Abstract
Atherosclerosis, the primary cause of cardiovascular disease (CVD), is a chronic inflammatory disorder in the walls of medium and large arteries. CVD is currently responsible for about one in three global deaths and this is expected to rise in the future due to an increase in the prevalence of obesity and diabetes. Current therapies for atherosclerosis mainly modulate lipid homeostasis and whilst successful at reducing the risk of a CVD-related death, they are associated with considerable residual risk and various side effects. There is therefore a need for alternative therapies aimed at regulating inflammation in order to reduce atherogenesis. This review will highlight the key role cytokines play during disease progression as well as potential therapeutic strategies to target them.
Keywords: Atherosclerosis, Cytokines, Chemokines, Neutralisation, Nanoparticles, Inflammation, Cardiovascular disease
1. Introduction
The World Health Organization (WHO) has estimated that one in three global deaths are as a result of cardiovascular disease (CVD)-related events such as myocardial infarction (MI) and stroke [1]. In 2012 there were approximately 17.5 million global deaths due to CVD-related events. As developing countries adopt a more westernized lifestyle and the incidences of diabetes and obesity continue to increase worldwide, the estimated number of CVD-related deaths is expected to rise to 23.3 million by 2030 [1]. CVD is a significant problem for the healthcare systems around the world and thus a major economic burden [2]. Therefore, there is a great need to discover new targets and to develop potential therapies for CVD.
Atherosclerosis is the primary cause of CVD-related events and associated morbidity and mortality from this disease. Atherosclerosis is a chronic inflammatory disease of the large and medium arteries that can be triggered by several risk factors including a diet rich in saturated fats, lack of exercise and smoking. The progression of atherosclerosis is influenced by the innate and adaptive immune system responses that are both regulated by a variety of cytokines [3,4]. Atherosclerosis is initiated by endothelial cell (EC) dysfunction/activation that is often triggered by the accumulation of low-density lipoprotein (LDL) and other apolipoprotein (Apo)B-containing lipoproteins in the walls of large and medium arteries [4–6]. An inflammatory response is triggered in the ECs neighbouring the LDL accumulation as it becomes oxidized into oxidized (ox)-LDL [4–6]. The activated ECs begin to release cytokines and chemokines into the blood stream as well as express cell adhesion molecules on their surface in order to recruit circulating monocytes and other immune cells to the site of oxLDL build-up [4–6]. Once the monocytes migrate into the walls of the arteries they differentiate into macrophages which are able to uptake oxLDL and form foam cells [4–6]. Atherosclerotic plaques develop due to the continuous and uncontrollable recruitment of macrophages and build-up of foam cells at the site of oxLDL accumulation and defective clearance of apoptotic cells/debris (efferocytosis) that leads to a chronic inflammatory response [4–6]. As the plaque continues to develop it can become unstable and rupture, leading to thrombosis, stroke or MI depending on the location of the rupture [4–7].
2. Cytokines are key orchestrators of inflammation in atherosclerosis
Cytokines are a board range of small proteins involved in cellular signaling pathways and can be divided into several categories including the interferons (IFN), colony-stimulating-factors (CSF), interleukins (IL), chemokines, transforming growth factors (TGF) and tumour necrosis factors (TNF) [3,5,6]. Furthermore, they can either aid atherosclerotic plaque development (pro-atherogenic) or attenuate plaque formation (anti-atherogenic). However some cytokines do not have a definite function and can be either pro- or anti-atherogenic depending on the surrounding environment [3,5,6]. More details regarding the role of specific cytokines and how they can be therapeutically targeted will be discussed in greater detail in the subsequent sections of this review. Due to their key roles in influencing the inflammatory response during atherosclerosis, targeting cytokines and their signaling pathways represents a promising therapeutic strategy for attenuating the development of this disease by inhibiting those that augment atherogenesisis as well as promoting those which retard plaque formation. A brief description of the key cytokines involved in atherosclerosis development and their signaling pathways are summarized in Table 1. This review will highlight some of the key cytokines and their roles in the different stages of atherosclerosis development and discuss the emerging therapeutics designed to target cytokines as well as their receptors. A comprehensive coverage of the roles of the full spectrum of cytokines in atherosclerosis is beyond the scope of the current article and the reader is directed to more recent reviews on this topic [3, 6].
Table 1.
The role and signaling pathway of crucial cytokines in atherosclerosis development.
| Cytokine | Pro- or anti-atherogenic | Role in atherosclerosis development | Signaling pathway | Reference |
|---|---|---|---|---|
| IFN-γ | Pro- | Can induce the pro-inflammatory M1 phenotype in macrophages. It is able to increase foam cell formation by attenuating the expression of key genes implicated in macrophage cholesterol efflux and increase the expression of those involved in the uptake of cholesterol. Contributes to the continuously growing plaque size by inducing foam cell apoptosis which causes them to ‘spill’ their lipid content into the core of the plaque. | JAK-STAT | [7–14] |
| CCL2 | Pro- | Also known as MCP-1. It is one of the major chemokines involved in monocyte recruitment during atherosclerosis development. Deficiency of CCL2 in atherosclerotic mouse models markedly attenuates disease development when CX3CL1 and CCL5 are also knocked out. Mouse models lacking CCL2 or its receptor develop smaller atherosclerotic plaques. | PKC/ERK1/2/NF-κB | [15,16] |
| IL-1β | Pro- | It is able to induce the pro-inflammatory M1 macrophage phenotype. oxLDL has been shown to stimulate IL-1β secretion in human macrophages. It is a major activator of the innate immune response. The expression of several pro-inflammatory genes can be induced by IL-1β. It also exerts an auto-inflammatory response by inducing its own expression. | NF-κB/JNK/p38 MAPK | [9–11,17–21] |
| TGF-β | Anti- | The anti-inflammatory M2 macrophage releases TGF-β. Its over expression in ApoE-/- mice results in smaller plaque, whereas the inverse occurs in ApoE-/- mice that are deficient for the TGF-β receptor. | SMAD-dependent or SMAD-independent | [9–11,22–24] |
| IL-33 | Anti- | IL-33 is capable of attenuating foam cell formation by stimulating macrophage cholesterol efflux and attenuating cholesterol uptake. ApoE-/- mice injected with IL-33 have a reduced number of foam cells present in their atherosclerotic lesions. Neutralization of IL-33 increases atherosclerosis. | ST2/IL-1RAcP/MyD88 or ERK1/2 or p38 MAPK or NF-κB | [6,25–29] |
ApoE, Apolipoprotein E; CCL, Chemokine (C-C motif) ligand; PKC, Protein kinase C; CX3CL, Chemokine (C-X3-C motif) ligand; ERK, Extracellular signal-regulated kinase; IFN, Interferon; IL, Interleukin; IL-1RAcP, IL-1 receptor accessory protein; JAK, Janus kinase; JNK, c-JUN N-terminal kinases; MAPK, Mitogen activated protein kinases; MCP-1, Monocyte chemoattractant protein-1; MyD88, Myeloid differentiation primary response gene 88; NF-κB, Nuclear factor kappa-light-chain-enhancer of activated B cells; oxLDL, oxidized low density lipoprotein; STAT, Signal transducer and activator of transcription; TGF, Transforming growth factor.
