Abstract
About 20 chemical elements are nutritionally essential for humans with defined molecular functions. Several essential and nonessential biometals are either functional nutrients with antidiabetic actions or can be diabetogenic. A key question remains whether changes in the metabolism of biometals and biominerals are a consequence of diabetes or are involved in its etiology. Exploration of the roles of zinc (Zn) in this regard is most revealing because 80 years of scientific discoveries link zinc and diabetes. In pancreatic β- and α-cells, zinc has specific functions in the biochemistry of insulin and glucagon. When zinc ions are secreted during vesicular exocytosis, they have autocrine, paracrine, and endocrine roles. The membrane protein ZnT8 transports zinc ions into the insulin and glucagon granules. ZnT8 has a risk allele that predisposes the majority of humans to developing diabetes. In target tissues, increased availability of zinc enhances the insulin response by inhibiting protein tyrosine phosphatase 1B, which controls the phosphorylation state of the insulin receptor and hence downstream signalling. Inherited diseases of zinc metabolism, environmental exposures that interfere with the control of cellular zinc homeostasis, and nutritional or conditioned zinc deficiency influence the patho-biochemistry of diabetes. Accepting the view that zinc is one of the many factors in multiple gene-environment interactions that cause the functional demise of β-cells generates an immense potential for treating and perhaps preventing diabetes. Personalized nutrition, bioactive food, and pharmaceuticals targeting the control of cellular zinc in precision medicine are among the possible interventions.
Keywords: biometals, zinc, diabetes, pancreas, insulin resistance
INTRODUCTION
A review article on selected trace elements and minerals [magnesium, manganese, iron, zinc (Zn), and chromium] in diabetes recognized (i) profound effects on metabolic processes, (ii) perturbed balances in diabetes and influences on the progression of the disease, and (iii) a need to define the nutritional status (1). The article specifically refrained from discussing whether imbalances of these micronutrients cause diabetes. Significant knowledge on the roles of these and other chemical elements in diabetes has been generated since the article was written more than 30 years ago. Definition of the nutritional status of trace elements and minerals in health and disease is an on-going endeavour and the issue of causation remains largely unresolved. A resolution would have significant potential for preventing and treating metabolic syndrome and diabetes.
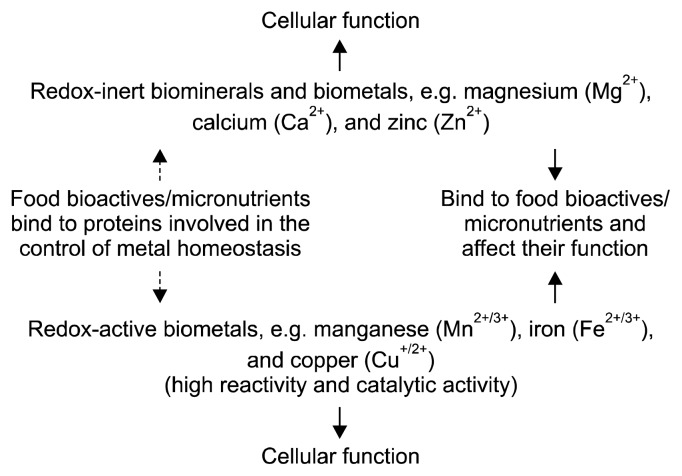
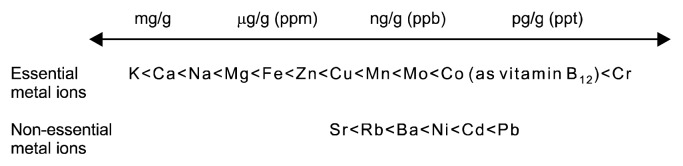
Biometal science is often viewed as a specialized field and largely ignored in many investigations despite of metal ions being omnipresent in food and biochemical reagents, and affecting the outcome of experiments (2). Many bioactive nutrients either bind metal ions or interact with metal/mineral metabolism indirectly by binding to proteins that control metal/mineral homeostasis (Fig. 1). A true understanding of trace metal and mineral metabolism is somewhat hindered by not separating quantity from quality (function). Cellular concentrations and amounts of minerals (a term often used for sodium, potassium, magnesium, and calcium) and trace metals (a term used for the other metals though the concentrations of iron and zinc are rather high and should not be considered as traces) cover many orders of magnitude (Fig. 2). The notion of a “trace” is sometimes understood as being less important or more specific. However, metal ions, even at very low concentrations, have potent effects by binding to macromolecules, being chemically reactive or catalytically active. Some are vitally important as micronutrients for the metabolism of macronutrients. Because “trace” and “ultratrace” are terms used in analytical chemistry, I shall refer to the biologically active metals/ minerals as biometals/biominerals instead. Two issues need to be considered. First, which chemical elements are essential should be stated with reference to a particular organism. For example, vanadium and nickel are not known to be essential for humans though they have well-defined molecular functions in some organisms. Specific organisms acquire metals such as tungsten, cadmium, or even lanthanides for functions. Second, the meaning of the