Mutations in HINT1 cause a common, autosomal-recessive, axonal Charcot-Marie-Tooth neuropathy, often with neuromyotonia. Peeters et al. summarize neurological aspects of the disease, epidemiology and mutation spectrum, and structural and functional characteristics of the affected protein. They propose guidelines to recognize and differentiate HINT1-neuropathy and suggest strategies to treat common symptoms.
Keywords: HINT1, CMT, neuropathy, neuromyotonia, clinical characteristics
Abstract
Recessive mutations in the gene encoding the histidine triad nucleotide binding protein 1 (HINT1) were recently shown to cause a motor-predominant Charcot–Marie–Tooth neuropathy. About 80% of the patients exhibit neuromyotonia, a striking clinical and electrophysiological hallmark that can help to distinguish this disease and to guide diagnostic screening. HINT1 neuropathy has worldwide distribution and is particularly prevalent in populations inhabiting central and south-eastern Europe. With 12 different mutations identified in more than 60 families, it ranks among the most common subtypes of axonal Charcot–Marie–Tooth neuropathy. This article provides an overview of the present knowledge on HINT1 neuropathy with the aim to increase awareness and spur interest among clinicians and researchers in the field. We propose diagnostic guidelines to recognize and differentiate this entity and suggest treatment strategies to manage common symptoms. As a recent player in the field of hereditary neuropathies, the role of HINT1 in peripheral nerves is unknown and the underlying disease mechanisms are unexplored. We provide a comprehensive overview of the structural and functional characteristics of the HINT1 protein that may guide further studies into the molecular aetiology and treatment strategies of this peculiar Charcot–Marie–Tooth subtype.
Introduction
Hereditary peripheral neuropathies are a clinically and genetically heterogeneous group of disorders, characterized by muscle weakness, wasting and sensory loss, starting in the distal parts of the limbs and slowly progressing in a length-dependent manner (Boerkoel et al., 2002; Patzko and Shy, 2012). In 2012, we identified recessive mutations in the gene encoding the histidine triad nucleotide binding protein 1 (HINT1) causing axonal, motor-predominant Charcot–Marie–Tooth (CMT) neuropathy, frequently associated with neuromyotonia (Zimon et al., 2012). HINT1 represents a global cause of CMT, with 79 patients of European, North American and Chinese ancestry identified to date (Zimon et al., 2012; Caetano et al., 2014; Zhao et al., 2014; Boaretto et al., 2015; Jerath et al., 2015; Lassuthova et al., 2015; Rauchenzauner et al., 2016; Veltsista and Chroni, 2016). The frequency of HINT1 mutations in a heterogeneous cohort of recessive CMT patients is ∼10% and rises to 80% when focusing on individuals with axonal neuropathy having the clinical hallmark of neuromyotonia (Zimon et al., 2012, 2015). Thus, HINT1-associated peripheral neuropathy represents a distinct clinical and genetic entity that needs to be differentiated among the numerous subtypes of CMT; and from myotonic dystrophy and the various channelopathies causing non-dystrophic forms of myotonia. This update summarizes the current knowledge on the clinical and electrophysiological aspects of the HINT1 neuropathy, the overlap with other clinical entities, the epidemiology and mutation spectrum, and the structural and functional characteristics of the encoded protein.
Epidemiology
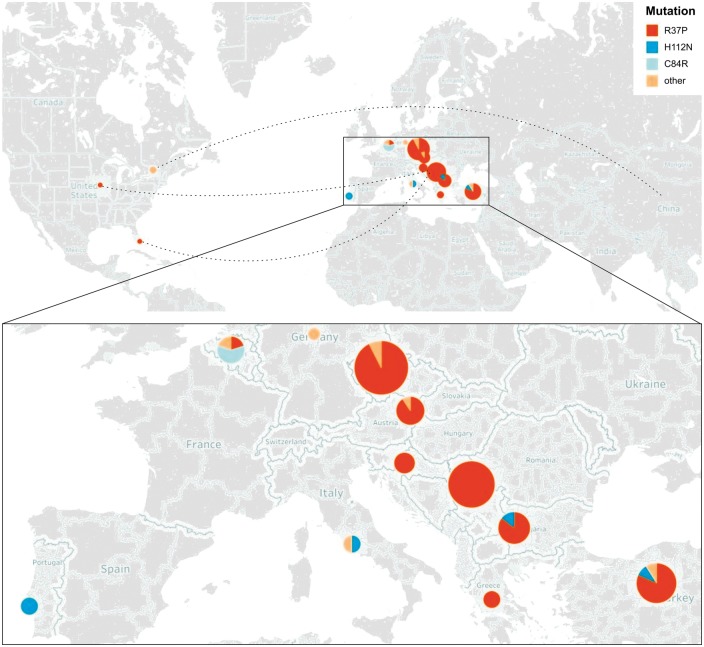
HINT1 neuropathy has a non-random distribution (Fig. 1). The majority of diagnosed individuals are of European origin, a fact attributed to three founder mutations (R37P, C84R, H112N). R37P is the most common among them, displaying a gradient of distribution increasing from west to east in Europe. Forty-eight families described to date carry this mutation, most of them inhabiting or originating from central or south-eastern Europe and Turkey (Fig. 1). The R37P carrier frequency in outbred populations living in this geographic area is as high as 1:67-182, making HINT1 neuropathy one of the most common autosomal recessive disorders in this part of the world (Zimon et al., 2012; Lassuthova et al., 2015). The high R37P carrier rate can even lead to ‘pseudo-dominant’ inheritance of CMT, with affected individuals in two consecutive generations due to the influx of unrelated heterozygous carriers (Jordanova A. and Tournev I., unpublished results). In the Czech population, HINT1 neuropathy is among the most frequent causes of inherited neuropathy, only surpassed by CMT1A/HNPP and mutations in GJB1 (previously known as Cx32) and MPZ (Lassuthova et al., 2015). Because 90% of the Czech HINT1 patients carry R37P, genetic diagnosis becomes straightforward. Moreover, the US-based patients homozygous for R37P have central European origin (Zimon et al., 2012; Jerath et al., 2015). H112N is another founder mutation, with five families reported of Italian, Turkish, Bulgarian and (Portuguese) Roma origin. Finally, C84R is present in homozygous or compound heterozygous state in four Belgian families. Overall, the genetic epidemiology suggests that HINT1 neuropathy should be considered in the diagnostic work-up of patients of European descent presenting with axonal CMT.
Figure 1.