3. Key cytokines associated with the inflammatory response in atherosclerosis
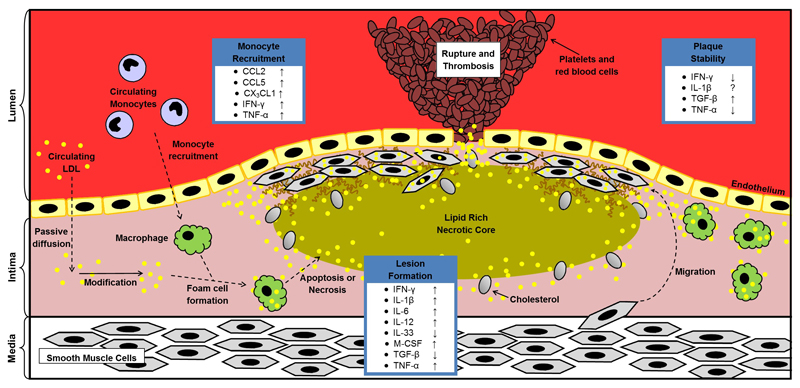
Cytokines are able to influence all stages of atherosclerosis development ranging from the initial recruitment of circulating monocytes and other immune cells from the bloodstream all the way through to mature plaque formation and stability (Figure 1). The use of atherosclerotic mouse models have greatly improved our understanding of the role of cytokines and their signaling during disease development. Normally wild type mice do not develop atherosclerosis, however ApoE knockout mice (ApoE-/-) and LDL receptor knockout mice (LDLr-/-) are able to develop atherosclerotic lesions while being fed a standard chow diet. Plaque formation in these mice can be accelerated by feeding them a high fat diet.
Figure 1. Atherosclerosis disease progression and the role of key cytokines.
The accumulation of modified LDL within the intima of the artery triggers an inflammatory response in the nearby endothelial cells. The activated endothelial cells begin to express cell-adhesion molecules on their surface as well as secrete pro-inflammatory cytokines and chemokines which are able to recruit circulating monocytes to the affected region. Once in the intima, the monocytes differentiate into macrophages and begin to take up modified LDL and become foam cells. Overtime these foam cells can accumulate and form the initial atherosclerotic lesion. During atherosclerotic plaque maturation, foam cells start to undergo apoptosis or necrosis, which causes the formation of a necrotic core. To stabilise the necrotic core, smooth muscle cells migrate from media to intima and form a fibrous cap over the core by depositing extra cellular matrix. The chronic inflammatory response of atherosclerosis eventually causes the mature atherosclerotic plaque to become unstable and rupture, leading to a thrombotic reaction, which can cause a myocardial infarction or stroke depending on the location of plaque formation. ↑, increase; ↓, decrease; ?, unclear. CCL, Chemokine (C-C motif) ligand; CX3CL1, Chemokine (C-X3-C motif) ligand; IFN, Interferon; IL, Interleukin; LDL, low-density lipoprotein; M-CSF, Macrophage colony stimulating factor; TGF, Transforming growth factor; TNF, Tumour necrosis factor.
3.1. Key role of cytokines in monocyte recruitment
During the initial formation of an atherosclerotic lesion, the reorganization of the actin and tubulin cytoskeletons of ECs can be triggered by IFN-γ and TNF-α [6]. This reorganization leads to changes in the shape of the ECs and creates gaps between neighbouring cells, making the endothelial layer permeable and allowing LDL to passively diffuse into to the walls of the arteries and accumulate. The LDL can then be oxidized to oxLDL which triggers an immune response in the nearby ECs. The activated ECs begin to release a variety of cytokines and chemokines. Chemokines is a term given to a sub-group of cytokines which are capable of attracting cells to a desired location [6]. Chemokines are able to exert their effects by interacting with cell surface receptors and activating heterotromeric G proteins and related intracellular signaling pathways [6].
In atherosclerosis development, chemokines play an important role in the recruitment of circulating monocytes and other immune cells to the site of oxLDL retention [5,30–32]. For circulating monocytes to enter into the intima of the artery they must first slow their speed through the blood by rolling along the endothelium before coming to rest and moving through the endothelium [30,31]. Two chemokines that are key to the initial rolling phase of monocyte recruitment are chemokine C-X3-C motif ligand (CX3CL)1 and chemokine C-C motif ligand (CCL)5 [6,30,31]. These chemokines interact with proteoglycans and P-selectins on the surface of ECs, allowing them to bind to their corresponding receptors on the circulating monocytes [6,30,31]. The rolling monocytes come to rest when adhesion molecules such as vascular cellular adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1 also bind to their receptors on the monocytes, creating a firm interaction between the monocyte and the endothelial layer and allowing the monocytes to migrate into the intima [6,30,31].
Targeting chemokines therapeutically can often be challenging as there is frequently functional redundancy in their signaling, with numerous interacting with several receptors or a receptor interacting with several agonists [33–35]. However there appears to be at least three major chemokines (and their corresponding receptors) whose deficiency completely abrogates the development of atherosclerosis in mouse model systems [6,36–38]. These chemokines are CCL2, CCL5 and CX3CL1 [6,30]. CCL2, also known as monocyte chemoattractant protein (MCP)-1, is thought to be a major chemokine involved in monocyte recruitment during atherosclerosis development. Atherosclerotic mouse models with attenuated expression of CCL2 or its receptor CCR2 resulted in smaller atherosclerotic lesions due to reduced monocyte recruitment [15,38,39]. A recent study has highlighted a possible new mechanism for the action of some chemokines which involves the remodelling of the actin cytoskeleton resulting in CD36 clustering, which increases foam cell formation by augmenting the responsiveness of the receptor for oxLDL [40].
Targeting CCL2, CX3CL1 and CCL5 or the chemokine network may represent promising therapeutic avenues in order to attenuate the development and progression of atherosclerosis. In addition, as cytokines are capable of influencing all stages of atherosclerosis development, it is important to understand the roles of key cytokines in further downstream events in the disease following the recruitment of immune cells, such as foam cell formation, and identify further therapeutic targets.
3.2. Key role of cytokines in lesion formation
Once in the intima of the arteries, the monocytes are exposed to several cytokines, including macrophage colony stimulating factor (M-CSF), and differentiate into macrophages [5]. The local microenvironment together with signaling from additional cytokines will affect whether the macrophages are classically activated (M1 phenotype) or alternatively activated (M2 phenotype) [5,9–11]. M1 macrophages are generally considered to be pro-inflammatory due to their role in promoting an inflammatory response to bacterial infection in the innate immune response [11]. The M1 phenotype has been found to be induced by the pro-inflammatory cytokines IFN-γ and IL-1β [9–11]. These M1 macrophages are able to exert their deleterious actions by releasing the pro-inflammatory cytokines IL-6, IL-12 and TNF-α [6]. M2 macrophages on the other hand are thought of as anti-inflammatory due to their role in repair and the resolution of inflammation [11]. Cytokines including IL-4 and IL-13 are able to induce the formation of M2 macrophages, which are then capable of producing the anti-inflammatory cytokines IL-10 and TGF-β [9–11]. A number of other macrophage phenotypes have also been identified though these are relatively poorly characterized [9,41]. These include M2b, M2c and M2d macrophages which can be induced by IL-1 receptor ligands, IL-10 and co-stimulation of Toll-like receptor together with adenosine A2A receptor agonists respectively [41]. The cytokine CXCL4 has been identified as being able to differentiate macrophages into a pro-inflammatory phenotype known as M4 in human atherosclerotic plaques [42].
The role cytokines play in macrophage polarization makes them a promising therapeutic target to switch the balance of M1:M2 formation in favour of the M2 phenotype in order to change the atherosclerotic plaque environment from a pro-inflammatory to an anti-inflammatory one to attenuate disease progression. It is important to try and achieve this due to the effects that are exerted by the cytokines they produce. For example increasing the expression of IL-6 in ApoE-/- mice by using a lentivirus resulted in an unstable plaque [43] whereas macrophage specific over expression of IL-10 in LDLr-/- mice resulted in reduced atherosclerosis development by a decrease in cholesteryl ester accumulation [44]. Such pro- and anti-inflammatory effects of the cytokines produced by the different macrophage phenotypes highlight the rationale for targeting cytokine signaling involved in macrophage polarization in order to inhibit M1 formation and promote the M2 phenotype. However it should be noted that one recent study has shown that M2 macrophages increase their expression of scavenger receptors (SRs) CD36 and SR-A1 following endoplasmic reticulum stress, which correlated to increased foam cell formation [45]. Further studies are required to fully determine the role of different macrophage phenotypes in atherosclerosis disease development.
