treatment with a combination of ASOs, and thus all ASO targets remain of interest for future research.
Conclusions and Future directions
Recent approval of Spinraza™ (Nusinersen) by US FDA as the first therapy for SMA is a major step forward for SMA patients worldwide. Spinraza™ also becomes the first antisense drug to restore the inclusion of an exon during pre-mRNA splicing. While invention of ISS-N1 was made in Singh laboratory more than a decade ago, credit of therapeutic development goes to several researchers who independently validated the therapeutic efficacy of ISS-N1 targeting ASOs. In particular, pioneering preclinical studies in the laboratory of Dr. Adrian Krainer at Cold Spring Harbor Laboratory in collaboration with Drs. Frank Bennet and Frank Rigo at Ionis Pharmaceuticals (formerly ISIS Pharmaceuticals) were critical for the Clinical development of Spinraza™. The exclusive licensing of ISS-N1-targeting ASOs from UMass Medical School allows IONIS Pharmaceuticals to develop additional drugs based on ISS-N1 target. Studies in the laboratories of Dr. Arthur Burghes at The Ohio State University and Dr. Francesco Muntoni at University College London independently validate the efficacy of ISS-N1-targeting morpholino ASOs [53–59]. SMA patients will tremendously benefit if additional antisense drugs based on morpholino and other chemistries are developed. This could be particularly important for patients who cannot tolerate the chemistry of Spinraza™. As we move forward with ASO-based therapy of SMA, there will be a need to develop noninvasive procedures for an effective delivery of drug into brain and spinal cord. With the FDA approval of Spinraza™, SMA disease transitions to the next phase in which long-term efficacy of Spinraza™ will be carefully monitored. We hope for a positive outcome that will have a transformative effect on the development of the next generation of the antisense drugs for SMA as well as for several other genetic diseases.
Acknowledgement
The nonprofit organization Cure SMA (formerly Families of SMA) supported the initial studies in Dr. Ravindra Singh laboratory that led the discovery of ISS-N1 target at University Massachusetts Medical School, Worcester, MA (USA). US National Institutes of Health (NIH) continues to support Singh laboratory to understand the mechanism of splicing regulation by ISS-N1 target, a complex regulatory element (NIH R01 NS055925). Author acknowledges critical comments and valuable suggestions of Dr. Ravindra Singh on this report. Author is partly supported by grants from NIH (NS055925), Iowa Center of Advanced Neurotoxicology (ICAN) and Salsbury Endowment at Iowa State University.
References
- [1].Lunn M.R., Wang C.H.. Spinal muscular atrophy. Lancet. 2008;371:2120–2133. doi: 10.1016/S0140-6736(08)60921-6. [DOI] [PubMed] [Google Scholar]
- [2].Ahmad S., Bhatia K., Kannan A., Gangwani L.. Molecular Mechanisms of Neurodegeneration in Spinal Muscular Atrophy. J. Exp. Neuroscience. 2016;10:39–49. doi: 10.4137/JEN.S33122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lefebvre S., Bürglen L., Reboullet S., Clermont O., Burlet P., Viollet L.. et al. Identification and characterization of a spinal muscular atrophydetermining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- [4].Wirth B.. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA) Hum. Mut. 2000;15:228–237. doi: 10.1002/(SICI)1098-1004(200003)15:3<228::AID-HUMU3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- [5].Singh R.N., Howell M.D., Ottesen E.W., Singh N.N.. Diverse role of Survival Motor Neuron protein. BBA Gene Reg. Mech. doi: 10.1016/j.bbagrm.2016.12.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lorson C.L., Hahnen E., Androphy E.J., Wirth B.. