Abstract
Background
Delayed encephalopathy after acute carbon monoxide (CO) poisoning (DEACMP) is one of the most serious complications after CO poisoning. This study was conducted to explore the efficacy of the combined application of N-Butylphthalide and hyperbaric oxygenation therapy (HBO) on cognitive dysfunction in patients with DEACMP.
Material/Methods
A total of 184 patients with DEACMP were randomly assigned to either receive HBO or N-Butylphthalide and HBO. Meanwhile, all patients received conventional treatment. The total remission rate (RR) was used to assess the clinical efficacy. The Mini-Mental State Examination (MMSE) was used to assess the cognitive function, and the National Institutes of Health Stroke Scale (NIHSS) was used to assess the neurological function.
Results
Finally, there were 90 and 94 patients in the control and experimental groups, respectively. After eight weeks of treatment, the total RR in the experimental group (47.9%) was significantly higher than that in the control group (33.3%). Compared to the control group, significantly more patients in the experimental group had MMSE scores of 24–30. The lower NIHSS score in the experimental group showed that N-Butylphthalide had the effect of preservation and restoration of neurological function. No obvious drug toxicity or liver and kidney dysfunction was observed, and there was no significant change in the level of blood glucose and blood lipids.
Conclusions
These results indicated that the combined application of N-Butylphthalide and HBO could significantly improve the cognitive dysfunction of patients with DEACMP and have great clinical efficacy, which should be further studied.
MeSH Keywords: Carbon Monoxide, Hyperbaric Oxygenation, Patients
Background
Carbon monoxide (CO) is an odorless, colorless, and tasteless gas, which is highly difficult to detect when escaping. It is toxic to humans when its concentration is higher than 35 ppm. This gas is the leading cause of poisoning in the United States [1]. Nowadays, among the fatal air poisonings, CO poisoning is the most common and causes a series of serious health problems for people in many countries [2]. Delayed encephalopathy after acute CO poisoning (DEACMP) is one of the most serious complications [3]. The incidence rate of this disease ranges from 0.2% to 47% [4]. DEACMP is a common cause of clinical neurological complications and could result in memory impairment, unresponsiveness, visceral autonomic nervous system dysfunction, Parkinson’s syndrome, cognitive dysfunction, and behavioral disorders in patients.
Many factors are associated with the occurrence of DEACMP. A previous study reported that the elderly people might have a higher incidence of DEACMP due to the decrease in hypoxia tolerance of nervous tissue in brain [5]. Also, some studies found that a prolonged duration of coma after acute CO poisoning was the risk factor for DEACMP [6,7]. In addition, Choi et al. reported that lung infection and urinary system infection promoted the occurrence of DEACMP, which might be because the presence of infection increased the oxygen consumption of brain [8]. Additionally, the brains of mental workers often need more oxygen and are more sensitive to hypoxia, which might make them more likely to have DEACMP after CO poisoning compared to manual workers [9].
In regards to the treatments, hyperbaric oxygen therapy (HBO) was often recommended by previous studies to treat patients with acute CO poisoning [10,11]. Researchers also reported that the HBO had significant effects on DEACMP [12]. Recently, N-Butylphthalide was found to have a neuroprotective role in brain damage after acute CO poisoning [13,14]. Therefore, we conducted this study to explore the effect of N-Butylphthalide and HBO on the cognitive dysfunction in patients with DEACMP.
Material and Methods
Recruited patients
The purpose and design of this work were reviewed and approved by the Institutional Review Board (IRB) of our hospital. Before the treatment, all of the recruited patients provided written informed consent. This work was conducted between January 2012 and July 2015. We used the Mini-Mental State Examination (MMSE) to assess the cognitive function of 301 patients with DEACMP. An MMSE score ≤24 indicated cognitive dysfunction [15]. Finally, 215 patients with cognitive dysfunction were recruited.