In order to become foam cells the macrophages must take up modified LDL, in particular oxLDL [4,6,7]. There are several mechanisms by which macrophages are able to take up lipoproteins including macropinocytosis, pinocytosis and phagocytosis, however the major route for the uptake of oxLDL and other modified forms of this lipoprotein is via SR-mediated endocytosis [5,6]. Unlike LDL uptake via LDLr which is highly regulated by a negative feedback loop by intracellular cholesterol levels, oxLDL uptake by SRs such as SR-A1 and CD36, is unregulated resulting in uncontrolled uptake [5,6]. Foam cells begin to form when the balance of cholesterol uptake and efflux is tipped in favour of uptake and retention [5,6]. Cytokines are important in maintaining this balance as they are able to influence the expression of several key genes involved in cholesterol uptake and efflux [5].
The pro-inflammatory cytokine IFN-γ can induce foam cell formation by increasing cholesterol retention by attenuating the expression of ATP-binding cassette, sub-family A (ABCA1), a key transporter in cholesterol efflux, as well as inducing the expression of acyl-CoA acyltransferase 1 (ACAT1), which is involved in cholesterol esterification [6]. Human THP-1 and primary macrophages stimulated with IFN-γ showed an increase in the expression of key SRs, such as SR-A1, leading to an increase in oxLDL uptake [12,13]. Additionally, ApoE-/- mice also deficient for TNF-α develop smaller atherosclerotic lesions due to a decrease in lipid accumulation after 6 weeks [46]. Furthermore the expression of pro-inflammatory cytokines and adhesion molecules such as IFN-γ, ICAM-1, VCAM-1 and CCL2, were significantly decreased at the mRNA level in these mice [46]. These genes were also found to be down regulated after 8 weeks feeding of a high fat diet in ApoE-/- mice which also had the p55 TNF receptor (p55 TNFR) knocked out [47]. The lesions that developed in these mice were also smaller compared to controls [47], highlighting the pro-apoptotic role TNF-α plays during early lesion formation.
The cytokines IL-1α, IL-β and IL-18 are pro-inflammatory and play a major role in atherosclerosis disease progression [6,18]. ApoE-/- mice which were also deficient for IL-1α and IL-β were found to develop smaller atherosclerotic lesions compared to the control mice [48]. Furthermore a bone marrow transfer (BMT) of IL-1+/+, IL-1α-/- or IL-β-/- cells into ApoE-/- mice found that those receiving IL-1+/+ cells developed larger plaques by approximately 52% and 32% when compared to those mice that received IL-1α-/- and IL-β-/- cells respectively [48]. Similar results were observed in ApoE-/- mice which also lacked the IL-1 receptor type-1 (IL-1R1) [49]. Smaller atherosclerotic lesions were found in mice which lacked IL-1R1 compared to the ApoE-/- control mice. This correlated to reduced expression of monocyte recruitment genes including CCL2, ICAM-1 and VCAM-1 in the vascular wall [49], demonstrating the pro-inflammatory effects of IL-1 during atherosclerosis disease development. One study using ApoE-/- mice fed a standard chow diet while receiving daily injections of recombinant IL-18 developed larger and more unstable lesions by increasing the expression of CD36 and matrix metalloproteinase (MMP)-9, possibly via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [50]. However another study which fed ApoE-/-IL-18-/- mice a high fat diet for 12 weeks developed more and larger lesions compared to ApoE-/- mice [51]. These lesions were also less stable due to an increased lipid content [51], indicating that IL-18 may actually attenuate atherosclerosis development. Other pro-inflammatory cytokines that promote foam cell formation include IL-6 [52], IL-15 [53], TNF-related weak inducer of apoptosis (TWEAK) [54] and TNF-like protein 1A (TL1A) [55].
The over expression of TGF-β in ApoE-/- mice leads to smaller and more stable plaques compared to controls after receiving a high fat diet for 24 weeks [23]. Inhibition of TGF-β signaling by introducing a functional inactivation mutation in the TGF-β receptor II (TGFβR2) in ApoE-/- mice resulted in a two fold increase in atherosclerotic lesion size, as well as increasing the presence of inflammatory markers such as IFN-γ [24]. Although LDLr-/- mice which were also deficient for Smad7 (an inhibitor of TGF-β signaling) developed larger lesions, their fibrous caps contained more collagen which correlates to a more stable plaque [56]. Therefore targeting TGF-β signaling may represent a promising therapeutic avenue to reduce early lesion formation while simultaneously making already developed plaques more stable. However a recent study found that specific inhibition of TGF-β signaling in T cells had no effect on atherosclerosis development [57]. TGF-β also attenuates macrophage foam cell formation by inhibiting the expression of key genes implicated in the uptake of lipoproteins and stimulating those that are involved in the efflux of cholesterol [58].
Another anti-inflammatory cytokine capable of attenuating lipid uptake is IL-33. ApoE-/- mice injected with IL-33 twice a day for 6 weeks had decreased build-up of foam cells compared to the control group [26]. Furthermore the same study also found that IL-33 was capable of increasing the expression of key genes implicated in cholesterol efflux, including ABCA1 and ApoE, as well as attenuating the expression of SR genes such as CD36 [26]. This altered pattern of gene expression correlated to a decrease in modified LDL uptake and intracellular cholesterol accumulation in addition to an increase in cholesterol efflux in vitro [26]. In addition, studies in ApoE-/- mice in which the action of IL-33 was inhibited by injection of a soluble decoy receptor or augmented by injection of the cytokine revealed its protective role in atherosclerosis [29].
Other cytokines such as IL-10 and IL-13 have also shown cardiovascular protective effects. Overexpressing IL-10 in LDLr-/- mice resulted in an increase in cholesterol efflux, reduced apoptosis as well as a reduction in the expression of pro-inflammatory molecules including TNF-α and CCL2 [44]. These results indicate an attenuation of the disease progression, which correlates to the observed reduction in atherosclerotic lesion size observed in these mice [44]. Biweekly injections of IL-13 in LDLr-/- mice which were fed a high fat diet for 16 weeks showed a reduced number of macrophages and increased collagen content in their plaques compared to control mice [59], two indicators of plaque stability. Furthermore, it was found that the IL-13 injections resulted in a decrease in the pro-inflammatory M1 macrophage and increased M2 macrophage differentiation. The greatest evidence for the anti-atherogenic properties of IL-13 comes from LDLr-/- mice which are also deficient for IL-13. These mice developed larger atherosclerotic plaques, which correlated to an increase in the size of the necrotic core, as well as a reduction in the number of anti-inflammatory M2 macrophages found within the plaque compared to the control mice [59].
3.3. Key role of cytokines in plaque stability
Although anti-inflammatory cytokines such as TGF-β can promote plaque stability, a prolonged inflammatory response and continuous stimulation in the plaque by pro-inflammatory cytokines can lead to an unstable plaque which can rupture and cause a MI or stroke [6]. Pro-inflammatory cytokines such as TNF-α, IFN-γ and IL-1β can induce macrophage and foam cell apoptosis, causing them to release their lipid content into the intima of the artery and contribute towards the size of the lipid core in the plaque [6,14]. As well as lipids the macrophages also release enzymes that can influence extracellular matrix remodelling including MMPs and their inhibitors known as TIMPs (tissue inhibitor of metalloproteinases) [60]. The activity of both MMPs and TIMPs are highly regulated by a range of cytokines [60]. Inhibition of IFN-γ signaling postnatally in ApoE-/- mice by over expressing a soluble decoy receptor also resulted in smaller lesions as well as increasing mature plaque stability by increasing the number of SMCs present in the fibrous cap [61]. However, the relationship between pro-inflammatory cytokines and plaque instability is not always clear-cut. For example, ApoE-/- mice which are also deficient in the IL-1 receptor type 1 (IL-1R1) developed plaques with lower amounts of collagen and fewer smooth muscle cells (SMCs), both of which are indicators of increased plaque instability [62].