Worldwide distribution of HINT1 mutations. Pie chart size represents the number of patients identified per country and colours indicate which founder HINT1 mutations they are carrying. Dashed lines point out the country of origin of the identified patients. Enlarged panel below shows the regions in Europe where most patients are clustered. Note the gradient of distribution for the most common HINT1 mutation (R37P), increasing in central and south-eastern Europe.
Clinical features
The phenotype initially related to mutations in HINT1 encompasses axonal, motor-greater-than-sensory polyneuropathy with an onset mostly in the first decade of life, combined with action neuromyotonia (more pronounced in the hands) and neuromyotonic or myokymic discharges on needle EMG (Zimon et al., 2012; Caetano et al., 2014; Lassuthova et al., 2015; Rauchenzauner et al., 2016). The identification of additional patients extended the clinical spectrum; including a later disease onset (up to 28 years of age) (Zhao et al., 2014), asymmetric gait involvement (Rauchenzauner et al., 2016) or a pure distal motor neuropathy (dHMN) without neuromyotonia (Zhao et al., 2014; Boaretto et al., 2015).
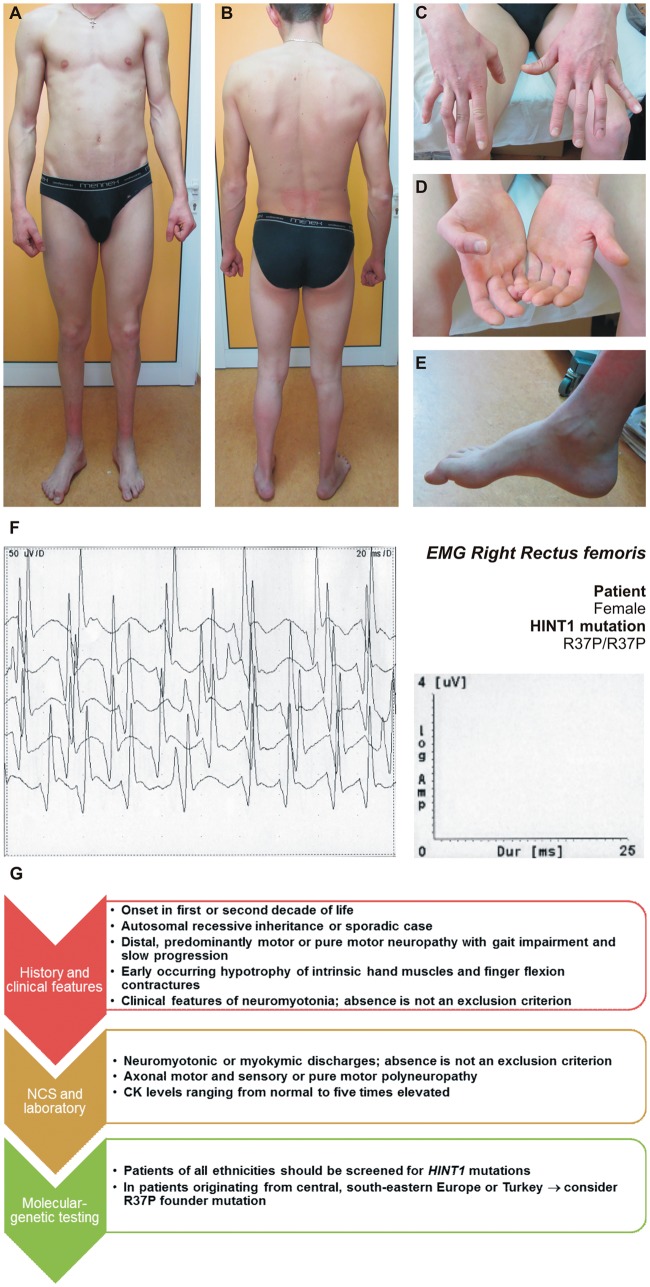
The initial complaints are distal lower limb weakness with gait impairment, combined with muscle stiffness, fasciculations and cramps in hands and legs, worsened by cold. When specifically asked, most patients report difficulties in releasing grip after a strong voluntary hand contraction, dating back from childhood. The disorder is slowly progressive; none of the reported patients lose ambulation until the sixth decade of life. Upon clinical examination, foot/toe extension and flexion weakness to plegia are present in almost all cases (Zimon et al., 2012; Caetano et al., 2014; Boaretto et al., 2015; Lassuthova et al., 2015). Achilles tendon reflexes are diminished to absent, depending on the stage of progression. Upper limbs become involved later in the disease course, usually in the first or second decade. Calf and intrinsic hand and foot muscle wasting is almost always observed to a variable degree (Fig. 2A–E). The hypotrophy of the intrinsic hand muscles, particularly of the hypothenar and thenar eminence is pronounced, leading to flexion contractures of the fingers, even in cases with mild muscle weakness (Fig. 2D and E). Mild distal sensory impairment can be present (Zimon et al., 2012; Caetano et al., 2014; Lassuthova et al., 2015).
Figure 2.
Clinical presentation of HINT1 patients. (A–E) A 29-year-old male patient (genotype R37P/R37P) showing bilateral calf muscle atrophy (A and B), flexion contractures of the fingers (C), intrinsic hand muscle wasting (D), and pes cavus (E). (F) Neuromyotonic discharges, recorded with a concentric needle electrode in the right m. rectus femoris of a 27-year-old female patient (genotype R37P/R37P). (G) Diagnostic guidelines for HINT1 neuropathy.
Neuromyotonia
Neuromyotonia is present in 70–80% of patients and is a diagnostic hallmark. It is characterized by spontaneous muscular activity at rest (myokymia), impaired muscle relaxation (pseudomyotonia), and contractures of hands and feet (Maddison, 2006); and can be observed with or without overt peripheral neuropathy (Hahn et al., 1991, 2000). In contrast to myotonia, in which abnormal muscle activity occurs only after voluntary or induced muscle contraction, neuromyotonia results from spontaneously occurring peripheral nerve discharges often accentuated by voluntary muscle contraction (Rauchenzauner et al., 2016). This phenomenon was comprehensively characterized in two sibs of a Canadian family (Hahn et al., 1991), where subsequently HINT1 mutations were identified (Zimon et al., 2012). The abnormal electrical activity can be enhanced by nerve ischaemia, but not by mechanical or electrical stimulation of the nerve supplying the muscle, thus suggesting that the nerve hyperexcitability is a generalized phenomenon related to a functional or structural abnormality of the axonal membrane. The neuronal origin of neuromyotonia was subsequently proven by regional neuromuscular blockade with curare and nerve block with xylocaine. HINT1 patients display action myotonia (delayed muscle relaxation of the hands after strong flexion of the fingers), while percussion myotonia of the thenar eminence is not typical (Zimon et al., 2012; Caetano et al., 2014; Boaretto et al., 2015; Lassuthova et al., 2015). Unfortunately, the symptoms of peripheral nerve excitability can be easily missed from patients’ history or from the neurological examination.