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Monani U.R., Lorson C.L., Parsons D.W., Prior T.W., Androphy E.J., Burghes A.H.M.. et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- [8].Cho S.C., Dreyfuss G.. A degron created by SMN2 exon 7 skipping is a principal contributor to spinal muscular atrophy severity. Genes Dev. 2010;24:438–442. doi: 10.1101/gad.1884910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Singh N.N., Seo J., Rahn S.J., Singh R.N.. A Multi-Exon-Skipping Detection Assay Reveals Surprising Diversity of Splice Isoforms of Spinal Muscular Atrophy Genes. Plos One. 2012;7:17. doi: 10.1371/journal.pone.0049595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Seo J., Singh N.N., Ottesen E.W., Sivanesan S., Shishimorova M., Singh R.N.. Oxidative Stress Triggers Body-Wide Skipping of Multiple Exons of the Spinal Muscular Atrophy Gene. Plos One. 2016;11:31. doi: 10.1371/journal.pone.0154390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Seo J., Singh N.N., Ottesen E.W., Lee B.M., Singh R.N.. A novel humanspecific splice isoform alters the critical C-terminus of Survival Motor Neuron protein. Sci. Rep. 2016;6:14. doi: 10.1038/srep30778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Setola V., Terao M., Locatelli D., Bassanini S., Garattini E., Battaglia G.. Axonal-SMN (a-SMN), a protein isoform of the survival motor neuron gene, is specifically involved in axonogenesis. Proc. Natl. Acad. Sci. U. S.A. 2007;104:1959–1964. doi: 10.1073/pnas.0610660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ottesen E.W., Howell M.D., Singh N.N., Seo J., Whitley E.M., Singh R.N.. Severe impairment of male reproductive organ development in a low SMN expressing mouse model of spinal muscular atrophy. Sci. Rep. 2016;6:17. doi: 10.1038/srep20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cartegni L., Krainer A.R.. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat. Genet. 2002;30:377–384. doi: 10.1038/ng854. [DOI] [PubMed] [Google Scholar]
- [15].Kashima T., Manley J.L.. A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat. Genet. 2003;34:460–463. doi: 10.1038/ng1207. [DOI] [PubMed] [Google Scholar]
- [16].Singh N.N., Androphy E.J., Singh R.N.. An extended inhibitory context causes skipping of exon 7 of SMN2 in spinal muscular atrophy. Biochem. Biophys. Res. Commun. 2004;315:381–388. doi: 10.1016/j.bbrc.2004.01.067. [DOI] [PubMed] [Google Scholar]
- [17].Singh N.N., Androphy E.J., Singh R.N.. In vivo selection reveals combinatorial controls that define a critical exon in the spinal muscular atrophy genes. RNA. 2004;10:1291–1305. doi: 10.1261/rna.7580704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Singh N.N., Androphy E.J., Singh R.N.. The regulation and regulatory activities of alternative splicing of the SMN gene. Crit. Rev. Eukaryot. Gene Expr. 2004;14:271–285. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.30. [DOI] [PubMed] [Google Scholar]
- [19].Singh R.N.. Unfolding the mystery of alternative splicing through a unique method of in vivo selection, Front. Biosci. 2007;12:3263–3272. doi: 10.2741/2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Singh N.N., Singh R.N.. Alternative splicing in spinal muscular atrophy underscores the role of an intron definition model. RNA Biol. 2011;8:600–606. doi: 10.4161/rna.8.4.16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Singh N.N., Howell M.D., Singh R.N.. Transcriptional and Splicing Regulation of Spinal Muscular Atrophy Genes Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Charlotte S.J., Paushkin S., Ko C.-P.. Elsevier Inc. 2016 [Google Scholar]
- [22].Seo J., Howell M.D., Singh N.N., Singh R.N.. Spinal muscular atrophy: An update on therapeutic progress. Biochim. Biophys. Acta-Mol. Basis Dis. 2013;1832:2180–2190. doi: 10.1016/j.bbadis.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Awano T., Kim J.K., Monani U.. Spinal Muscular Atrophy: Journeying From Bench to Bedside. NeuroRx. 2014;11:786–795. doi: 10.1007/s13311-014-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Howell M.D., Singh N.N., Singh R.N.. Advances in therapeutic development for spinal muscular atrophy. Future Med. Chem. 2014;6:1081–1099. doi: 10.4155/fmc.14.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mohseni J., Zabidi-Hussin Z., Sasongko T.H.. Histone deacetylase inhibitors as potential treatment for spinal muscular atrophy. Genet. Mol. Biol. 2013;36:299–307. doi: 10.1590/S1415-47572013000300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Branchu J., Biondi O., Chali F., Collin T., Leroy F., Mamchaoui K.. et al. Shift from Extracellular Signal-Regulated Kinase to AKT/cAMP Response Element-Binding Protein Pathway Increases Survival-Motor-Neuron Expression in Spinal-Muscular-Atrophy-Like Mice and Patient Cells. J. Neurosci. 