4. Cytokine targeted therapeutic approaches
Statins are a class of cholesterol lowering therapeutics which target and inhibit the enzyme 3-hydroxy-3-methylglutaryl-CoA (HMG CoA) reductase. This enzyme plays a major role in the rate limiting step of cholesterol biosynthesis. Therefore targeting HMG CoA reductase with statins results in a decrease in serum cholesterol levels, in particular LDL, reducing an individual’s risk of suffering a CVD-related event. In addition, statins have other pleotropic effects such as acting in an anti-inflammatory manner and reducing endothelial cell dysfunction [63]. However long term usage of statins is known to cause several side effects ranging from minor effects, such as nose bleed and headaches, to major ones including an increased risk of developing diabetes and muscle pain [64–66]. In addition, statin therapy is associated with considerable residual risk for CVD. Furthermore there is a significant minority of individuals who are unable to achieve recommended plasma cholesterol levels even with the maximum dose of statins [5].
Due to the pro-inflammatory characteristics of some cytokines and their key roles in atherosclerosis development they represent promising therapeutic targets in the treatment of this disease. An excellent example of cytokine therapeutics is a neutralizing antibody targeted against IL-1β. This cytokine is produced by the activation of the inflammasome and is a key regulator of the innate immune response [18,19]. Not only can IL-1β induce the expression of several pro-inflammatory mediators, it can also induce its own expression due to its auto-inflammatory nature [20]. Therefore IL-1β represents an ideal cytokine to target and retard the progression of atherosclerosis.
A human monoclonal antibody against IL-1β known as canakinumab provides some hope for specifically targeting cytokines. Canakinumab is currently approved for use in two auto-inflammatory diseases, Muckle Wells syndrome and cryopyrin-associated periodic syndrome [20]. The promising outcome lead to its use in a phase II clinical trial involving 556 diabetic participants with high risk for developing CVD [67]. Although this study found that canakinumab did not alter LDL or HDL concentrations, it did reduce the levels of key downstream pro-inflammatory mediators including IL-6 and C-reactive protein (CRP) in a concentration dependent manner. CRP is a well-known and recognized indicator of inflammation that often correlates with CVD [68]. Furthermore canakinumab has been found to exert long term anti-inflammatory effects due to decreased levels of IL-6, CRP and IL-1β several months after individuals receive only a single dose [67]. To further investigate the effectiveness of canakinumab, a large scale trial known as Canakinumab Anti-inflammatory Thrombosis Outcomes study (CANTOS) was set up in 2011 [20]. The trial involves 10,065 post-myocardial patients worldwide who receive either canakinumab or a placebo every 3 months [20]. The CANTOS trial is expected to end in 2017. There is hope that this trial will provide the first evidence of targeting IL-1β directly using neutralizing antibodies reduces an individuals’ risk of suffering a CVD-related event and open up similar avenues with other cytokines. Another ongoing study is the Cardiovascular Inflammation Reduction Trial (CRIT) [69]. Due to the success of using low dose methotrexate therapy in patients with rheumatoid arthritis and psoriasis to dampen the inflammatory response, it is now being investigated as a potential therapeutic to reduce a patient’s risk of suffering a CVD-disease related event. As a generic anti-inflammatory compound, which is likely to affect the expression of several cytokines, the CRIT trial may open multiple avenues for broad anti-inflammatory agents.
Several approaches have been pursued in relation to IFN-γ due to the central role of this cytokine in atherosclerosis disease progression. The strategies can be broadly defined as targeting the IFN-γ pathway or neutralizing the cytokine directly [7]. IFN-γ signals through the Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) pathway which requires the phosphorylation of STAT1 at tyrosine 701 and serine 727 for maximal activity [7,70]. Some potential therapeutics have been designed to inhibit the phosphorylation of STAT1 in order to prevent the pro-inflammatory effects of IFN-γ. For example adenosine is known to inhibit the phosphorylation of serine 727 on STAT1 in human macrophages [7]. This has led to the creation of a novel compound called thio-CI-IB-MECA, which is designed to be an agonist for the adenosine A3 receptor and has been found to reduce IFN-γ-induced, STAT1-dependent gene expression [71]. Furthermore in vitro work using human macrophages has shown that phosphorylation of tyrosine 701 or serine 727 on STAT1 as well as JAK2 activation can be inhibited by resveratrol, which is a phenol compound that occurs naturally in plant extracts [72].
Unlike IL-1β neutralization that directly targets the cytokine with antibodies, studies on IFN-γ neutralization so far involves the use of a soluble decoy receptor. The soluble IFN-γ receptor (sIFN-γR) works by out competing the normal IFN-γR for binding to the cytokine and thereby preventing the activation of the JAK-STAT pathway [7]. This then prevents the downstream expression of IFN-γ-induced pro-inflammatory mediators. The first use of a sIFN-γR was in ApoE-/- mice which were fed a high fat diet for 8 weeks, while also receiving intramusclular injections containing a sIFN-γR encoding plasmid on weeks 4 and 6 [61]. The sIFN-γR treated mice developed smaller atherosclerotic plaques when compared to the control mice [61]. Furthermore, due to increased levels of collagen accumulation and vascular smooth muscle cells (VSMCs) in the fibrous cap, the plaques which developed were more stable [61]. Soluble decoy receptors have also been used to inhibit IL-6 signalling [52]. Soluble glycoprotein 130 (sgp130) has been used in LDLr-/- mice to attenuate IL-6 signaling which resulted in decreased atherosclerosis disease progression due to reduced monocyte recruitment and EC activation [52]. These studies show the potential of using a cytokine decoy receptor to attenuate atherosclerosis development.
Due to the importance of the chemokines CCL2, CCL5 and CX3CL1 during the initial monocyte recruitment phase of atherosclerosis development, targeting their corresponding receptors pharmaceutically in order to prevent activation and reduce disease progression has also been explored. Several approaches have shown success in mouse model systems, including viral proteins that act as broad spectrum inhibitors [73,74], pharmacological inhibitors [75], neutralizing antibodies against chemokines or their receptors [33–35], decoy ligands that bind to the receptors without causing activation [33–35] and RNA intereference-mediated knockdown strategies [76]. A major problem with targeting chemokine and their receptors is that they are also crucial for the resolution of an inflammatory response triggered by infection or other stresses. It will therefore be necessary to target them specifically to the arterial wall by use of nanoparticles. ApoE-/- mice which also expressed human CCR2 did not develop smaller atherosclerotic lesions following a high fat diet for 5 weeks while receiving a daily dose of a novel CCR2 antagonist [77]. However the mice did have reduced macrophage content within their plaques, an indicator of increased plaque stability [77]. Specifically targeting CCR2 in monocytes with a siRNA linked to a nanoparticle was used to reduce monocyte recruitment during MI inflammation in ApoE-/- mice [76]. A phase II clinical trial involving 112 patients at risk of CVD who received the antibody MLN1202, which is capable of directly blocking CCR2, found that their circulating CRP levels were reduced compared to the placebo group [78]. Targeting CCR5 with an antagonist retroviral drug, known as maraviroc, reduced both ritonavir-induced atherosclerotic plaque size and the number of macrophages recruited to the plaque in ApoE-/- mice [79]. Furthermore the expression of VCAM-1, ICAM-1, CCL2 and TNF-α were all significantly reduced within the plaque [79].