Various types of skeletal deformities are noted in HINT1 patients. Foot deformities (pes cavus, pes equinovarus, pes cavovarus or Achilles tendon shortening) are present in a great proportion of cases (Zimon et al., 2012; Lassuthova et al., 2015). Flexion contractures of the fingers are typical, occurring up to several years after the lower limb involvement (Tournev I., unpublished results). Scoliosis is reported in one-third of the patients (Lassuthova et al., 2015; Jerath et al., 2015).
In some patients, mild-to-moderate elevation of creatine kinase levels is observed (Zimon et al., 2012; Jerath et al., 2015), probably related to the chronic neurogenic muscle atrophy in combination with the neuromyotonia.
Electrophysiology
Electrophysiological studies of peripheral nerves are compatible with axonal polyneuropathy; either motor-and-sensory (42/64; 66%) (Zimon et al., 2012; Lassuthova et al., 2015) or pure motor (22/64; 34%) (Zimon et al., 2012; Zhao et al., 2014; Boaretto et al., 2015). Conduction velocities of motor and sensory fibres are (nearly) normal, while the amplitudes of compound muscle action potential or sensory nerve action potential are decreased. No markers of demyelination (conduction slowing, temporal dispersion or conduction block) are present. Needle EMG shows increased amplitude of motor unit action potentials and reduction of recruitment pattern with temporal summation.
Concentric needle EMG from proximal and distal muscles often displays neuromyotonic discharges (Fig. 2F) occurring spontaneously or provoked by needle movement or muscle contraction (Zimon et al., 2012; Lassuthova et al., 2015). They are characterized by high frequency (150–200 Hz), decrementing, repetitive discharges of a single motor unit with motor unit action potential morphology. Myokymic discharges, representing rhythmic, grouped discharges of the same motor unit, are also observed. The firing frequency within the burst is 2–60 Hz followed by a short period (up to a few seconds) of silence, and then recurrence of the burst at regular intervals (Kucukali et al., 2015). Hyperexcitability and ectopic impulse generation can occur along the whole length of the axons, including the terminal arborizations (Hahn et al., 1991). Although considered an EMG hallmark, neuromyotonic or myokymic discharges are absent in around 20–30% of patients, thus complicating the differential diagnosis (Zimon et al., 2012; Zhao et al., 2014; Boaretto et al., 2015). Moreover, they may occur in the later stages of the disease (Zimon et al., 2012; Caetano et al., 2014; Boaretto et al., 2015; Lassuthova et al., 2015).
Nerve biopsy
The changes observed in the sural nerve of five HINT1 patients are consistent with an axonal neuropathy, even when no clinical features of sensory neuropathy are present (Zimon et al., 2012).
Differential diagnosis
The diagnosis of HINT1-associated hereditary neuropathy requires consideration whether the phenotype is genetic or acquired. Due to the recessive pattern of inheritance this is not always straightforward, especially in sporadic cases. Detailed genealogy, neurological examination, nerve conduction studies and EMG are crucial. Diagnostic guidelines to recognize HINT1 neuropathy are represented in Fig. 2G.
The differential diagnosis includes several acquired and inherited disease entities, associated with abnormal spontaneous muscle/nerve hyperexcitability and/or weakness (Table 1). As neuromyotonia can be absent or under-recognized, other types of hereditary axonal CMT and pure motor neuropathies should be considered (Rossor et al., 2012; Zimon et al., 2012; Zhao et al., 2014).
Table 1.
Differential diagnosis of HINT1 neuropathy
| Clinical entity | Aetiology | Clinical findings | Electrodiagnostic findings |
|---|---|---|---|
| Acquired disorders | |||
| Isaacs syndrome | VGKC antibodies | Onset predominantly in the mid-40s Continuous muscle twitching and myokymia, muscle hypertrophy, weight loss, hyperhidrosis Preserved muscle strength and tendon reflexes | Normal sensory and motor NCS, except for after-discharges Neuromyotonic and myokymic discharges, doublets or triplets or multiplets, fasciculation potentials, fibrillation potentials, and cramp discharges, occurring spontaneously or activated by voluntary muscle contraction on needle EMG |
| Morvan syndrome | VGKC antibodies | Similar to Isaacs syndrome plus CNS: encephalopathy, headaches, drowsiness, and hallucinations | Similar to Isaacs syndrome |
| Cramp fasciculation syndrome | Uncertain | Muscle cramps, exercise intolerance, and muscle twitching | After-discharges on repetitive nerve stimulation and fasciculation potentials on needle EMG |
| Other | Toxins: lead, silver and gold | Myotonic discharges on needle EMG | |
| Inherited disorders | |||
| Episodic ataxia type 1 | Mutations in KCNA1 | Attacks of generalized ataxia, persistent myokymia | Neuromyotonic and myokymic discharges on needle EMG |
| Schwartz–Jampel syndrome | Mutations in HSPG2 | Myotonia, typical facial appearance (blepharophimosis) and skeletal deformities | Myotonic discharges on needle EMG |
| Rippling muscle disease | Mutations in CAV3 | Rolling movements of muscles after stretching or percussion | Percussion induced activity |
| Myotonic dystrophy type 1 | Trinucleotide expansion in DMPK | Predominant distal muscle weakness, cataracts, cardiac conduction disturbances, cognitive impairment, endocrine disturbances | Rarely neuropathy, secondary to endocrine disturbances Myopathic changes and myotonic discharges on needle EMG |
| Non-dystrophic myotonias | Mutations in CLNC1 and SCN4A | Muscle stiffness as well as pain, weakness and fatigue | Normal NCS and myotonic discharges on needle EMG |
| Axonal CMT | Slowly progressive muscle weakness, wasting and sensory loss, starting in the distal parts of the limbs, deformities | NCV within normal range or slightly reduced, reduced CMAPs and SNAPs Positive waves, polyphasic potentials, or fibrillations on needle EMG | |
| Distal hereditary motor neuropathies | Slowly progressive muscle weakness, wasting, starting in the distal parts of the limbs, deformities | NCV within normal range or slightly reduced, reduced CMAPs Positive waves, polyphasic potentials, or fibrillations on needle EMG | |
CMAPs = compound muscle action potentials; NCS = nerve conduction studies; SNAPs = sensory nerve action potentials; VGKC = voltage-gated potassium channel.