2013;33:4280-+. doi: 10.1523/JNEUROSCI.2728-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Baughan T.D., Dickson A., Osman E.Y., Lorson C.L.. Delivery of bifunctional RNAs that target an intronic repressor and increase SMN levels in an animal model of spinal muscular atrophy. Hum. Mol. Genet. 2009;18:1600–1611. doi: 10.1093/hmg/ddp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hua Y., Sahashi K., Rigo F., Hung G., Horev G., Bennett C.F.. et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–126. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sivanesan S., Howell M.D., DiDonato C.J., Singh R.N.. Antisense oligonucleotide mediated therapy of spinal muscular atrophy. Transl. Neurosci. 2013;4:1–7. doi: 10.2478/s13380-013-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Singh N.N., Lee B.M., DiDonato C.J., Singh R.N.. Mechanistic principles of antisense targets for the treatment of spinal muscular atrophy. Fut. Med. Chem. 2015;7:1793–1808. doi: 10.4155/fmc.15.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Heier C.R., DiDonato C.J.. Translational readthrough by the aminoglycoside geneticin (G418) modulates SMN stability in vitro and improves motor function in SMA mice in vivo. Hum. Mol. Genet. 2009;18:1310–1322. doi: 10.1093/hmg/ddp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hsu S.H., Lai M.C., Er T.K., Yang S.N., Hung C.H., Tsai H.H.. et al. Ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) regulates the level of SMN expression through ubiquitination in primary spinal muscular atrophy fibroblasts. Clin. Chim. Acta. 2010;411:1920–1928. doi: 10.1016/j.cca.2010.07.035. [DOI] [PubMed] [Google Scholar]
- [33].Han K.J., Foster D.G., Zhang N.Y., Kanisha K., Dzieciatkowska M., Sclafani R.A.. et al. Ubiquitin-specific Protease 9x Deubiquitinates and Stabilizes the Spinal Muscular Atrophy Protein-Survival Motor Neuron. J. Biol. Chem. 2012;287:43741–43752. doi: 10.1074/jbc.M112.372318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Seo J., Ottesen E.W., Singh R.N.. Antisense Methods to Modulate PremRNA Splicing. Meth. Mol. Biol. 2014;1126:271–283. doi: 10.1007/978-1-62703-980-2_20. [DOI] [PubMed] [Google Scholar]
- [35].Hua Y., Vickers T.A., Okunola H.L., Bennett C.F., Krainer A.R.. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am. J. Hum. Genet. 2008;82:834–848. doi: 10.1016/j.ajhg.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Singh N.N., Seo J.B., Ottesen E.W., Shishimorova M., Bhattacharya D., Singh R.N.. TIA1 Prevents Skipping of a Critical Exon Associated with Spinal Muscular Atrophy. Mol. Cell. Biol. 2011;31:935–954. doi: 10.1128/MCB.00945-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Singh N.N., Hollinger K., Bhattacharya D., Singh R.N.. An antisense microwalk reveals critical role of an intronic position linked to a unique long-distance interaction in pre-mRNA splicing. RNA. 2010;16:1167–1181. doi: 10.1261/rna.2154310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Singh N.N., Lawler M.N., Ottesen E.W., Upreti D., Kaczynski J.R., Singh R.N.. An intronic structure enabled by a long-distance interaction serves as a novel target for splicing correction in spinal muscular atrophy. Nucl. Acids Res. 2013;41:8144–8165. doi: 10.1093/nar/gkt609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Singh N.N., Lee B.M., Singh R.N.. Splicing regulation in spinal muscular atrophy by an RNA structure formed by long-distance interactions. DNA Habitats and Their RNA Inhabitants. 2015;1341:176–187. doi: 10.1111/nyas.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cartegni L., Krainer A.R.. Correction of disease-associated exon skipping by synthetic exon-specific activators. Nat. Struct. Biol. 2003;10:120–125. doi: 10.1038/nsb887. [DOI] [PubMed] [Google Scholar]
- [41].Meyer K., Marquis J., Trub J., Nlend R.N., Verp S., Ruepp M.D.. et al. Rescue of a severe mouse model for spinal muscular atrophy by U7 snRNA-mediated splicing modulation. Hum. Mol. Genet. 2009;18:546–555. doi: 10.1093/hmg/ddn382. [DOI] [PubMed] [Google Scholar]