A recent in vitro study has found that a known HIV infection blocking anti-body, RoAb13, is also capable of reducing monocyte migration towards CCR5 stimuli in a transwell system [80]. An antagonist for CX3CR1 known as F1 is capable of reducing the lesion size which develops in ApoE-/- mice following treatment three times a week for 10 weeks after the mice had already been receiving a high fat diet for 5 weeks [81]. The reduction in plaque size was due to a decrease in the number of monocytes and macrophages recruited to the site of lesion formation [81]. These studies highlight the possibility of specifically targeting chemokine receptors to attenuate monocyte recruitment during atherosclerosis progression and reduce the size of the plaques that develop. Targeting the chemokine receptors would potentially attenuate atherosclerosis plaque formation before initial lesions have time to mature into large plaques. Another anti-atherogenic strategy being pursued is the blocking of chemokine activity by viral proteins [73,74]. The vaccinia virus produces a generic CC chemokine inhibitor known as 35K. ApoE-/- mice which were fed a standard chow diet and injected with a lentivirus encoding 35K had reduced chemokine activity, correlating to a decrease in atherosclerotic plaque size compared to the control mice after 12 weeks [74]. Viral proteins may be a potential therapeutic avenue capable of providing long-term attenuation of chemokine activity in order to slow atherosclerosis progression.
Although targeting cytokines and chemokines appears to be an exciting therapeutic avenue to explore in order to reduce atherosclerosis progression there are major limitations that must be first overcome. The biggest limitation to targeting cytokines is the potential consequences of systemic inhibition. Due to the major role cytokines play during the immune response [82–84], targeting them therapeutically may result in severe adverse effects. For example using antibodies to target pro-inflammatory cytokines systemically will result in a dampening of the patients’ inflammatory response causing them to be at a greater risk of developing infections. Furthermore cytokines act in short range cell to cell signaling manner, therefore the administration of anti-inflammatory cytokines would result in unspecified systemic responses and would also need to be given at potentially toxic levels in order to mimic the physiological paracrine response at the intended target location [85]. Therefore the use of therapies that target cytokines systemically should be reserved for those considered high risk of suffering a CVD-related event. The development of nanoparticles and micelles (a spherical structure which is formed of lipid molecules when they are in aqueous solutions due to their hydrophobic nature) has led to the ability of directly targeting atherosclerotic plaques for imaging and therapeutic delivery [86–89]. Although nanoparticles and micelles are capable of directing antibodies and antagonists for cell surface receptors to atherosclerotic plaques for imaging [86,87], very little has been developed for directing cytokine therapies [88]. However nanoparticles have been shown to be able to target the anti-inflammatory cytokine IL-10 and improve ex vivo imaging of plaques from AopE-/- mice [90]. This study demonstrates that it is possible to target cytokines with nanoparticles and highlight the potential to use them to deliver cytokine-targeted therapies directly to atherosclerotic plaques. Indeed this potential therapeutic avenue has started to be explored. A recent study which treated LDLr-/- mice with a nanoparticle-directed short peptide designed to mimic annexin A1, resulted in improved inflammation resolution [91]. The advanced plaques in the LDLr-/- mice developed a thicker fibrous cap as well as reducing oxidative stress and necrosis within the plaque, indicating improved lesion stability compared to the control mice [91].
Another potential strategy to slow atherosclerosis disease progression is to accelerate the resolution of inflammation. This can be done by using natural products derived from food sources known as nutraceuticals [92–94]. Fish oils, which contain omega-3 polyunsaturated fatty acids (ω-3 PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have many anti-atherogenic actions [95–98]. Monocyte migration has shown to be attenuated both in vitro and in vivo with ω-3 PUFA treatment [95]. Furthermore, foam cell formation is reduced by ω-3 PUFAs, due to their ability to alter the expression of key genes implicated in the uptake and efflux of cholesterol [96,97]. There are several murine and human studies which highlight the importance of using nutraceuticals in the resolution and prevention of atherosclerosis [95,99,100]. Both EPA and DHA have been found to be metabolised via the COX and lipoxygenase pathways into a new class of lipid mediators known as resolvins [101]. Resolvins are powerful anti-inflammatory agents thought to be capable of accelerating the resolution of inflammation [101], highlighting one possible mechanism by which ω-3 PUFAs are capable of exerting their anti-inflammatory effects. It should be noted that although the consumption of ω-3 PUFAs has shown some promise in attenuating atherosclerosis disease progression, their effectiveness as nutraceuticals is still debated in the current literature. Several meta-analyses have been unable to demonstrate any association between ω-3 PUFA consumption and reduced risk of suffering a CVD-related event [102–104]. Numerous other nutraceuticals including flavanols [105,106], phytosterols [107] and polyphenols [108,109] have also been shown to exert anti-inflammatory actions. Furthermore, a unique combination of nutraceuticals has recently been shown to exert anti-inflammatory effects on several key processes associated with atherosclerosis in vitro [110]. Due to the anti-inflammatory effects of nutraceuticals and their ability to reduce the expression of pro-inflammatory cytokines, they can potentially be given without the systemic-inhibition risks associated with cytokine targeting therapies. In addition nutraceuticals can also be taken safely over an individual’s lifetime, a major advantage when compared to traditional pharmaceutical therapies which are only prescribed once an individual is considered to be at high risk of suffering a CVD-related event.
5. Conclusion
Due to cytokines being the main orchestrators of atherosclerosis development and the driving force behind many of the key steps involved in disease progression, including immune cell recruitment, foam cell formation and plaque stability, they represent promising therapeutic targets. As cytokines and their receptors have functional redundancy, the majority of current cytokine targeted therapies use antibodies to neutralize and prevent pro-inflammatory cytokine signaling in order to dampen the inflammatory response observed in atherosclerotic lesions and slow plaque growth. However the administration of anti-cytokine antibodies and potentially decoy receptors in patients would mean the cytokine would be targeted systemically and leave the individual immunocompromised and at a greater risk of infections. Therefore their use should be limited to those who are considered to be at high risk of suffering a CVD-related event. Despite the current limitations of cytokine-targeted therapies, they are still a therapeutic avenue worth pursuing and provide hope of attenuating atherosclerosis development by targeting the key drivers of disease progression. The development of drug delivery systems which will direct cytokine therapies to an atherosclerotic plaque will make anti-cytokine therapies an even more viable therapeutic option. However to achieve the full potential of cytokine-targeted therapies, more studies focused on developing a greater understanding of both the role and signaling pathways of cytokines in atherosclerosis development must be carried out. Once a more complete insight into cytokine signaling pathways has been developed, novel therapeutic targets may be identified.
Future Perspective.
Over the next 5 to 10 years as drug delivery systems such as nanoparticles and micelles continue to be developed and refined there will be an explosion in atherosclerotic targeted therapies. This will be particularly true for cytokine-targeted therapies. Drug delivery systems will allow for the use of cytokine-targeted therapies without the risk of potential systemic side effects. Not only will they allow the direct delivery of antibodies, decoy receptors and inhibitors of pro-inflammatory cytokine signaling directly to the plaque without the risks of systemic inhibition, they will also allow for the delivery of anti-inflammatory cytokines/agents at non-toxic levels in order to accelerate the resolution of inflammation. Furthermore, over the next 5 to 10 years there will be an increase in the number of studies focusing on nutraceuticals in order to speed up the resolution of inflammation. Nutraceuticals such as omega-3 fatty acids, flavanols, polyphenols and combinations of nutraceuticals have already been shown to exert anti-atherosclerotic effects with little to no side effects. As previously discussed some patients taking current pharmaceutical therapies are unable to achieve lower plasma cholesterol levels and sustained use of traditional pharmaceutical therapies can result in side effects, stressing the need to investigate alternative therapies such as nutraceuticals and cytokine targeted therapies.
Executive Summary.
Atherosclerosis
Atherosclerosis is characterized as the build-up of fatty deposits in the walls of medium and larger arteries.
This build-up triggers an inflammatory response which recruits immune cells to the affected site.
Over time the inflammatory response becomes chronic and a plaque begins to form.
If this plaque ruptures it can lead to a myocardial infarction or stroke.
Role of cytokines in disease development
Cytokines are small protein molecules involved in cellular signalling and can be broadly classified as either pro- or anti-inflammatory (with some able to exert characteristics of both).