word “essential” should be specified. It is understood here as essential for the survival of an organism. But a wider definition is also employed: the presence of some chemical elements optimizes specific functions without the element being essential for survival (3). Such beneficial functions are documented for chemical elements such as fluorine, boron, or vanadium (4). An unresolved issue is whether or not chromium(III) is essential for humans. Whilst accepted as an essential trace metal in many government guidelines, the premise that it is essential came under scrutiny recently (5). The arguments surrounding this issue are involved and cannot be addressed in this short article. Suffice it to say that we cannot claim that we know the full complement of chemical elements that are essential for humans. A reminder of our lack of knowledge is that a molecular function for bromine in humans was established only two years ago (6).
Fig. 1.
Biometals/biominerals interact with food bioactives and micronutrients. Metal ions bind to molecules affecting the metabolism of both. For those metal ions with high affinity binding, actions can be at very low concentrations (Fig. 2). In addition to the interaction with biomolecules, some free (unbuffered) metal ions have unique chemical reactivities. Bioactive food is rarely screened for metal content and metal binding capacity nor are the effects on control of metal homeostasis addressed.
Fig. 2.
The cellular concentrations of biometals and biominerals. Essential metal ions occur at concentrations that cover many orders of magnitude. Controlled fluctuations make them important regulators of biological function, especially the redox-inert metal ions calcium and zinc as intracellular messengers/signalling ions. Some non-essential metal ions occur at concentrations commensurate with those of essential metal ions. ppm, parts per million; ppb, parts per billion; ppt, part per trillion.
More than 20 chemical elements are essential for life (7). Thus an organic chemistry-centric view of life is insufficient. Inorganic–“not deriving from organic matter”– chemistry is a misnomer when discussing biochemistry, where a considerable amount of metallobiochemistry that is traditionally within the purview of inorganic chemistry is critical for life processes. Not addressing the inorganic aspects of life processes omits a large number of functional micronutrients. Another striking fact is that in addition to the essential chemical elements many other elements are present in human tissues. Some of them, e.g. rubidium, strontium, titanium, and aluminium, occur at higher concentrations than those of some essential elements. Their biological functions are largely unknown. They are not chemically inert. Metals such as cadmium and lead accumulate with age with adverse, but not yet fully understood consequences for our health (8, 9). It would seem prudent to monitor the concentrations of these additional chemical elements in human tissues, in particular the ones that are known to be toxic and to interact with the functions of the essential elements. However, this is done only in exceptional cases when poisoning is suspected. The status of essential chemical elements influences the functions of toxic elements and vice versa. Interactions are multimodal. For example, iron deficiency (anaemia) increases the uptake of cadmium, which then interferes with the functions of zinc. Likewise, essential chemical elements interact. High zinc intake in the diet lowers copper uptake from the diet; high molybdenum in the diet also interferes with copper metabolism, though such molybdenosis is rare in humans.
BIOMETALS IN DIABETES
Some metal ions have antidiabetic functions, e.g. zinc and vanadium at certain concentrations, while others are diabetogenic, e.g. iron under conditions of its overload or cadmium. With few exceptions, there is little insight into how human cells allow or restrict the access of non-essential metal ions to tissues. Cadmium and nickel can be measured in human pancreatic islets. At concentrations corresponding to environmental exposures, cadmium affects functions of murine β-cells (10). While cadmium ions can be transported into cells, human zinc exporters of the ZnT family prevent their export (11), suggesting a possible pathway for accumulation in the cell but also for protection of subcellular compartments such as the dense core insulin granules of β-cells from cadmium toxicity.