- [42].Owen N., Zhou H.Y., Malygin A.A., Sangha J., Smith L.D., Muntoni F.. et al. Design principles for bifunctional targeted oligonucleotide enhancers of splicing. Nucl. Acids Res. 2011;39:7194–7208. doi: 10.1093/nar/gkr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Singh R.N., Singh N.N., Singh N.K., Androphy E.J.. Spinal muscular atrophy (SMA) treatment via targeting of SMN2 splice site inhibitory sequences. US patent publication # US7838657. 2010 Also published as US8110560, US8586559, US9476042, US20070292408, US20100087511, US20120165394, US20140066492. [Google Scholar]
- [44].Singh N.K., Singh N.N., Androphy E.J., Singh R.N.. Splicing of a critical exon of human survival motor neuron is regulated by a unique silencer element located in the last intron. Mol. Cell. Biol. 2006;26:1333–1346. doi: 10.1128/MCB.26.4.1333-1346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Buratti E., Baralle M., Baralle F.E.. Defective splicing, disease and therapy: searching for master checkpoints in exon definition. Nucl. Acids Res. 2006;34:3494–3510. doi: 10.1093/nar/gkl498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Singh N.N., Seo J., Singh R.N. Stamm S., Smith C. W. J., Lührmann R. Identification of Splicing cis-Elements Through an Ultra-Refined Antisense Microwalk, in Alternative pre-mRNA Splicing: Theory and Protocols. Wiley-VCH Verlag GmbH & Co., KGaA, Weinheim; Germany: 2012. [Google Scholar]
- [47].Singh R.N., Singh N.N. Stamm S., Smith C. W. J., Lührmann R. Functional Analysis of Large Exonic Sequences Through Iterative In Vivo Selection, in Alternative pre-mRNA Splicing: Theory and Protocols. Wiley-VCH Verlag GmbH & Co., KGaA, Weinheim; Germany: 2012. [Google Scholar]
- [48].Singh N.N., Singh R.N., Androphy E.J.. Modulating role of RNA structure in alternative splicing of a critical exon in the spinal muscular atrophy genes. Nucl. Acids Res. 2007;35:371–389. doi: 10.1093/nar/gkl1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Williams J.H., Schray R.C., Patterson C.A., Ayitey S.O., Tallent M.K., Lutz G.J.. Oligonucleotide-Mediated Survival of Motor Neuron Protein Expression in CNS Improves Phenotype in a Mouse Model of Spinal Muscular Atrophy. J. Neurosci. 2009;29:7633–7638. doi: 10.1523/JNEUROSCI.0950-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hua Y.M., Sahashi K., Hung G.N., Rigo F., Passini M.A., Bennett C.F.. et al. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 2010;24:1634–1644. doi: 10.1101/gad.1941310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Passini M.A., Bu J., Richards A.M., Kinnecom C., Sardi S.P., Stanek L.M.. et al. Antisense Oligonucleotides Delivered to the Mouse CNS Ameliorate Symptoms of Severe Spinal Muscular Atrophy. Sci. Transl. Med. 2011;3:11. doi: 10.1126/scitranslmed.3001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Osman E.Y., Yen P.F., Lorson C.L.. Bifunctional RNAs Targeting the Intronic Splicing Silencer N1 Increase SMN Levels and Reduce Disease Severity in an Animal Model of Spinal Muscular Atrophy. Mol. Ther. 2012;20:119–126. doi: 10.1038/mt.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Porensky P.N., Mitrpant C., McGovern V.L., Bevan A.K., Foust K.D., Kaspar B.K.. et al. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum. Mol. Genet. 2012;21:1625–1638. doi: 10.1093/hmg/ddr600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhou H., Janghra N., Mitrpant C., Dickinson R.L., Anthony K., Price L.. et al. A novel morpholino oligomer targeting ISS-N1 improves rescue of severe spinal muscular atrophy transgenic mice. Hum. Gene. Ther. 2013;24:331–342. doi: 10.1089/hum.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mitrpant C., Porensky P., Zhou H.Y., Price L., Muntoni F., Fletcher S.. et al. Improved Antisense Oligonucleotide Design to Suppress Aberrant SMN2 Gene Transcript Processing: Towards a Treatment for Spinal Muscular Atrophy. Plos One. 2013;8:10. doi: 10.1371/journal.pone.0062114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhou H.Y., Meng J.H., Marrosu E., Janghra N., Morgan J., Muntoni F.. Repeated low doses of morpholino antisense oligomer: an intermediate mouse model of spinal muscular atrophy to explore the window of therapeutic response. Hum. Mol. Genet. 