Chemokines play a major role during the initial development of atherosclerosis. They are the main driver of monocyte and other immune cell recruitment to the fatty deposit build-up.
Once in the walls of the arteries, these monocytes become exposed to pro-inflammatory cytokines which causes them to differentiate into the pro-inflammatory M1 macrophage phenotype.
Cytokines are then able to influence modified lipoprotein uptake and cholesterol efflux, leading to increased intracellular cholesterol levels and the development of foam cells.
These foam cells then undergo apoptosis or necrosis and “spill” their lipid-rich content into the walls of the arteries and contribute to the size of the atherosclerotic plaque.
The stability of the plaque is then influenced by cytokines, with pro-inflammatory cytokines leading to the increased release of MMPs from macrophages which can lead to plaque destabilization.
Targeting cytokines therapeutically
Due to the inability of statins to reduce cholesterol levels in some individuals and potential side effects of long-term usage, targeting cytokines represents a promising therapeutic strategy.
Neutralizing pro-inflammatory cytokine signaling by targeting either the cytokine or receptor is showing some promise.
Neutralization can be achieved with antibodies against the cytokine/receptor to stop the two interacting and triggering the signalling pathway or by outcompeting the receptor by using a decoy receptor.
However due to the major role of cytokines in the immune response, systemic administration of cytokine targeted therapies may result in severe adverse effects.
To overcome this problem, the use of drug delivery systems such as nanoparticles may allow the delivery of cytokine therapies directly to the atherosclerotic plaque without any systemic side effects.
Acknowledgements
We apologize to all the authors whose work could not be cited because of space limitations. Research in our laboratory was supported by grants from the British Heart Foundation (grants PG/10/55/28467 and PG/12/50/29691).
References
- 1.WHO. World Health Organization Fact Sheet 317. 2015 [Internet]. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(5):969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 4.Buckley ML, Ramji DP. The influence of dysfunctional signaling and lipid homeostasis in mediating the inflammatory responses during atherosclerosis. Biochim Biophys Acta Mol Basis Dis. 2015;1852(7):1498–1510. doi: 10.1016/j.bbadis.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 5.McLaren JE, Michael DR, Ashlin TG, Ramji DP. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog Lipid Res. 2011;50(4):331–347. doi: 10.1016/j.plipres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Ramji DP, Davies TS. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015;26(6):673–685. doi: 10.1016/j.cytogfr.2015.04.003. [** Comprehensive and up-to-date coverage on the roles of different cytokines and chemokines in the pathogenesis of atherosclerosis with focus on studies using mouse model systems.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss JWE, Ramji DP. Interferon-γ: Promising therapeutic target in atherosclerosis. World J Exp Med. 2015;5(3):154–159. doi: 10.5493/wjem.v5.i3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boshuizen MCS, de Winther MPJ. Interferons as essential modulators of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(7):1579–88. doi: 10.1161/ATVBAHA.115.305464. [DOI] [PubMed] [Google Scholar]
- 9.Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12(1):10–7. doi: 10.1038/nrcardio.2014.173. [** Describes in detail our current understanding of the different macrophage subsets and their roles in atherosclerosis.] [DOI] [PubMed] [Google Scholar]
- 10.Wolfs IMJ, Donners MMPC, de Winther MPJ. Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb Haemost. 2011;106(5):763–771. doi: 10.1160/TH11-05-0320. [DOI] [PubMed] [Google Scholar]
- 11.Leitinger N, Schulman IG. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33(6):1120–1126. doi: 10.1161/ATVBAHA.112.300173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 14.Andres V, Pello OM, Silvestre-Roig C. Macrophage proliferation and apoptosis in atherosclerosis. Curr Opin Lipidol. 2012;23(5):429–438. doi: 10.1097/MOL.0b013e328357a379. [DOI] [PubMed] [Google Scholar]
- 15.Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Russo HM, Eitzman DT. Monocyte chemoattractant protein-1 deficiency protects against visceral fat-induced atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(6):1151–8. doi: 10.1161/ATVBAHA.110.205914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411(21-22):1570–9. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3(105) doi: 10.1126/scisignal.3105cm1. cm1. [DOI] [PubMed] [Google Scholar]
- 18.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–32. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Tassell BW, Toldo S, Mezzaroma E, Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128(17):1910–23. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: Moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145–156. doi: 10.1161/CIRCRESAHA.115.306656. [** Describes in detail the importance of inflammatory markers such as interleukins-1/6 and C-reactive protein in cardiovascular disease and the rationale for the CANTOS trial for targeting inflammation in atherosclerosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W, Yin Y, Zhou Z, He M, Dai Y. OxLDL-induced IL-1 beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflamm Res. 2014;63(1):33–43. doi: 10.1007/s00011-013-0667-3. [DOI] [PubMed] [Google Scholar]
- 22.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reifenberg K, Cheng F, Orning C, et al. Overexpression of TGF-β1 in macrophages reduces and stabilizes atherosclerotic plaques in ApoE-deficient mice. PLoS One. 2012;7(7):e40990. doi: 10.1371/journal.pone.0040990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lievens D, Habets KL, Robertson A-K, et al. Abrogated transforming growth factor beta receptor II (TGFβRII) signalling in dendritic cells promotes immune reactivity of T cells resulting in enhanced atherosclerosis. Eur Heart J. 2013;34(48):3717–27. doi: 10.1093/eurheartj/ehs106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–18. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaren JE, Michael DR, Salter RC, et al. IL-33 reduces macrophage foam cell formation. J Immunol. 2010;185:1222–1229. doi: 10.4049/jimmunol.1000520. [DOI] [PubMed] [Google Scholar]
- 27.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7(10):827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali S, Mohs A, Thomas M, et al. The dual function cytokine IL-33 interacts with the transcription factor NF-κB to dampen NF-κB-stimulated gene transcription. J Immunol. 2011;187(4):1609–16. doi: 10.4049/jimmunol.1003080. [DOI] [PubMed] [Google Scholar]
- 29.Miller AM, Xu D, Asquith DL, et al. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205(2):339–46. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore K, Sheedy F, Fisher E. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [* Comprehensive coverage of the role of macrophages in atherosclerosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soehnlein O, Drechsler M, Döring Y, et al. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013;5(3):471–81. doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–22. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 34.Koenen RR, Weber C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat Rev Drug Discov. 2010;9(2):141–53. doi: 10.1038/nrd3048. [DOI] [PubMed] [Google Scholar]