Experimental methods
Patients who signed the written informed consent were randomly assigned into the experimental group or the control group. The random number table was applied to perform randomization in this study. Both the patients and the clinicians were blind to the allocation. All patients received conventional treatment, including sputum suction, using antibiotics, maintaining unobstructed airway, reducing or avoiding respiratory tract infection, regulating water and electrolytes, maintaining stable blood pressure and blood glucose, preventing urinary tract infection, and giving HBO. Patients were placed in an oxygen chamber with 0.25 MPa absolute pressure for 80 minutes per day. Meanwhile, the patients in the experimental group also received N-Butylphthalide. The dose of N-Butylphthalide was set to 0.2 g orally, three times per day. N-Butylphthalide was provided 5 days per week for eight weeks.
Outcomes assessment
A rater who was blinded to the treatments conducted the assessment at the 4th and 8th weeks. Before the treatment, the cognitive function of all patients was assessed using the MMSE score. In addition, the National Institute of Health Stroke Scale (NIHSS) was used to assess the neurological function of patients. These two scales were also used at the 4th and 8th weeks. According to the MMSE score, complete recovery (CR) was defined as the recovery of intelligence and language ability, and availability to do some simple work (MMSE >24); partial recovery (PR) was defined as the partial disappearance of signs and symptoms (MMSE of ≥10 to ≤24); no relief (NR) was defined as the no improvement of signs and symptoms (MMSE <10). The remission rate (RR) was calculated as the number of CRs divided by the number of patients.
Statistical analysis
The continuous data were expressed as mean ± standard deviation. Student’s t test and the chi-squared test were applied to investigate the differences between the two groups on demographic and baseline clinical variables. Analysis of covariance (ANCOVA) was conducted to test the effect of treatments on the post-treatment MMSE and NIHSS scores. This method used their baseline scores as the covariate to eliminate the effect of each parameter’s initial value. If patients withdrew from treatment, then their latest assessments were viewed as the final assessments. The intention-to-treat (ITT) analysis was used in this study. All procedures were 2-tailed and the significance was set at a P value less than 0.05. All analyses were conducted with SPSS 19.0 software.
Results
Demographic characteristics
A total of 215 patients with cognitive dysfunction were recruited at first. Before the treatment, 31 patients were excluded because they did not agree to randomization. After randomization, there were 90 and 94 patients in the control and experimental groups, respectively. After four weeks of treatment, 6 patients who required other treatment methods in the control group and 5 patients who required other treatment methods in the experimental group did not continue this study. Finally, 173 patients completed the 8-week treatment. There were no significant differences between the control and experimental groups on any key demographic characteristics, such as gender, educational level, age, work type, body mass index (BMI), CO exposure time, carboxyhemoglobin levels, or latent phase. Therefore, our findings were be influenced by these demographic factors. The detailed information is shown in Table 1.
Table 1.
Demographic characteristics of the included patients.
| Variable | Control | Experiment | t/χ2 | p-Value |
|---|---|---|---|---|
| Number | 90 | 94 | – | – |
| Female/male | 41/49 | 42/52 | 0.014 | 0.902 |
| Age (years) | 51.44±10.82 | 52.35±12.43 | −0.527 | 0.599 |
| BMI (kg/m2) | 22.24±2.95 | 22.47±3.27 | −0.486 | 0.627 |
| Education level | 11.10±4.24 | 11.01±4.41 | 0.140 | 0.889 |
| Work type (Me/Ma) | 63/27 | 70/24 | 0.458 | 0.498 |
| CO exposure time | 5.31±0.72 | 5.08±0.67 | 0.679 | 0.574 |
| COHb levels (%) | 21.05±18.57 | 23.72±20.24 | −0.874 | 0.686 |
| WBC count (/μ) | 14527.55±4057.34 | 14384.04±4231.80 | 0.554 | 0.409 |
| LDH (U/L) | 384.33±265.07 | 397.24±270.61 | −0.354 | 0.689 |
| Creatine kinase (IU/L) | 4409.25±4095.22 | 4511.29±4139.02 | −0.527 | 0.810 |
| Latent phase | 19.11±8.94 | 21.23±9.22 | −0.603 | 0.611 |
| Hypertension | 10 | 7 | 0.736 | 0.391 |
| Diabetes | 3 | 6 | 0.919 | 0.498 |
| Coronary heart disease | 9 | 12 | 0.348 | 0.555 |
| MMSE | 8.93±6.09 | 8.89±5.85 | 0.045 | 0.964 |
| NIHSS | 17.23±4.64 | 17.28±4.68 | −0.063 | 0.950 |
BMI – body mass index; MMSE – Mini-Mental State Examination; NIHSS – National Institute of Health Stroke Scale; Me – mental worker; Ma – manual worker; COHb – carboxyhemoglobin; WBC – white blood cell; LDH – lactate dehydrogenase.