There is a rather extensive literature on an altered status of biometals and biominerals in diabetic patients (12). Hypomagnesemia and hypozincemia have been noted repeatedly (13–15). Concentrations in blood plasma, which is the source for most clinical tests, do not necessarily reflect the status of biometals or biominerals in tissues. For example, an increase of zinc in blood cells was noted in insulin-dependent diabetes mellitus (IDDM) patients (16). With regard to zinc, only about 0.1% of total body zinc is in blood and it is not an indicator of zinc status in tissues, for which there is no established biomarker. There are several pathways for re-distribution of metal ions in diabetes. First, infections and inflammation trigger an acute phase response that removes metal ions from the circulatory system. As a result an inverse relationship exists, namely increasing cellular concentrations of some metal ions while decreasing them systemically. Second, oxidative stress affects thiols of cysteines that are involved in the binding of many biometals. The reduced binding capacity of proteins for metal ions increases available metal ion concentrations with either functional effects at sites where metal ions do not normally bind or export of metal ions from the cell. Third, diuresis and compromised kidney function contribute to an overall loss of metal ions. An ensuing deficiency of an essential biometal may be treated by supplementation. However, supplementation does not always restore the metal balance and improve function. Reasons why it can be ineffective involve additional factors, e.g. not treating an underlying oxidative stress or the occurrence of compensatory changes in the control of metal homeostasis through altered gene expression.
This short review focuses on some particular aspects of diabetes related to the functions of insulin and zinc in the pancreas and in target tissues. Diabetes is a complex metabolic disease. In 1936, Himsworth distinguished two forms: type 1 and type 2 (17). Type 1 is an autoimmune disease that destroys the insulin-producing pancreatic islet β-cells resulting in an absolute requirement for insulin (IDDM, mostly juvenile). Type 2 accounts for 90+% of all diabetes cases and was originally adult-onset but is now more frequently diagnosed in younger people. It develops from insulin resistance in peripheral tissues, hence its designation as non-IDDM. When type 2 diabetes progresses the capacity of β-cells to produce insulin is eventually exhausted and the patient also becomes dependent on insulin. Thus impaired insulin secretion from β-cells is critical to the etiology of both types of diabetes. A prediabetic state, metabolic syndrome–defined by the occurrence of three out of five factors, i.e. abdominal obesity, high blood pressure, increased serum triglycerides and fasting plasma glucose, and decreased high-density lipoproteins–can develop into type 2 diabetes. The immense health risks arising from uncontrolled blood glucose (hyperglycemia) are diabetic complications leading to microvascular disease with kidney failure (nephropathy), blindness (retinopathy), nerve damage (neuropathy), macrovascular disease with amputations, cardiovascular disease, and stroke. Diabetes is also a risk factor for developing neurodegenerative diseases (Alzheimer’s and Parkinson’s) and cancer. It has reached epidemic proportions globally, is a major public health issue, and poses a largely unresolved challenge to existing health care systems.
THE SIGNIFICANCE OF ZINC
Zinc receives increasing attention with regard to diabetes in the scientific community. Zinc behaves like a type 2 nutrient, i.e., one that is needed for general metabolism (18). Its importance can be understood in terms of its large number of functions in an estimated 3,000 human proteins affecting almost any aspect of cellular biology (19). Zinc has catalytic, structural, and regulatory functions in proteins. Catalytic functions in about 1,000 human enzymes of all six enzyme classes are used predominantly in hydrolytic reactions and in some committing steps in intermediary metabolism. Zinc has structural functions when it stabilizes global protein structure or organizes local protein domains that interact with other proteins, nucleic acids, or lipids. Regulatory functions have received attention only rather recently when controlled release of zinc ions in the cell and from some cells was observed.
Characteristic fluctuations make zinc a signalling ion on par with calcium in intracellular communication. Zinc ions also participate in intercellular communication. While bioinformatics has provided realistic estimates of the number of proteins with catalytic and structural zinc sites, the number of regulatory sites in proteins is unknown and is expected to increase the protein/zinc interactions beyond the ones in the 3,000 human zinc proteins. Regulatory functions require an additional level of homeostatic control, namely the control of fluctuating zinc ion concentrations. This control is critical for the balance between health and disease. At least three dozen proteins participate in this process: zinc transporters of the ZnT (SLC30A) and Zip (SLC39A) families and cytosolic transport proteins such as metallothioneins (MTs). The realization that such a complex network of proteins is necessary for controlling cellular and subcellular zinc ion concentrations emphasizes the importance of this biometal for cellular function and shifts the attention from zinc in the diet to the many proteins that control zinc.