Treatment strategies
There is no curative treatment for patients with HINT1 neuropathy, therefore regular physical therapy, ankle–foot orthoses and/or special shoes remain mandatory. In the stage of limb deformities, surgical orthopaedic corrections are beneficial. These include soft-tissue procedures (plantar fascia release, tendon release or transfer), osteotomy (metatarsal, midfoot, calcaneal), and joint-stabilizing procedures (triple arthrodesis) (Caetano et al., 2014; Boaretto et al., 2015; Lassuthova et al., 2015; and Tournev I., unpublished results). Additionally, to decrease the symptoms of neuromyotonia and the abnormal spontaneous discharges on EMG, a favourable therapeutic response has been elicited with medications blocking sodium channels, such as antiepileptic drugs (diphenylhydantoin and carbamazepine) (Hahn et al., 1991; Tournev I., unpublished results) and anti-arrhythmics (tocainid) (Hahn et al., 1991).
HINT1 structure and enzymatic activity
HINT1 is a member of the histidine triad (HIT) protein family, sharing a characteristic HIT motif (His-x-His-x-His-x-x, where x is a hydrophobic residue) in the catalytic pocket (Seraphin, 1992; Brenner, 2002). Mammalian HINT1 orthologues are nearly identical, and even though sequence similarity is lower with other eukaryotes, HINT1 function is evolutionary conserved (Bieganowski et al., 2002). The protein is ubiquitous, but highly expressed in brain and spinal cord (Barbier et al., 2007; Liu et al., 2008; Zimon et al., 2012), suggesting its important role in the nervous system.
HINT1 is a globular protein of 13.7 kDa that acts as a homodimer and binds purine nucleosides and nucleotides (Gilmour et al., 1997). Each monomer has a nucleotide-binding cleft containing the HIT motif (Brenner et al., 1997). The nucleotide-contacting residues in this cleft are strictly conserved throughout the HIT superfamily (Brenner et al., 1997), but substrate specificity is dependent on the sequence of the C-terminal loop (Chou et al., 2007). Furthermore, dimerization is required to maintain sufficient catalytic activity (Chou and Wagner, 2007).
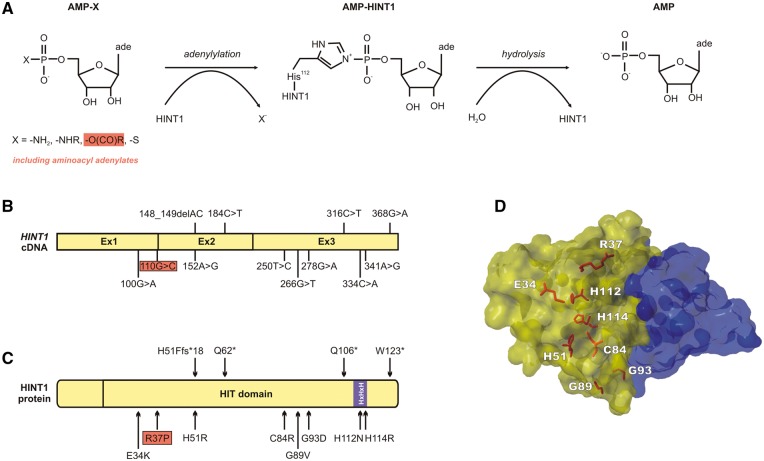
The endogenous substrate(s) of HINT1 remain unknown; nevertheless, it appears to be a promiscuous enzyme in vitro (Gilmour et al., 1997; Bieganowski et al., 2002; Chou and Wagner, 2007; Ozga et al., 2010; Wang et al., 2012). The general formula of HINT1 substrates is NMP-X [X = -NHR, -OC(O)R, -S] (Krakowiak et al., 2014) (Fig. 3A). HINT1 preferentially hydrolyses adenosine derivatives with a single phosphate, including phosphoramidates (AMP-NH2, AMP-N-ɛ-lysine, AMP-alanine) and aminoacyl adenylates (see below) (Bieganowski et al., 2002; Wang et al., 2012). Known HINT1 inhibitors are sulfamoyl adenosine and N-ethylsulfamyol adenosine (Krakowiak et al., 2004). Crystallographic analysis of HINT1 in complex with artificial substrates has elucidated its catalytic mechanism of action as follows (Lima et al., 1997; Krakowiak et al., 2004; Wang et al., 2012): (i) recognition of the NMP-X substrate: the nucleotide part binds in the HIT nucleotide-binding cleft and, in case the side-chain is an alkylamide, its alkyl portion binds the C-terminal Trp123 residue located across the dimer interface; (ii) nucleophilic attack by the His112 residue on the α-phosphate of the substrate; (iii) formation of a covalent nucleotidyl phospho-HINT1 intermediate (adenylylation); and (iv) hydrolysis of the nucleotide and release of the catalytic products (Fig. 3A).
Figure 3.
HINT1 structure and known mutations. (A) Reaction scheme for cleavage of AMP-linked compounds by the HINT1 enzyme, adapted from Krakowiak et al. (2014). (B and C). Position of the 12 known HINT1 mutations on the cDNA and protein structures, respectively. (D) 3D representation of the HINT1 dimer, highlighting the eight amino acid residues targeted by missense mutations. HINT1 monomers are shown in blue and yellow.
HINT1 functions
HINT1 hydrolyses aminoacyl adenylates, intermediary products of the charging reaction of tRNAs with their cognate amino acids by aminoacyl-tRNA synthetases (ARS); it was isolated in complexes with lysyl-tRNA synthetase (KARS) and transcription factors (Lee et al., 2004; Lee and Razin, 2005). In the presence of KARS and ATP, HINT1 is adenylated in a lysine-dependent manner, suggesting that the HINT1-AMP formation relies upon the production of lysyl-AMP by KARS (Chou and Wagner, 2007). Similarly, HINT1 reacts with other aminoacyl adenylates (Ala-AMP, Asp-AMP, Met-AMP, His-AMP) produced by their respective cognate (and no other) ARS (Wang et al., 2012). Thus, by hydrolysis of the aminoacyl adenylate intermediate, HINT1 might mediate ARS activity and influence the overall level of tRNA aminoacylation. Intriguingly, like HINT1, several ARS (YARS, GARS, AARS, KARS, MARS, HARS) are causal genes for CMT (Antonellis et al., 2003; Jordanova et al., 2006; Latour et al., 2010; McLaughlin et al., 2010; Gonzalez et al., 2013; Safka Brozkova et al., 2015). Since they form part of the same functional network, a common pathomechanism could exist, linking HINT1 and ARS jointly to disorders of the peripheral nervous system.