MMSE score
The MMSE assessment was performed at three time points (Figure 1). A rater blind to the treatment group conducted this assessment. Before the treatment, there was no significant difference in the MMSE scores between the two groups (P=0.964). After four weeks of treatment, the average MMSE scores were significantly increased in both the control (P<0.001) and experimental (P<0.001) groups. However, the results of ANCOVA showed that the effect of treatments on MMSE score was significantly different: the average MMSE score in the experimental group was significantly higher than that in the control group (P=0.004). At the end of 8th week, the increase in the average MMSE score was still smaller in the control group. And the results of ANCOVA showed that the experimental group had a significant higher average MMSE score compared to the control group (P=0.012).
Figure 1.
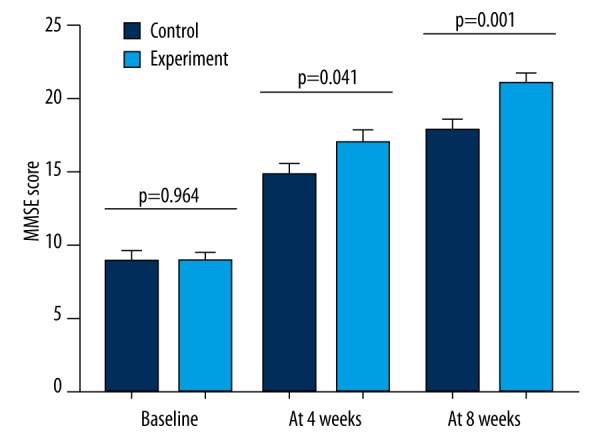
MMSE score of the patients in the experimental and control groups at different time points.
Remission rate
Before the treatment, the MMSE score in all patients was less than 24. The number of patients with an MMSE score of 10–24 was 31 and 29 in the experimental and control groups, respectively. The rest of the patients in the two groups had an MMSE score of 0–9. At the end of the 4th week, 33 and 19 patients in the experimental and control group, respectively, met the CR criteria (with MMSE score >24 after treatment); 42 and 28 patients met the PR criteria (with MMSE score of 10–24 after treatment). The RR was significantly different between the two groups (31.9% vs. 21.1%) (P=0.035). At the end of the 8th week, 45 and 30 patients in the experimental and control groups, respectively, met the CR criteria; 59 and 48 patients met the PR criteria (Table 2). The RR was still significantly different between the two groups (47.9% vs. 33.3%) (P=0.045).
Table 2.
Remission rate in the two groups at the end of 4th and 8th week.
| Time | Group | n | 0–9 | 10–24 | 25–30 | p-Value | RR | p-Value |
|---|---|---|---|---|---|---|---|---|
| Baseline | Experiment | 94 | 63 | 31 | 0 | 0.912 | – | – |
| Control | 90 | 61 | 29 | 0 | – | – | ||
| 4th week | Experiment | 94 | 21 | 40 | 33 | 0.025 | 31.9% | 0.035 |
| Control | 90 | 35 | 36 | 19 | 21.1% | |||
| 8th week | Experiment | 94 | 4 | 45 | 45 | 0.021 | 47.9% | 0.045 |
| Control | 90 | 13 | 47 | 30 | 33.3% |
RR – remission rate.