A remarkable number of mutations in zinc transporters and MTs demonstrate genetic variations in zinc requirements and utilization and are associated with various diseases, including hypertension with reference to metabolic syndrome (20,21). While many of these transporters have specific roles in processes relevant to diabetes in pancreatic islets, I will focus only on one zinc transporter in islet cells, for which an association with diabetes risk is firmly established.
ZINC AND DIABETES
As far back as in the 1930s, autopsies revealed that only about half the amount of zinc is present in the pancreas of diabetic patients compared to a healthy pancreas (22). The involvement of zinc in carbohydrate, lipid, and protein metabolism then established multiple interactions with metabolic disease and diabetes (23,24), which were discussed at a “Zinc and Diabetes” symposium that I convened during the BioMetals 2004 conference and published as key presentations (25). In the following sections, I will discuss some additional aspects that have developed over the last 10 years. They concern the role of zinc in the insulin-producing β-cells and in insulin signal transduction in cells targeted by insulin, and the importance of the general relationship between zinc and redox metabolism for diabetes.
ZINC IN PANCREATIC ISLET PHYSIOLOGY
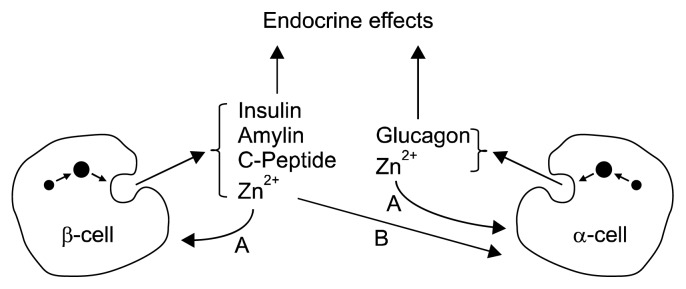
The secretory granules of pancreatic β-cells are a hub of biochemical activity (26). Proteomics studies demonstrate the presence of >600 proteins and low molecular weight compounds such as adenine nucleotides and calcium, zinc, magnesium, and phosphate ions in the granules (27,28). In humans, insulin is stored as a crystalline hexamer containing two zinc ions and one calcium ion. When interacting with a phenolic ligand in vitro, the zinc coordination changes and insulin undergoes an allosteric transition from the T to the R state (29). Whether such ligand interactions occur in vivo is not known. Zinc cosecreted with insulin has an autocrine effect on the β-cell, a paracrine effect on glucagon secretion from α-cells, and possibly an endocrine effect on the liver by inhibiting hepatic insulin clearance (30,31) (Fig. 3). Zinc binding may prevent amyloidogenesis of proteins in and secreted from β-cell granules. In vitro, zinc inhibits the fibrillation of monomeric insulin and the formation of the dimer of the human islet amyloid polypeptide (amylin), which is co-secreted with insulin and can be detected as amyloid fibers in the pancreas of diabetic patients. The dissociation constant of zinc from monomeric insulin is 0.4 μM (32). Zinc interacts with C-peptide and amylin, presumably through binding to Glu-27 of C-peptide (33) and His-18 of the amylin monomer, inhibiting the formation of toxic amylin oligomers (34). Zinc binding does not affect the structural features of the amylin oligomer but binds only to specific classes of oligomers, thus decreasing the number of polymorphic forms (35). C-peptide and amylin form 1:1 heterodimers with insulin, thus removing insulin oligomers and suppressing aggregation (36). Zinc ions localize to the granules of α-cells, where a zinc-specific stain detects them at the membrane (37). Their secretion is thought to have an autocrine effect on α-cells as well (30).
Fig. 3.
Complexity of zinc circuits in pancreatic islet biology. In addition to intracellular functions in about 3,000 proteins, zinc ions have hormone-like functions in islet biology. They are secreted from matured insulin and glucagon vesicles in β- and α-cells by exocytosis and have autocrine functions. Zinc ions secreted from β-cell together with insulin affect glucagon secretion from α-cells (paracrine function) and have been suggested to have an endocrine function on hepatic insulin clearance. A, autocrine effects; B, paracrine effect.