HINT1 desulphurates nucleoside 5’-O-monophosphorothioates (NMPS) in vivo (Krakowiak et al., 2014), producing free H2S inside the cell. By influencing the levels of this signalling molecule, HINT1 might regulate physiological processes, like post-translational protein modification, targeting of ATP-sensitive potassium channels and modulation of vascular tone (Koenitzer et al., 2007).
HINT1 acts as a transcriptional suppressor via direct binding to transcription factors. The HINT1–transcription factor complex can be dissociated through the sequestering of HINT1, consequently leading to transcriptional activation. Known sequesters of HINT1 are the diadenosine tetraphosphate (AP4A), a side-product of KARS activity (Brevet et al., 1982) and the N-terminal intracellular domain of teneurin-1 (TEN1-ICD), a cleaved-off peptide that translocates to the nucleus (Scholer et al., 2015). Basal AP4A and TEN1-ICD levels thus determine the delicate balance between HINT1-mediated transcriptional repression and activation.
Known transcription factors directly regulated by HINT1 are MITF, USF2, pontin and reptin (Razin et al., 1999; Lee and Razin, 2005; Weiske and Huber, 2005). Independent of its enzymatic activity, HINT1 has an overall repressive effect on the T cell factor (TCF)-4-β-catenin transcriptional activity, neutralizing the activating effect of pontin and strengthening the transcriptional repression by reptin (Weiske and Huber, 2006). Also unrelated to its catalytic activity, HINT1 inhibits the activator protein-1 (AP1) transcription factor by binding to the POSH-JNK2 complex and inhibiting c-Jun phosphorylation (Wang et al., 2007). Finally, HINT1 interacts with the cyclin-dependent kinase 7 (CDK7), part of the TFIIK component of the general transcription factor TFIIH (Keogh et al., 2002). It is presumed that catalysis by HINT1 is a prerequisite for proper formation of the TFIIH complex (Bieganowski et al., 2002).
Loss of HINT1 increases susceptibility to carcinogenesis in mice (Su et al., 2003; Li et al., 2006), suggesting a role as a tumor suppressor. Hint1−/− mouse embryonic fibroblasts display an augmented growth rate, spontaneous immortalization and an increased resistance to ionizing radiation (Su et al., 2003). In some cancer cell lines, HINT1 expression is decreased due to epigenetic silencing and its subsequent upregulation then halts cell proliferation, independent of the HINT1 catalytic activity (Wang et al., 2007; Zhang et al., 2009).
HINT1 interacts with the μ-opioid receptor (MOR), the major molecular target for morphine analgesia (Guang et al., 2004; Rodriguez-Munoz et al., 2011). HINT1 functions as an adaptor coupling protein kinase C gamma (PKCγ) to the MOR to downregulate its signalling capacity (Ajit et al., 2007). The role of HINT1 in the MOR pathway is unrelated to its enzymatic activity, as this function does not depend on HINT1 dimerization (Rodriguez-Munoz et al., 2008).
HINT1 is implicated in the regulation of mood and behaviour, suggesting an additional role in the CNS. HINT1 levels are increased in dorsolateral prefrontal cortex of patients with major depression disorder (Martins-de-Souza et al., 2012) and, adversely, decreased in the same brain regions of patients with schizophrenia (Varadarajulu et al., 2012). Furthermore, association studies reveal HINT1 as a susceptibility gene for schizophrenia (Chen et al., 2008; Kurotaki et al., 2011), bipolar disorder (Elashoff et al., 2007) and nicotine dependence (Jackson et al., 2011; Fang et al., 2014). So far, CMT patients carrying HINT1 mutations have not been neuropsychiatrically evaluated. Such examinations could help revealing putative common pathomechanisms for disorders of the peripheral and CNS.
CMT mutations cause loss of HINT1 function
The 12 known CMT-causing mutations (Zimon et al., 2012; Zhao et al., 2014; Boaretto et al., 2015; Lassuthova et al., 2015; Rauchenzauner et al., 2016) (Fig. 3B–D) cause loss of functional HINT1 protein, because they: (i) affect residues critical for the catalytic activity of HINT1 (H112N, H114R) (Bieganowski et al., 2002; Ozga et al., 2010); (ii) putatively lead to nonsense-mediated decay of the mutant transcript (H51Ffs*18, Q62*); or (iii) are proven to cause protein instability and subsequent proteasome-mediated degradation (R37P, H51R, C84R, W123*) (Zimon et al., 2012). Five of the mutations (R37P, H51R, C84R, H112N, W123*) were modelled in a yeast strain that is deficient for the orthologous gene, HNT1 (Zimon et al., 2012). This strain does not grow on synthetic galactose-containing media at 39°C (Bieganowski et al., 2002). Under standard culturing conditions, however, HNT1 knockout strain is perfectly viable and indistinguishable from the wild-type, indicating that HNT1 is a non-essential gene. Unlike wild-type human HINT1, the CMT-causing proteins cannot complement the growth phenotype of this strain, thus providing further evidence that loss of functional HINT1 leads to peripheral neuropathy. It is currently unclear which one of the multiple HINT1 functions is mostly affected by the CMT mutations. However, it is likely that this function in dependent on enzymatic activity of the protein, as stable, but catalytically inactive HINT1 versions (e.g. H112N), are capable of causing the neuropathy.
Hint1 knockout mice do not show signs of peripheral neuropathy
Homozygous and heterozygous Hint1 knockout mice display normal foetal and adult development and appearance. Yet, they are more susceptible to chemically induced carcinogenesis and to spontaneous tumour development on ageing (Su et al., 2003; Li et al., 2006). These findings are indicative of the role of HINT1 as a haploinsufficient tumour suppressor. Additionally, ablation of HINT1 leads to major reprogramming of lipid homeostasis (Beyoglu et al., 2014) likely due to increased proliferative signalling and reduced pro-apoptotic signalling in the liver of Hint1 knockout mice.
Furthermore, Hint1−/− mice exhibit anxiety-related (Varadarajulu et al., 2011; Jackson et al., 2012), aggressive (Dang et al., 2015), and depression-related behaviour (Barbier and Wang, 2009), some of which can be reversed by valproate treatment (Barbier and Wang, 2009). Additionally, the animals respond differently to treatment with CNS-acting compounds, such as morphine (Guang et al., 2004), amphetamine (Barbier et al., 2007), and nicotine. PKCγ protein levels are elevated in Hint1−/− mouse brains (Varadarajulu et al., 2011), yet, the PKCγ activation response upon psycho-stimulation with amphetamine is attenuated (Zhang et al., 2015). These combined findings suggest a role for HINT1 and PKCγ in modulating, among others, anxiogenic and stress-coping behaviour in mice.