NIHSS score
The NIHSS assessment was performed at three time points (Figure 2). A rater blind to the treatment group conducted this assessment. Before the treatment, there was no significant difference in the NIHSS scores between the two groups (P=0.950). After four weeks of treatment, the average NIHSS scores were significantly decreased in both the control (P=0.004) and experimental (P<0.001) groups. However, the results of ANCOVA showed that the effect of treatments on NIHSS score was significantly different: the average NIHSS score in the experimental group was significantly lower than that in the control group (P=0.011). At the end of 8th week, the reduction in the average NIHSS score was still smaller in the control group. And the results of ANCOVA showed that the experimental group had a significant lower average NIHSS score compared to the control group (P=0.004).
Figure 2.
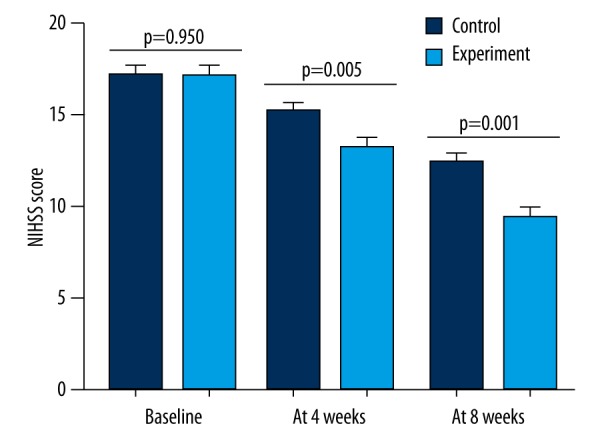
NIHSS score of the patients in the experimental and control groups at different time points.
Adverse events
None of these patients experienced obvious drug toxicity or liver and kidney dysfunction. The blood glucose and blood lipids were not significantly changed during the whole treatment period. In the experimental group, two patients had mild abdominal discomfort, one patients experienced mild nausea, and three patients had minor skin irritations. However, these patients recovered by themselves and needed no additional medications.
Discussion
DEACMP is a serious worldwide disease that often causes great damage to the human body. Also, it is considered as one of the important factors leading to cognitive dysfunction. Here, we found that both HBO and the combined application of N-Butylphthalide and HBO could significantly increase the average MMSE scores after short-term treatment, but the efficacy of the latter was greater than that of the former. Compared to the control group, the RR in the experimental group was also significantly higher. These results demonstrated that the combined application of N-Butylphthalide and HBO could significantly improve the cognitive function of patients with DEACMP and produce better clinical efficacy. Moreover, the greater reduction of NIHSS score in the experimental group indicated that the addition of N-Butylphthalide during disease management might yield better efficacy in the protection and restoration of neurological function. Based on the aforementioned results, the clinical applicability of the combined application of N-Butylphthalide and HBO showed greater promise and should be explored further.
A previous study reported that N-Butylphthalide had strong anti-cerebral ischemia effects and could decrease brain edema [16]. It could inhibit neuronal cell death by improving brain energy metabolism and increasing the microcirculation and blood flow in the ischemic brain regions [17]. In addition, it had anti-platelet, anti-apoptotic, anti-inflammatory, and anti-thrombotic properties [18]. In addition, it could significantly increase the local blood flow of the brain and improve the memory function of specific nerve cells, which could effectively reverse or improve the cognitive dysfunction [19]. However, the molecular mechanism of the effects of N-Butylphthalide still remains unclear. Also, HBO could increase the blood oxygen to remove the hypoxia state in brain tissues, which is beneficial to the function recovery of the damaged brain cells. Therefore, the combined application of N-Butylphthalide and HBO could be a potential effective therapy in treating cognitive dysfunction for patients with DEACMP.