- 35.Zernecke A, Weber C. Chemokines in atherosclerosis: proceedings resumed. Arterioscler Thromb Vasc Biol. 2014;34(4):742–50. doi: 10.1161/ATVBAHA.113.301655. [DOI] [PubMed] [Google Scholar]
- 36.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32(10):470–7. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zernecke A, Weber C. Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc Res. 2010;86(2):192–201. doi: 10.1093/cvr/cvp391. [DOI] [PubMed] [Google Scholar]
- 38.Combadière C, Potteaux S, Rodero M, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117(13):1649–57. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 39.Liu XL, Zhang PF, Ding SF, et al. Local gene silencing of monocyte chemoattractant protein-1 prevents vulnerable plaque disruption in apolipoprotein E-knockout mice. PLoS One. 2012;7(3):e33497. doi: 10.1371/journal.pone.0033497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong HS, Jaumouillé V, Freeman SA, et al. Chemokine signaling enhances CD36 responsiveness toward oxidized low-density lipoproteins and accelerates foam cell formation. Cell Rep. 2016;14(12):2859–71. doi: 10.1016/j.celrep.2016.02.071. [* A study describing a new role for chemokines that involves enhancement of CD36 responsiveness to oxidized LDL and subsequent acceleration of foam cell formation.] [DOI] [PubMed] [Google Scholar]
- 41.Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262(1):153–66. doi: 10.1111/imr.12218. [DOI] [PubMed] [Google Scholar]
- 42.Erbel C, Tyka M, Helmes CM, et al. CXCL4-induced plaque macrophages can be specifically identified by co-expression of MMP7+S100A8+ in vitro and in vivo. Innate Immun. 2015;21(3):255–65. doi: 10.1177/1753425914526461. [DOI] [PubMed] [Google Scholar]
- 43.Zhang K, Huang X, Li X, et al. Interleukin 6 destabilizes atherosclerotic plaques by downregulating prolyl-4-hydroxylase α1 via a mitogen-activated protein kinase and c-Jun pathway. Arch Biochem Biophys. 2012;528(2):127–33. doi: 10.1016/j.abb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Han X, Kitamoto S, Wang H, Boisvert WA. Interleukin-10 overexpression in macrophages suppresses atherosclerosis in hyperlipidemic mice. FASEB J. 2010;24(8):2869–80. doi: 10.1096/fj.09-148155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh J, Riek AE, Weng S, et al. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem. 2012;287(15):11629–41. doi: 10.1074/jbc.M111.338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao N, Yin M, Zhang L, et al. Tumor necrosis factor-alpha deficiency retards early fatty-streak lesion by influencing the expression of inflammatory factors in apoE-null mice. Mol Genet Metab. 2009;96(4):239–244. doi: 10.1016/j.ymgme.2008.11.166. [DOI] [PubMed] [Google Scholar]
- 47.Xanthoulea S, Thelen M, Pöttgens C, Gijbels MJJ, Lutgens E, de Winther MPJ. Absence of p55 TNF receptor reduces atherosclerosis, but has no major effect on angiotensin II induced aneurysms in LDL receptor deficient mice. PLoS One. 2009;4(7):e6113. doi: 10.1371/journal.pone.0006113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamari Y, Shaish A, Shemesh S, et al. Reduced atherosclerosis and inflammatory cytokines in apolipoprotein-E-deficient mice lacking bone marrow-derived interleukin-1α. Biochem Biophys Res Commun. 2011;405(2):197–203. doi: 10.1016/j.bbrc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Shemesh S, Kamari Y, Shaish A, et al. Interleukin-1 receptor type-1 in non-hematopoietic cells is the target for the pro-atherogenic effects of interleukin-1 in apoE-deficient mice. Atherosclerosis. 2012;222(2):329–36. doi: 10.1016/j.atherosclerosis.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Bhat OM, Kumar PU, Giridharan NV, Kaul D, Kumar MJM, Dhawan V. Interleukin-18-induced atherosclerosis involves CD36 and NF-κB crosstalk in Apo E-/- mice. J Cardiol. 2015;66(1):28–35. doi: 10.1016/j.jjcc.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Pejnovic N, Vratimos A, Lee SH, et al. Increased atherosclerotic lesions and Th17 in interleukin-18 deficient apolipoprotein E-knockout mice fed high-fat diet. Mol Immunol. 2009;47(1):37–45. doi: 10.1016/j.molimm.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 52.Schuett H, Oestreich R, Waetzig GH, et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32(2):281–90. doi: 10.1161/ATVBAHA.111.229435. [DOI] [PubMed] [Google Scholar]
- 53.van Es T, van Puijvelde GHM, Michon IN, et al. IL-15 aggravates atherosclerotic lesion development in LDL receptor deficient mice. Vaccine. 2011;29(5):976–83. doi: 10.1016/j.vaccine.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 54.Sastre C, Fernández-Laso V, Madrigal-Matute J, et al. Genetic deletion or TWEAK blocking antibody administration reduce atherosclerosis and enhance plaque stability in mice. J Cell Mol Med. 2014;18(4):721–34. doi: 10.1111/jcmm.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLaren JE, Calder CJ, McSharry BP, et al. The TNF-like protein 1A-death receptor 3 pathway promotes macrophage foam cell formation in vitro. J Immunol. 2010;184(10):5827–34. doi: 10.4049/jimmunol.0903782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gistera A, Robertson AK, Andersson J, et al. Transforming growth factor-beta signaling in t cells promotes stabilization of atherosclerotic plaques through an interleukin-17 dependent pathway. Atherosclerosis. 2014;235(2):e88–e89. doi: 10.1126/scitranslmed.3006133. [DOI] [PubMed] [Google Scholar]
- 57.Reifenberg K, Cheng F, Twardowski L, et al. T cell-specific overexpression of TGFß1 fails to influence atherosclerosis in apoe-deficient mice. PLoS One. 2013;8(12):e81444. doi: 10.1371/journal.pone.0081444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michael DR, Salter RC, Ramji DP. TGF-β inhibits the uptake of modified low density lipoprotein by human macrophages through a Smad-dependent pathway: a dominant role for Smad-2. Biochim Biophys Acta. 2012;1822(10):1608–16. doi: 10.1016/j.bbadis.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardilo-Reis L, Gruber S, Schreier SM, et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4(10):1072–86. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siasos G, Tousoulis D, Kioufis S, et al. Inflammatory mechanisms in atherosclerosis: the impact of matrix metalloproteinases. Curr Top Med Chem. 2012;12(10):1132–48. doi: 10.2174/1568026611208011132. [DOI] [PubMed] [Google Scholar]
- 61.Koga M, Kai H, Yasukawa H, et al. Inhibition of progression and stabilization of plaques by postnatal interferon-gamma function blocking in ApoE-knockout mice. Circ Res. 2007;101(4):348–356. doi: 10.1161/CIRCRESAHA.106.147256. [DOI] [PubMed] [Google Scholar]
- 62.Alexander MR, Moehle CW, Johnson JL, et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122(1):70–9. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18(11):1519–30. doi: 10.2174/138161212799504803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127(1):96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 66.NHS. Statins - Side effects. 2016 [Internet]. Available from: http://www.nhs.uk/Conditions/Cholesterol-lowering-medicines-statins/Pages/Side-effects.aspx.