Intriguingly, unlike in humans, Hint1−/− mice do not have overt signs of neuropathy (Seburn et al., 2014). Thorough examination for relevant neurological phenotypes, including motor performance, nerve, muscle and neuromuscular junction anatomy, nerve conduction studies and EMG does not show any evidence of axonal degeneration or neuromyotonia. Mice were aged to more than 1 year and, additionally, they were subjected to external stressors such as low temperature and a potassium channel-blocking agent to provoke neuromyotonia (Shillito et al., 1995); yet all without the appearance of neuropathy-related phenotypes. This finding supports the notion that, similar to yeast, HINT1 is a non-essential gene in mammals and that alternative pathways exist that can functionally complement organismal HINT1 deficiency. This suggests that activation or upregulation of such pathways in patients with HINT1 mutations may provide an attractive route for the development of therapeutic strategies for HINT1 neuropathy.
Conclusion
Recessive, loss-of-function mutations in HINT1 cause an early-onset, axonal form of motor-predominant peripheral neuropathy, often accompanied by the characteristic feature of neuromyotonia. The considerable prevalence of the disorder, especially in patients of European ancestry, is largely due to the existence of founder mutations, of which R37P is by far the most frequent. Here, we propose guidelines to recognize and differentiate HINT1-related neuropathy and suggest treatment strategies to manage common symptoms.
As a recent player in the field of hereditary neuropathies, the function of HINT1 in peripheral nerves is still completely unexplored. The gene is ubiquitously expressed, playing a role in manifold transcriptional and signalling pathways. Moreover, previous studies have indicated a relation of HINT1 to CNS functioning and pathology, yet, it was highly unexpected to find this housekeeping gene causing a disorder affecting the peripheral nerves exclusively. The high prevalence and significant burden of the HINT1 neuropathy warrant further investigations into its underlying pathomechanisms, with the aim of finding therapeutic strategies to treat this incurable disorder.
Funding
This work was funded in part by the Research Fund of the University of Antwerp (grant #TOP-BOF-29069 to A.J.); the Fund for Scientific Research-Flanders (grants FWO#G0543.13N, G0784.14N, G0D77.13N to A.J.); the Association Belge contre les Maladies Neuromusculaires (ABMM); the French Association for Neuromuscular Disorders (AFM#16197 to A.J.) the Bulgarian Ministry of Education and Science (grants #DTK-02/67, DFNI-B02/3 to A.J., I.T.).
Glossary
Abbreviations
- AMP
adenosine monophosphate
- ARS
aminoacyl-tRNA synthetase
- CMT
Charcot-Marie-Tooth disease
- HIT
histidine triad
References
- Ajit SK, Ramineni S, Edris W, Hunt RA, Hum WT, Hepler JR. et al. RGSZ1 interacts with protein kinase C interacting protein PKCI-1 and modulates mu opioid receptor signaling. Cell Signal 2007; 19: 723–30. [DOI] [PubMed] [Google Scholar]
- Antonellis A, Ellsworth RE, Sambuughin N, Puls I, Abel A, Lee-Lin SQ. et al. Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V. Am J Hum Genet 2003; 72: 1293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E, Wang JB. Anti-depressant and anxiolytic like behaviors in PKCI/HINT1 knockout mice associated with elevated plasma corticosterone level. BMC Neurosci 2009; 10: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E, Zapata A, Oh E, Liu Q, Zhu F, Undie A. et al. Supersensitivity to amphetamine in protein kinase-C interacting protein/HINT1 knockout mice. Neuropsychopharmacology 2007; 32: 1774–82. [DOI] [PubMed] [Google Scholar]
- Beyoglu D, Krausz KW, Martin J, Maurhofer O, Dorow J, Ceglarek U. et al. Disruption of tumor suppressor gene Hint1 leads to remodeling of the lipid metabolic phenotype of mouse liver. J Lipid Res 2014; 55: 2309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieganowski P, Garrison PN, Hodawadekar SC, Faye G, Barnes LD, Brenner C. Adenosine monophosphoramidase activity of Hint and Hnt1 supports function of Kin28, Ccl1, and Tfb3. J Biol Chem 2002; 277: 10852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boaretto F, Cacciavillani M, Mostacciuolo ML, Spalletta A, Piscosquito G, Pareyson D. Novel loss-of-function mutation of the HINT1 gene in a patient with distal motor axonal neuropathy without neuromyotonia. Muscle Nerve 2015; 52: 588–9. [DOI] [PubMed] [Google Scholar]
- Boerkoel CF, Takashima H, Garcia CA, Olney RK, Johnson J, Berry K, Russo P. et al. Charcot-Marie-Tooth disease and related neuropathies: mutation distribution and genotype-phenotype correlation. Ann Neurol 2002; 51: 190–201. [DOI] [PubMed] [Google Scholar]
- Brenner C. Hint, Fhit, and GalT: function, structure, evolution, and mechanism of three branches of the histidine triad superfamily of nucleotide hydrolases and transferases. Biochemistry 2002; 41: 9003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C, Garrison P, Gilmour J, Peisach D, Ringe D, Petsko GA. et al. Crystal structures of HINT demonstrate that histidine triad proteins are GalT-related nucleotide-binding proteins. Nat Struct Biol 1997; 4: 231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevet A, Plateau P, Cirakoglu B, Pailliez JP, Blanquet S. Zinc-dependent synthesis of 5',5'-diadenosine tetraphosphate by sheep liver lysyl- and phenylalanyl-tRNA synthetases. J Biol Chem 1982; 257: 14613–15. [PubMed] [Google Scholar]
- Caetano JS, Costa C, Baets J, Zimon M, Venancio M, Saraiva J. et al. Autosomal recessive axonal neuropathy with neuromyotonia: a rare entity. Pediat Neurol 2014; 50: 104–7. [DOI] [PubMed] [Google Scholar]
- Chen Q, Wang X, O'Neill FA, Walsh D, Kendler KS, Chen X. Is the histidine triad nucleotide-binding protein 1 (HINT1) gene a candidate for schizophrenia? Schizophr Res 2008; 106: 200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TF, Sham YY, Wagner CR. Impact of the C-terminal loop of histidine triad nucleotide binding protein1 (Hint1) on substrate specificity. Biochemistry 2007; 46: 13074–9. [DOI] [PubMed] [Google Scholar]