ZnT8, a member of the cation diffusion facilitator family of zinc transporters, SLC30A, transports zinc into the secretory insulin granules of β-cells (38). It is also found in α-cells where it is required for hypoglycemica-induced glucagon secretion (39). ZnT8 is an autoantigen in type 1 diabetes (40). A strong association between a mutation in ZnT8 and type 2 diabetes was noticed by many investigators and investigated extensively in different populations (41). An Arg instead of a Trp at position 325 in the cytoplasmic domain of ZnT8 increases the risk for developing diabetes. The risk allele is the one prevalent in populations (55% in Asians, 75% in Europeans, and 95% in Africans). In addition, rare N-terminal truncations of the protein lead to haploinsufficiency and are associated with a loss-of-function (42). The Arg mutant has higher zinc transport activity (43). It is a matter of intense research efforts with practical implications for improving β-cell function to determine how the two amino acids in the cytoplasmic domain differentially affect zinc transport in the transmembrane domain and how higher zinc transport is associated with a risk for developing diabetes (44).
ZINC IN THE PHYSIOLOGY OF CELLS TARGETED BY INSULIN
Already 50 years ago it was observed that zinc-deficient rats secrete less insulin and have reduced insulin sensitivity (45). Zinc stimulates lipogenesis and glucose uptake in isolated adipocytes (46). In some cultured hybridoma cell lines, zinc can replace insulin in serum-free media (47). The effects of zinc are variously referred to as insulin-mimetic, insulin-like, or insulin-sparing. Effects of zinc are intracellular and resemble the effects of reactive oxygen species, namely inhibiting the dephosphorylation of the insulin/insulin-like growth factor-1 receptor by protein tyrosine phosphatases (PTPs) and maintaining the receptor in an active state for downstream signalling (48,49). Zinc and reactive oxygen species inhibit PTPs by different mechanisms. PTPs are not recognized as metalloenzymes. However, both metal cations and metal oxyanions bind to their active sites and modulate their enzymatic activity. At physiological concentrations of zinc ions, inhibition occurs only when an oxyanion is already bound in the active site and the protein is in the closed conformation with the catalytic aspartate in a position to provide a donor atom for zinc coordination (50). This novel and so far unique mechanism is different from the established redox regulation of PTP activity in the open conformation. Zinc inhibits PTP1B, which controls the phosphorylation state of the insulin and leptin receptors, with an IC50 value of 15 nM (48,51). The inhibition explains why zinc-dependent changes in phosphorylation of proteins in signalling downstream of the insulin receptor have been observed. For example, zinc affects phosphoinositide 3-kinase (PI3K) and protein kinase B (Akt), enhancing glucose uptake (52). Fluctuations of zinc ion concentrations for modulation of PTP activity can be induced by insulin through nicotinamide adenine dinucleotide phosphate oxidase-generated hydrogen peroxide production resulting in zinc release from MT or by zinc release from the endoplasmic reticulum by the zinc channel Zip7 (53,54).
THE ROLE OF ZINC IN REDOX HOMEOSTASIS
A relatively large body of literature describes a role of oxidative stress in developing the complications of uncontrolled diabetes (55). Oxidative stress has a role in β-cell pathophysiology (56) and in insulin resistance in target tissues (57). Increased and decreased cellular zinc concentrations elicit oxidative stress (58). Considering zinc as an antioxidant, however, is chemically not correct because zinc is redox-inert in biology. Observed effects are indirect and depend on the zinc concentrations, making zinc either a pro-antioxidant or a pro-oxidant with a role in redox homeostasis and redox signalling (59,60). Zinc deficiency and zinc overload are pro-oxidant conditions leading to oxidative damage of biomolecules. The pro-antioxidant effects of zinc have been explained with (i) protecting sulfhydryls against oxidation, (ii) competing with redox-active transition metal ions and thereby avoiding the production of damaging free radicals, and (iii) activating metal response element (MRE)-binding transcription factor (MTF)-1 and NF-E2-related factor (Nrf-2) transcription factors and thus expression of antioxidant enzymes, MT, and enzyme(s) of glutathione metabolism. Increased zinc ion concentrations induce the production of free radicals by inhibiting antioxidant enzymes and the mitochondrial respiratory chain. The threshold at which zinc ion concentrations switch from pro-antioxidant/cyto-protective to pro-oxidant/cytotoxic effects depends on the cellular zinc buffering capacity of cells–the ratio between bound zinc and free zinc ions. Oxidative stress decreases