Currently, the pathogenesis of DEACMP is still unclear. Because the central nervous system is the most sensitive tissue to oxygen, some researchers thought that the hypoxia caused by CO poisoning might be the primary factor inducing DEACMP [20,21]. But this theory could not explain the whole clinical manifestation and pathological changes of DEACMP, especially the many symptoms occurring after the recovery of carbonyl hemoglobin level. Gorman et al. reported that the reactive oxygen radicals and peroxides were significantly increased in brain cells after CO poisoning, but the antioxidants, such as catalase, glutathione peroxidase, and vitamin E, were significantly decreased [22]. Then, a great number of free radicals were produced, which could enhance the lipid peroxidation in the cell membrane. Consequently, damage, such as demyelination of nerve cells and secondary neuronal cell death, happened [23]. Other researchers reported that the DEACMP was immune-mediated, and the delayed CO-mediated neuropathology was associated with an adaptive immunological response to chemically modified myelin basic protein [24]. Additionally, Ischiropoulos et al. reported that the CO poisoning changed the nitric oxide (NO) level and NO synthase activity, which indicated that the NO might be involved in brain tissue damage [25]. All in all, DEACMP is caused by the interaction and common effects of various factors.
Limitations of this study should be mentioned here: (1) a relatively small number of DEACMP patients with cognitive dysfunction were included; (2) only the short-term efficacy of the combined application of N-Butylphthalide and HBO was examined; thus, whether this method had the similar long-term efficacy was unclear; and (3) all of the included patients were from the same city; thus, the site-specific bias could not be ruled out. Further studies recruiting heterogeneous populations to study the efficacy of this method are required.
Conclusions
This study found that compared with HBO, the combined application of N-Butylphthalide and HBO in disease management could significantly increase the MMSE score and decrease the NIHSS score, and obtain significantly higher RR, in DEACMP patients with cognitive dysfunction. However, future studies recruiting more patients from different sites are needed to verify and support the efficacy of this method.
Footnotes
Disclosure of conflict of interest
None.
Source of support: Departmental sources
References
- 1.Friedman P, Guo XM, Stiller RJ, et al. Carbon monoxide exposure during pregnancy. Obstet Gynecol Surv. 2015;70:705–12. doi: 10.1097/OGX.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 2.Hampson NB, Weaver LK. Carbon monoxide poisoning: A new incidence for an old disease. Undersea Hyperb Med. 2007;34:163–68. [PubMed] [Google Scholar]
- 3.Goldstein M. Carbon monoxide poisonin. J Emerg Nurs. 2008;34:538–42. doi: 10.1016/j.jen.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Guzman JA. Carbon monoxide poisoning. Crit Care Clin. 2012;28:537–48. doi: 10.1016/j.ccc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Schega L, Peter B, Brigadski T, et al. Effect of intermittent normobaric hypoxia on aerobic capacity and cognitive function in older people. J Sci Med Sport. 2016 doi: 10.1016/j.jsams.2016.02.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Pan R, Tang YM, Rong XM, et al. Analysis of clinical features and risk factors for delayed encephalopathy after carbon monoxide poisoning. Chinese J Pract Intern Med. 2012;32:787–90. [Google Scholar]
- 7.Yang XF, Nie BG, Huang SR. The role of electroencephalogram in delayed encephalopathy after carbon monoxide poisoning. West China Med J. 2005;20:665–66. [Google Scholar]
- 8.Choi IS, Kim SK, Choi YC, et al. Evaluation of outcome after acute carbon monoxide poisoning by brain CT. Korean Med Sci. 1993;8:78–86. doi: 10.3346/jkms.1993.8.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geehr EC, Salluzzo R, Bosco S, et al. Emergency health impact of a severe storm. Am J Emerg Med. 1989;7:598–604. doi: 10.1016/0735-6757(89)90282-9. [DOI] [PubMed] [Google Scholar]