- Chou TF, Wagner CR. Lysyl-tRNA synthetase-generated lysyl-adenylate is a substrate for histidine triad nucleotide binding proteins. J Biol Chem 2007; 282: 4719–27. [DOI] [PubMed] [Google Scholar]
- Dang YH, Liu P, Ma R, Chu Z, Liu YP, Wang JB. et al. HINT1 is involved in the behavioral abnormalities induced by social isolation rearing. Neurosci Lett 2015; 607: 40–5. [DOI] [PubMed] [Google Scholar]
- Elashoff M, Higgs BW, Yolken RH, Knable MB, Weis S, Webster MJ. et al. Meta-analysis of 12 genomic studies in bipolar disorder. J Mol Neurosci 2007; 31: 221–43. [DOI] [PubMed] [Google Scholar]
- Fang J, Wang X, He B. Association between common genetic variants in the opioid pathway and smoking behaviors in Chinese men. Behav Brain Funct 2014; 10: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour J, Liang N, Lowenstein JM. Isolation, cloning and characterization of a low-molecular-mass purine nucleoside- and nucleotide-binding protein. Biochem J 1997; 326 (Pt 2): 471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, McLaughlin H, Houlden H, Guo M, Yo-Tsen L, Hadjivassilious M. et al. Exome sequencing identifies a significant variant in methionyl-tRNA synthetase (MARS) in a family with late-onset CMT2. J Neurol Neurosurg Psychiatry 2013; 84: 1247–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guang W, Wang H, Su T, Weinstein IB, Wang JB. Role of mPKCI, a novel mu-opioid receptor interactive protein, in receptor desensitization, phosphorylation, and morphine-induced analgesia. Mol Pharmacol 2004; 66: 1285–92. [DOI] [PubMed] [Google Scholar]
- Hahn AF, Ainsworth PJ, Naus CC, Mao J, Bolton CF. Clinical and pathological observations in men lacking the gap junction protein connexin 32. Muscle Nerve Suppl 2000; 9: S39–48. [PubMed] [Google Scholar]
- Hahn AF, Parkes AW, Bolton CF, Stewart SA. Neuromyotonia in hereditary motor neuropathy. J Neurol Neurosurg Psychiatry 1991; 54: 230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Chen Q, Chen J, Aggen SH, Kendler KS, Chen X. Association of the histidine-triad nucleotide-binding protein-1 (HINT1) gene variants with nicotine dependence. Pharmacogenomics J 2011; 11: 251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Wang JB, Barbier E, Chen X, Damaj MI. Acute behavioral effects of nicotine in male and female HINT1 knockout mice. Genes Brain Behav 2012; 11: 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerath NU, Shy ME, Grider T, Gutmann L. A case of neuromyotonia and axonal motor neuropathy: A report of a HINT1 mutation in the United States. Muscle Nerve 2015; 52: 1110–13. [DOI] [PubMed] [Google Scholar]
- Jordanova A, Irobi J, Thomas FP, Van DP, Meerschaert K, Dewil M. et al. Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot-Marie-Tooth neuropathy. Nat Genet 2006; 38: 197–202. [DOI] [PubMed] [Google Scholar]
- Keogh MC, Cho EJ, Podolny V, Buratowski S. Kin28 is found within TFIIH and a Kin28-Ccl1-Tfb3 trimer complex with differential sensitivities to T-loop phosphorylation. Mol Cell Biol 2002; 22: 1288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP. et al. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol 2007; 292: H1953–60. [DOI] [PubMed] [Google Scholar]
- Krakowiak A, Pace HC, Blackburn GM, Adams M, Mekhalfia A, Kaczmarek R. et al. Biochemical, crystallographic, and mutagenic characterization of hint, the AMP-lysine hydrolase, with novel substrates and inhibitors. J Biol Chem 2004; 279: 18711–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak A, Pawlowska R, Kocon-Rebowska B, Dolot R, Stec WJ. Interactions of cellular histidine triad nucleotide binding protein 1 with nucleosides 5'-O-monophosphorothioate and their derivatives - Implication for desulfuration process in the cell. Biochim Biophys Acta 2014; 1840: 3357–66. [DOI] [PubMed] [Google Scholar]
- Kucukali CI, Kurtuncu M, Akcay HI, Tuzun E, Oge AE. Peripheral nerve hyperexcitability syndromes. Rev Neurosci 2015; 26: 239–51. [DOI] [PubMed] [Google Scholar]
- Kurotaki N, Tasaki S, Mishima H, Ono S, Imamura A, Kikuchi T. et al. Identification of novel schizophrenia loci by homozygosity mapping using DNA microarray analysis. PloS One 2011; 6: e20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassuthova P, Brozkova DS, Krutova M, Neupauerova J, Haberlova J, Mazanec R. et al. Mutations in HINT1 are one of the most frequent causes of hereditary neuropathy among Czech patients and neuromyotonia is rather an underdiagnosed symptom. Neurogenetics 2015; 16: 43–54. [DOI] [PubMed] [Google Scholar]
- Latour P, Thauvin-Robinet C, Baudelet-Mery C, Soichot P, Cusin V, Faivre L. et al. A major determinant for binding and aminoacylation of tRNA(Ala) in cytoplasmic Alanyl-tRNA synthetase is mutated in dominant axonal Charcot-Marie-Tooth disease. Am J Hum Genet 2010; 86: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YN, Nechushtan H, Figov N, Razin E. The function of lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcepsilonRI-activated mast cells. Immunity 2004; 20: 145–51. [DOI] [PubMed] [Google Scholar]
- Lee YN, Razin E. Nonconventional involvement of LysRS in the molecular mechanism of USF2 transcriptional activity in FcepsilonRI-activated mast cells. Mol Cell Biol 2005; 25: 8904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang Y, Su T, Santella RM, Weinstein IB. Hint1 is a haplo-insufficient tumor suppressor in mice. Oncogene 2006; 25: 713–21. [DOI] [PubMed] [Google Scholar]
- Lima CD, Klein MG, Hendrickson WA. Structure-based analysis of catalysis and substrate definition in the HIT protein family. Science 1997; 278: 286–90. [DOI] [PubMed] [Google Scholar]
- Liu Q, Puche AC, Wang JB. Distribution and expression of protein kinase C interactive protein (PKCI/HINT1) in mouse central nervous system (CNS). Neurochem Res 2008; 33: 1263–76. [DOI] [PubMed] [Google Scholar]