the zinc buffering capacity because about one third of this zinc buffering capacity is based on sulfhydryl donors as ligands of zinc. Any chemistry that oxidizes or modifies these sulfhydryls lowers the zinc buffering capacity and increases the availability of free zinc ions. Free zinc ion concentrations are higher in cells from diabetic cardiac cells/tissues (61), decrease under hypoxia in β-cells (62), and double when β-cells are exposed to higher glucose concentrations (63). When free zinc ion concentrations increase zinc can bind to macromolecules/proteins that otherwise would not interact with zinc and affect their functions. Increases of free zinc ion concentrations may lead to a loss of zinc from tissues. Cellular zinc deficiency exacerbates the oxidative stress, increases inflammation, and compromises immunity. Another pathway leading to increased free zinc ion concentrations involves glycation of proteins in diabetic hyperglycemia, decomposition of advanced glycation end products to generate so-called carbonyl stress, and the reaction of carbonyls with sulfhydryl groups and lowering the zinc buffering capacity (64). This reactivity has been shown for MT, a family of at least a dozen human proteins that participate in cellular zinc and redox buffering (65). Their zinc/thiolate coordination environments confer redox-activity on the protein. Oxidation of their cysteine sulfhydryls results in zinc dissociation while reduction of the oxidized sulfhydryls restores the capacity to bind zinc. Accordingly, higher amounts of MT protect the pancreas and other tissues against the chemical stress and hence tissue injury in diabetes (66). A polymorphism of MT1A, an Asn27Thr substitution, which is thought to change the zinc-binding properties of the protein, is associated with type 2 diabetes and coronary heart disease (67).
NUTRITIONAL APPROACHES TO PREVENT AND TREAT DIABETES
Micronutrient deficiencies can be treated by supplementing the micronutrient. Yet, occasional failures to restore the nutritional status through supplementation of single bioactive micronutrients influenced a widespread belief that orthomolecular therapy–a termed coined by Pauling (68)– is ineffective. Accordingly biometals in diabetes have received limited attention. Biometals such as zinc have central roles in both islet biology and insulin sensitivity of peripheral tissues. Our thinking about how to approach metal deficiencies and overloads in diabetes is too simplistic. A lack of understanding of the consequences of genetic variability and of suitable biomarkers means that we cannot reliably determine the requirement for supplementation. Not the underlying biochemistry but the particular interventions are at issue. Zinc supplementation may not restore control of zinc homeostasis under conditions of sustained oxidative stress. In fact, additional zinc may harm rather than benefit because it binds to proteins that are normally not targeted if control of redox homeostasis is compromised. Restoration of proper redox homeostasis is not straightforward as redox signalling is necessary in cells, in particular in cells with specific requirements such as β-cells that rely on glucose-stimulated mitochondrial adenosine triphosphate generation for insulin release. The risks and benefits of zinc supplementation need to be evaluated carefully with not only zinc itself in mind but also the factors that affect its proper usage in tissues (69).
Zinc supplementation has insulin-enhancing effects, lowers blood glucose, and improves β-cell function in preclinical investigations. There is ample evidence for zinc compounds having antidiabetogenic and insulinomimetic properties (70,71). In humans, zinc supplementation alleviates markers of oxidative stress (72). Yet, despite all of these insights, including urinary loss of zinc in diabetic patients, and some positive effects of zinc supplementation in zinc-deficient humans, epidemiological studies and systematic reviews of prospective cohort studies are inconclusive as to whether zinc supplementation prevents type 2 diabetes (73–75). The consensus is, however, that more well-designed intervention studies are needed to evaluate this important aspect of public health.
Novel approaches to prevent and treat diabetes are direly needed. Targeting the proteins that control systemic and cellular homeostasis of biometals and biominerals is an avenue worth pursuing. The zinc/diabetes interactions described here have far reaching implications for prevention and treatment of metabolic disease in settings such as primary zinc deficiency in developing countries and in specific populations such as the elderly, changes in zinc metabolism in genetically predisposed individuals, and environmental exposures that lead to perturbations of zinc homeostasis.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The author declares no conflict of interest.