2015;24:6265–6277. doi: 10.1093/hmg/ddv329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sintusek P., Catapano F., Angkathunkayul N., Marrosu E., Parson S.H., Morgan J.E.. et al. Histopathological Defects in Intestine in Severe Spinal Muscular Atrophy Mice Are Improved by Systemic Antisense Oligonucleotide Treatment. Plos One. 2016;11:15. doi: 10.1371/journal.pone.0155032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Szunyogova E., Zhou H.Y., Maxwell G.K., Powis R.A., Francesco M., Gillingwater T.H.. et al. Survival Motor Neuron (SMN) protein is required for normal mouse liver development. Sci. Rep. 2016;6:14. doi: 10.1038/srep34635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hammond S.M., Hazell G., Shabanpoor F., Saleh A.F., Bowerman M., Sleigh J.N.. et al. Systemic peptide-mediated oligonucleotide therapy improves long-term survival in spinal muscular atrophy. Proc. Natl. Acad. Sci. U. S. A. 2016;113:10962–10967. doi: 10.1073/pnas.1605731113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chiriboga C.A., Swoboda K.J., Darras B.T., Iannaccone S.T., Montes J., De Vivo D.C.. et al. Results from a phase 1 study of nusinersen (ISIS-SMNRx) in children with spinal muscular atrophy. Neurol. 2016;86:890–897. doi: 10.1212/WNL.0000000000002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Finkel R.S., Chiriboga C.A., Vajsar J., Day J.W., Montes J., De Vivo D.C.. et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study, Lancet. 2017;388:3017–3026. doi: 10.1016/S0140-6736(16)31408-8. Epub 2016/12/07. [DOI] [PubMed] [Google Scholar]
- [62].Zhang Z.X., Pinto A.M., Wan L.L., Wang W., Berg M.G., Oliva I.. et al. Dysregulation of synaptogenesis genes antecedes motor neuron pathology in spinal muscular atrophy. Proc. Natl. Acad. Sci. U. S. A. 2013;110:19348–19353. doi: 10.1073/pnas.1319280110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Heier C.R., Satta R., Lutz C., DiDonato C.J.. Arrhythmia and cardiac defects are a feature of spinal muscular atrophy model mice. Hum. Mol. Genet. 2010;19:3906–3918. doi: 10.1093/hmg/ddq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Shababi M., Habibi J., Yang H.T., Vale S.M., Sewell W.A., Lorson C.L.. Cardiac defects contribute to the pathology of spinal muscular atrophy models. Hum. Mol. Genet. 2010;19:4059–4071. doi: 10.1093/hmg/ddq329. [DOI] [PubMed] [Google Scholar]
- [65].Gombash S.E., Cowley C.J., Fitzgerald J.A., Iyer C.C., Fried D., McGovern V.L.. et al. SMN deficiency disrupts gastrointestinal and enteric nervous system function in mice. Hum. Mol. Genet. 2015;24:3847–3860. doi: 10.1093/hmg/ddv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Thomson A.K., Somers E., Powis R.A., Shorrock H.K., Murphy K., Swoboda K.J.. et al. Survival of motor neurone protein is required for normal postnatal development of the spleen. J. Anat. 2016 doi: 10.1111/joa.12546. Epub 2016/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Miyajima H., Miyaso H., Okumura M., Kurisu J., Imaizumi K.. Identification of a cis-acting element for the regulation of SMN exon 7 splicing. J. Biol. Chem. 2002;277:23271–23277. doi: 10.1074/jbc.M200851200. [DOI] [PubMed] [Google Scholar]
- [68].Singh N.N., Shishimorova M., Cao L.C., Gangwani L., Singh R.N.. A short antisense oligonucleotide masking a unique intronic motif prevents skipping of a critical exon in spinal muscular atrophy. RNA Biol. 2009;6:341–350. doi: 10.4161/rna.6.3.8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Keil J.M., Seo J., Howell M.D., Hsu W.H., Singh R.N.. DiDonato CJ., A Short Antisense Oligonucleotide Ameliorates Symptoms of Severe Mouse Models of Spinal Muscular Atrophy. Mol. Ther.-Nucleic Acids. 2014;3:10. doi: 10.1038/mtna.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].d’Ydewalle C., Ramos D.M., Pyles N.J., Ng S.Y., Gorz M., Pilato C.M.. et al. The Antisense Transcript SMN-AS1 Regulates SMN Expression and Is a Novel Therapeutic Target for Spinal Muscular Atrophy. Neuron. 2016 doi: 10.1016/j.neuron.2016.11.033. Epub 2016/12/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Pao P.W., Wee K.B., Yee W.C., DwiPramono Z.A.. Dual Masking of Specific Negative Splicing Regulatory Elements Resulted in Maximal Exon 7 Inclusion of SMN2 Gene. Mol. Ther. 2014;22:854–861. doi: 10.1038/mt.2013.276. [DOI] [PMC free article] [PubMed] [Google Scholar]