- Maddison P. Neuromyotonia. Clin Neurophysiol 2006; 117: 2118–27. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Guest PC, Harris LW, Vanattou-Saifoudine N, Webster MJ, Rahmoune H. et al. Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl Psychiatry 2012; 2: e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin HM, Sakaguchi R, Liu C, Igarashi T, Pehlivan D, Chu K. et al. Compound heterozygosity for loss-of-function lysyl-tRNA synthetase mutations in a patient with peripheral neuropathy. Am J Hum Genet 2010; 87: 560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozga M, Dolot R, Janicka M, Kaczmarek R, Krakowiak A. Histidine triad nucleotide-binding protein 1 (HINT-1) phosphoramidase transforms nucleoside 5'-O-phosphorothioates to nucleoside 5'-O-phosphates. J Biol Chem 2010; 285: 40809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzko A, Shy ME. Charcot-Marie-Tooth disease and related genetic neuropathies. Continuum 2012; 18: 39– 59. [DOI] [PubMed] [Google Scholar]
- Rauchenzauner M, Fruhwirth M, Hecht M, Kofler M, Witsch-Baumgartner M, Fauth C. A Novel variant in the HINT1 gene in a girl with autosomal recessive axonal neuropathy with neuromyotonia: thorough neurological examination gives the clue. Neuropediatrics 2016; 47: 119–22. [DOI] [PubMed] [Google Scholar]
- Razin E, Zhang ZC, Nechushtan H, Frenkel S, Lee YN, Arudchandran R. et al. Suppression of microphthalmia transcriptional activity by its association with protein kinase C-interacting protein 1 in mast cells. J Biol Chem 1999; 274: 34272–6. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, de la Torre-Madrid E, Sanchez-Blazquez P, Wang JB, Garzon J. NMDAR-nNOS generated zinc recruits PKCgamma to the HINT1-RGS17 complex bound to the C terminus of Mu-opioid receptors. Cell Signal 2008; 20: 1855–64. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, Bailon C, Martin-Aznar B, Garzon J. The histidine triad nucleotide-binding protein 1 supports mu-opioid receptor-glutamate NMDA receptor cross-regulation. Cell Mol Life Sci 2011; 68: 2933–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossor AM, Kalmar B, Greensmith L, Reilly MM. The distal hereditary motor neuropathies. J Neurol Neurosurg Psychiatry 2012; 83: 6–14. [DOI] [PubMed] [Google Scholar]
- Safka Brozkova D, Deconinck T, Griffin LB, Ferbert A, Haberlova J, Mazanec R. et al. Loss of function mutations in HARS cause a spectrum of inherited peripheral neuropathies. Brain 2015; 138: 2161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholer J, Ferralli J, Thiry S, Chiquet-Ehrismann R. The intracellular domain of teneurin-1 induces the activity of microphthalmia-associated transcription factor (MITF) by binding to transcriptional repressor HINT1. J Biol Chem 2015; 290: 8154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seburn KL, Morelli KH, Jordanova A, Burgess RW. Lack of neuropathy-related phenotypes in hint1 knockout mice. J Neuropathol Exp Neurol 2014; 73: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraphin B. The HIT protein family: a new family of proteins present in prokaryotes, yeast and mammals. DNA Seq 1992; 3: 177–9. [DOI] [PubMed] [Google Scholar]
- Shillito P, Molenaar PC, Vincent A, Leys K, Zheng W, van den Berg RJ. et al. Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol 1995; 38: 714–22. [DOI] [PubMed] [Google Scholar]
- Su T, Suzui M, Wang L, Lin CS, Xing WQ, Weinstein IB. Deletion of histidine triad nucleotide-binding protein 1/PKC-interacting protein in mice enhances cell growth and carcinogenesis. Proc Natl Acad Sci USA 2003; 100: 7824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajulu J, Lebar M, Krishnamoorthy G, Habelt S, Lu J, Bernard Weinstein I. et al. Increased anxiety-related behaviour in Hint1 knockout mice. Behav Brain Res 2011; 220: 305–11. [DOI] [PubMed] [Google Scholar]
- Varadarajulu J, Schmitt A, Falkai P, Alsaif M, Turck CW, Martins-de-Souza D. Differential expression of HINT1 in schizophrenia brain tissue. Eur Arch Psychiatry Clin Neurosci 2012; 262: 167–72. [DOI] [PubMed] [Google Scholar]
- Veltsista D, Chroni E. A first case report of HINT1-related axonal neuropathy with neuromyotonia in a Greek family. Clin Neurol Neurosurg 2016; 148: 85–7. [DOI] [PubMed] [Google Scholar]
- Wang J, Fang P, Schimmel P, Guo M. Side chain independent recognition of aminoacyl adenylates by the Hint1 transcription suppressor. J Phys Chem B 2012; 116: 6798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Y, Li H, Xu Z, Santella RM, Weinstein IB. Hint1 inhibits growth and activator protein-1 activity in human colon cancer cells. Cancer Res 2007; 67: 4700–8. [DOI] [PubMed] [Google Scholar]
- Weiske J, Huber O. The histidine triad protein Hint1 interacts with Pontin and Reptin and inhibits TCF-beta-catenin-mediated transcription. J Cell Sci 2005; 118: 3117–29. [DOI] [PubMed] [Google Scholar]
- Weiske J, Huber O. The histidine triad protein Hint1 triggers apoptosis independent of its enzymatic activity. J Biol Chem 2006; 281: 27356–66. [DOI] [PubMed] [Google Scholar]
- Zhang F, Fang Z, Wang JB. Hint1 knockout results in a compromised activation of protein kinase C gamma in the brain. Brain Res 2015; 1622: 196–203. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Li H, Wu HC, Shen J, Wang L, Yu MW. et al. Silencing of Hint1, a novel tumor suppressor gene, by promoter hypermethylation in hepatocellular carcinoma. Cancer Lett 2009; 275: 277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Race V, Matthijs G, De JP, Robberecht W, Lambrechts D. et al. Exome sequencing reveals HINT1 mutations as a cause of distal hereditary motor neuropathy. Eur J Hum Genet 2014; 22: 847–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimon M, Baets J, Almeida-Souza L, De VE, Nikodinovic J, Parman Y. et al. Loss-of-function mutations in HINT1 cause axonal neuropathy with neuromyotonia. Nat Genet 2012; 44: 1080–3. [DOI] [PubMed] [Google Scholar]
- Zimon M, Battaloglu E, Parman Y, Erdem S, Baets J, De VE. et al. Unraveling the genetic landscape of autosomal recessive Charcot-Marie-Tooth neuropathies using a homozygosity mapping approach. Neurogenetics 2015; 16: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]