- 10.Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347:1057–67. doi: 10.1056/NEJMoa013121. [DOI] [PubMed] [Google Scholar]
- 11.Annane D, Chadda K, Gajdos P, et al. Hyperbaric oxygen therapy for acute domestic carbon monoxide poisoning: Two randomized controlled trials. Intensive Care Med. 2011;37:486–92. doi: 10.1007/s00134-010-2093-0. [DOI] [PubMed] [Google Scholar]
- 12.Mao M, Rao P, Mou X, et al. Clinical observation on delayed encephalopathy after carbon monoxide poisoning treated with acupuncture to restore consciousness combined with hyperbaric oxygen treatment. Zhongguo Zhen Jiu. 2015;35:213–16. [PubMed] [Google Scholar]
- 13.Li Q, Cheng Y, Bi MJ, et al. Effects of N-Butylphthalide on the expressions of Nogo/NgR in rat brain tissue after carbon monoxide poisoning. Environ Toxicol Pharmacol. 2015;39:953–61. doi: 10.1016/j.etap.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Cheng Y, Bi M, et al. Effects of N-butylphthalide on the activation of Keap1/Nrf-2 signal pathway in rats after carbon monoxide poisoning. Environ Toxicol Pharmacol. 2015;40:22–29. doi: 10.1016/j.etap.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Shu AH, Wang Q, Chen XB. Effect of different depths of anesthesia on postoperative cognitive function in laparoscopic patients: A randomized clinical trial. Curr Med Res Opin. 2015;31:1883–87. doi: 10.1185/03007995.2015.1075968. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Wang L, Li J, et al. 2-(1-Hydroxypentyl)-benzoate increases cerebral blood flow and reduces infarct volume in rats model of transient focal cerebral ischemia. J Pharmacol Exp Ther. 2006;317:973–79. doi: 10.1124/jpet.105.098517. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Li Y, Ogle M, et al. DL-3-n-butylphthalide prevents neuronal cell death after focal cerebral ischemia in mice via the JNK pathway. Brain Res. 2010;1359:216–26. doi: 10.1016/j.brainres.2010.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng Y, Xing C, Lemere CA, et al. l-3-n-Butylphthalide ameliorates beta-amyloid-induced neuronal toxicity in cultured neuronal cells. Neurosci Lett. 2008;434:224–29. doi: 10.1016/j.neulet.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 19.Peng Y, Sun J, Hon S, et al. L-3-n-butylphthalide improves cognitive impairment and reduces amyloid-beta in a transgenic model of Alzheimer’s disease. J Neurosci. 2010;30:8180–89. doi: 10.1523/JNEUROSCI.0340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MS, Kim JS, Chung TS, et al. Measurements of cerebral blood flow in delayed carbon monoxide sequelae using xenon inhalation CT scan. Yonsei Med J. 1988;29:185–92. doi: 10.3349/ymj.1988.29.2.185. [DOI] [PubMed] [Google Scholar]
- 21.Prockop LD, Chichkova RI. Carbon monoxide intoxication: An updated review. Neurol Sci. 2007;262:122–30. doi: 10.1016/j.jns.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Gorman D, Drewry A, Huang YL. The clinical toxicology of carbon monoxide. Toxicol. 2003;187:25–38. doi: 10.1016/s0300-483x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 23.Nabeshima T, Katoh A, Ishim H, et al. Carbon monoxide-induced delayed amnesia, delayed neuronal death and change in acetylcholine concentration in mice. Pharmacol Exp Ther. 1991;256:378–84. [PubMed] [Google Scholar]
- 24.Thom SR, Bhopale VM, Fisher D, et al. D Delayed neuropathology after carbon monoxide poisoning is immune-mediated. Proc Natl Acad Sci USA. 2004;101:13660–65. doi: 10.1073/pnas.0405642101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ischiropoulos H, Beers MF, Ohnishi ST, et al. Nitric oxide production and perivascular nitration in brain after carbon monoxide poisoning in the rat. J Clin Invest. 1996;97:2260–67. doi: 10.1172/JCI118667. [DOI] [PMC free article] [PubMed] [Google Scholar]