- 67.Ridker PM, Howard CP, Walter V, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126(23):2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 68.Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: from improved risk prediction to risk-guided therapy. Int J Cardiol. 2013;168(6):5126–34. doi: 10.1016/j.ijcard.2013.07.113. [DOI] [PubMed] [Google Scholar]
- 69.Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166(2):199–207.e15. doi: 10.1016/j.ahj.2013.03.018. [* An in-depth description of the rationale for targeting cardiovascular inflammation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li N, McLaren JE, Michael DR, Clement M, Fielding CA, Ramji DP. ERK Is Integral to the IFN-y-mediated activation of STAT1, the expression of key genes implicated in atherosclerosis, and the uptake of modified lipoproteins by human macrophages. J Immunol. 2010;185:3041–3048. doi: 10.4049/jimmunol.1000993. [DOI] [PubMed] [Google Scholar]
- 71.Lee H, Chung H, Lee H, Jeong L, Lee S. Suppression of inflammation response by a novel A3 adenosine receptor agonist thio-Cl-IB-MECA through inhibition of Akt and NF-κB signaling. Immunobiology. 2011;216:997–1003. doi: 10.1016/j.imbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Voloshyna I, Hai O, Littlefield M, Carsons S, Reiss A. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARγ and adenosine. Eur J Pharmacol. 2013;698:299–309. doi: 10.1016/j.ejphar.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 73.White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation--therapeutic opportunities and pharmacological challenges. Pharmacol Rev. 2013;65(1):47–89. doi: 10.1124/pr.111.005074. [Internet] [DOI] [PubMed] [Google Scholar]
- 74.Bursill CA, McNeill E, Wang L, et al. Lentiviral gene transfer to reduce atherosclerosis progression by long-term CC-chemokine inhibition. Gene Ther. 2009;16(1):93–102. doi: 10.1038/gt.2008.141. [DOI] [PubMed] [Google Scholar]
- 75.Nie P, Li D, Hu L, et al. Atorvastatin improves plaque stability in ApoE-knockout mice by regulating chemokines and chemokine receptors. PLoS One. 2014;9(5):e97009. doi: 10.1371/journal.pone.0097009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Majmudar MD, Keliher EJ, Heidt T, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127(20):2038–2046. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olzinski AR, Turner GH, Bernard RE, et al. Pharmacological inhibition of C-C chemokine receptor 2 decreases macrophage infiltration in the aortic root of the human C-C chemokine receptor 2/apolipoprotein E-/-mouse: Magnetic resonance imaging assessment. Arterioscler Thromb Vasc Biol. 2010;30(2):253–259. doi: 10.1161/ATVBAHA.109.198812. [DOI] [PubMed] [Google Scholar]
- 78.Gilbert J, Lekstrom-Himes J, Donaldson D, et al. Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am J Cardiol. 2011;107(6):906–11. doi: 10.1016/j.amjcard.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Cipriani S, Francisci D, Mencarelli A, et al. Efficacy of the CCR5 antagonist maraviroc in reducing early, ritonavir-induced atherogenesis and advanced plaque progression in mice. Circulation. 2013;127(21):2114–24. doi: 10.1161/CIRCULATIONAHA.113.001278. [DOI] [PubMed] [Google Scholar]
- 80.Chain B, Arnold J, Akthar S, et al. A linear epitope in the n-terminal domain of CCR5 and its interaction with antibody. PLoS One. 2015;10(6):e0128381. doi: 10.1371/journal.pone.0128381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poupel L, Boissonnas A, Hermand P, et al. Pharmacological inhibition of the chemokine receptor, CX3CR1, reduces atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2013;33(10):2297–305. doi: 10.1161/ATVBAHA.112.300930. [DOI] [PubMed] [Google Scholar]
- 82.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 83.Striz I, Brabcova E, Kolesar L, Sekerkova A. Cytokine networking of innate immunity cells: a potential target of therapy. Clin Sci (Lond) 2014;126(9):593–612. doi: 10.1042/CS20130497. [DOI] [PubMed] [Google Scholar]
- 84.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16(4):343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christian DA, Hunter CA. Particle-mediated delivery of cytokines for immunotherapy. Immunotherapy. 2012;4(4):425–41. doi: 10.2217/imt.12.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chacko A-M, Hood ED, Zern BJ, Muzykantov VR. Targeted nanocarriers for imaging and therapy of vascular inflammation. Curr Opin Colloid Interface Sci. 2011;16(3):215–227. doi: 10.1016/j.cocis.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jayagopal A, Linton MF, Fazio S, Haselton FR. Insights into atherosclerosis using nanotechnology. Curr Atheroscler Rep. 2010;12(3):209–15. doi: 10.1007/s11883-010-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis DR, Kamisoglu K, York AW, Moghe PV. Polymer-based therapeutics: nanoassemblies and nanoparticles for management of atherosclerosis. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(4):400–20. doi: 10.1002/wnan.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chung EJ. Targeting and therapeutic peptides in nanomedicine for atherosclerosis. Exp Biol Med (Maywood) 2016 doi: 10.1177/1535370216640940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Almer G, Summers KL, Scheicher B, et al. Interleukin 10-coated nanoparticle systems compared for molecular imaging of atherosclerotic lesions. Int J Nanomedicine. 2014;9:4211–22. doi: 10.2147/IJN.S66830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fredman G, Kamaly N, Spolitu S, et al. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7(275):275ra20. doi: 10.1126/scitranslmed.aaa1065. [** An excellent study demonstrating the use of nanoparticles for targeting therapeutic agents in atherosclerosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr An Int Rev J. 2012;3(4):506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68(5):280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 94.Granados-Principal S, Quiles JL, Ramirez-Tortosa CL, Sanchez-Rovira P, Ramirez-Tortosa MC. Hydroxytyrosol: from laboratory investigations to future clinical trials. Nutr Rev. 2010;68(4):191–206. doi: 10.1111/j.1753-4887.2010.00278.x. [DOI] [PubMed] [Google Scholar]
- 95.Brown AL, Zhu X, Rong S, et al. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler Thromb Vasc Biol. 2012;32(9):2122–2130. doi: 10.1161/ATVBAHA.112.253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song Y, Zhang LJ, Li H, et al. Polyunsaturated fatty acid relatively decreases cholesterol content in THP-1 macrophage-derived foam cell: partly correlates with expression profile of CIDE and PAT members. Lipids Heal Dis. 2013;12(1):111. doi: 10.1186/1476-511X-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McLaren JE, Michael DR, Guschina IA, Harwood JL, Ramji DP. Eicosapentaenoic acid and docosahexaenoic acid regulate modified LDL uptake and macropinocytosis in human macrophages. Lipids. 2011;46(11):1053–1061. doi: 10.1007/s11745-011-3598-1. [DOI] [PubMed] [Google Scholar]
- 98.Niki T, Wakatsuki T, Yamaguchi K, et al. Effects of the addition of eicosapentaenoic acid to strong statin therapy on inflammatory cytokines and coronary plaque components assessed by integrated backscatter intravascular ultrasound. Circ J. 2016;80(2):450–60. doi: 10.1253/circj.CJ-15-0813. [DOI] [PubMed] [Google Scholar]
- 99.Franzese CJ, Bliden KP, Gesheff MG, et al. Relation of fish oil supplementation to markers of atherothrombotic risk in patients with cardiovascular disease not receiving lipid-lowering therapy. Am J Cardiol. 2015;115(9):1204–1211. doi: 10.1016/j.amjcard.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 100.Nakajima K, Yamashita T, Kita T, et al. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in ldl receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31(9):1963–1972. doi: 10.1161/ATVBAHA.111.229443. [DOI] [PubMed] [Google Scholar]
- 101.Calder PC. Mechanisms of Action of (n-3) Fatty Acids. J Nutr. 2012;142(3):592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 102.Enns JE, Yeganeh A, Zarychanski R, et al. The impact of omega-3 polyunsaturated fatty acid supplementation on the incidence of cardiovascular events and complications in peripheral arterial disease: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2014;14(1):70. doi: 10.1186/1471-2261-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kwak S, Myung S, Lee Y, Seo H, Korean Meta-analysis Study Group f. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: A meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med. 2012;172(9):686–694. doi: 10.1001/archinternmed.2012.262. [DOI] [PubMed] [Google Scholar]
- 104.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA. 2012;308(10):1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 105.Morrison M, van der Heijden R, Heeringa P, et al. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB in vivo. Atherosclerosis. 2014;233(1):149–156. doi: 10.1016/j.atherosclerosis.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 106.Sansone R, Rodriguez-Mateos A, Heuel J, et al. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: a randomised, controlled, double-masked trial: the Flaviola Health Study. Br J Nutr. 2015;114(8):1246–55. doi: 10.1017/S0007114515002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sabeva NS, McPhaul CM, Li X, Cory TJ, Feola DJ, Graf GA. Phytosterols differentially influence ABC transporter expression, cholesterol efflux and inflammatory cytokine secretion in macrophage foam cells. J Nutr Biochem. 2011;22(8):777–783. doi: 10.1016/j.jnutbio.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosignoli P, Fuccelli R, Fabiani R, Servili M, Morozzi G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J Nutr Biochem. 2013;24(8):1513–1519. doi: 10.1016/j.jnutbio.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 109.Fito M, Cladellas M, de la Torre R, et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur J Clin Nutr. 2008;62(4):570–574. doi: 10.1038/sj.ejcn.1602724. [DOI] [PubMed] [Google Scholar]
- 110.Moss JWE, Davies TS, Garaiova I, Plummer SF, Michael DR, Ramji DP. A unique combination of nutritionally active ingredients can prevent several key processes associated with atherosclerosis in vitro. PLoS One. 2016;11(3):e0151057. doi: 10.1371/journal.pone.0151057. [* A recent study demonstrating the efficacy of a novel combination nutritional products to attenuate several key steps associated with atherosclerosis in vitro.] [DOI] [PMC free article] [PubMed] [Google Scholar]