REFERENCES
- 1.Mertz W. Trace elements and minerals in diabetes. In: Brodoff BN, Bleicher SJ, editors. Diabetes Mellitus and Obesity. Williams & Wilkins; Baltimore, MD, USA: 1982. pp. 343–348. [Google Scholar]
- 2.Maret W. Metallomics: a primer of integrated biometal sciences. Imperial College Press; London, UK: 2016. [DOI] [Google Scholar]
- 3.Mertz W. The essential trace elements. Science. 1981;213:1332–1338. doi: 10.1126/science.7022654. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen FH. Should bioactive trace elements not recognized as essential, but with beneficial health effects, have intake recommendations. J Trace Elem Med Biol. 2014;28:406–408. doi: 10.1016/j.jtemb.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JB. The bioinorganic chemistry of chromium. John Wiley & Sons, Inc; Chichester, West Sussex, UK: 2013. [Google Scholar]
- 6.McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, Hudson BG. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell. 2014;157:1380–1392. doi: 10.1016/j.cell.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maret W. The metals in the biological periodic system of the elements: concepts and conjectures. Int J Mol Sci. 2016;17:66. doi: 10.3390/ijms17010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maret W, Moulis JM. The bioinorganic chemistry of cadmium in the context of its toxicity. In: Sigel A, Sigel H, Sigel RKO, editors. Metal Ions in Life Sciences. Vol. 11. Springer Science+ Business Media BV; Dordrecht, The Netherlands: 2013. pp. 1–29. [DOI] [PubMed] [Google Scholar]
- 9.Maret W. The bioinorganic chemistry of lead in the context of its toxicity. In: Sigel A, Sigel H, Sigel RKO, editors. Metal Ions in Life Sciences. Vol. 17. De Gruyter; Berlin, Germany: 2017. in press. [DOI] [PubMed] [Google Scholar]
- 10.El Muayed M, Raja MR, Zhang X, MacRenaris KW, Bhatt S, Chen X, Urbanek M, O'Halloran TV, Lowe WL., Jr Accumulation of cadmium in insulin-producing β cells. Islets. 2012;4:405–416. doi: 10.4161/isl.23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoch E, Lin W, Chai J, Hershfinkel M, Fu D, Sekler I. Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc Natl Acad Sci USA. 2012;109:7202–7207. doi: 10.1073/pnas.1200362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui K, Bawazeer N, Joy SS. Variation in macro and trace elements in progression of type 2 diabetes. Sci World J. 2014;2014:461591. doi: 10.1155/2014/461591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tosiello L. Hypomagnesemia and diabetes mellitus: a review of clinical implications. Arch Intern Med. 1996;156:1143–1148. doi: 10.1001/archinte.1996.00440100029005. [DOI] [PubMed] [Google Scholar]
- 14.Salgueiro MJ, Krebs N, Zubillaga MB, Weill R, Postaire E, Lysionek AE, Caro RA, De Paoli T, Hager A, Boccio J. Zinc and diabetes mellitus: is there a need of zinc supplementation in diabetes mellitus patients? Biol Trace Elem Res. 2001;81:215–228. doi: 10.1385/BTER:81:3:215. [DOI] [PubMed] [Google Scholar]
- 15.Praveeena S, Pasula S, Sameera K. Trace elements in diabetes mellitus. J Clin Diagn Res. 2013;7:1863–1865. doi: 10.7860/JCDR/2013/5464.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruse-Jarres JD, Rückgauer M. Trace elements in diabetes mellitus. Peculiarities and clinical validity of determinations in blood cells. J Trace Elem Med Biol. 2000;14:21–27. doi: 10.1016/S0946-672X(00)80019-X. [DOI] [PubMed] [Google Scholar]
- 17.Himsworth HP. Diabetes mellitus: its differentiation into insulin-sensitive and insulin-insensitive types. The Lancet. 1936;227:127–130. doi: 10.1016/S0140-6736(01)36134-2. [DOI] [Google Scholar]
- 18.King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr. 2011;94:679S–684S. doi: 10.3945/ajcn.110.005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogstrand C, Maret W. eLS: Essential for Life Science. John Wiley & Sons, Inc; Chichester, West Sussex, UK: 2016. Genetics of human zinc deficiencies; pp. 1–8. [DOI] [Google Scholar]
- 21.Raudenska M, Gumulec J, Podlaha O, Sztalmachova M, Babula P, Eckschlager T, Adam V, Kizek R, Masarik M. Metallothionein polymorphisms in pathological processes. Metallomics. 2014;6:55–68. doi: 10.1039/C3MT00132F. [DOI] [PubMed] [Google Scholar]
- 22.Scott DA, Fisher AM. The insulin and the zinc content of normal and diabetic pancreas. J Clin Invest. 1938;17:725–728. doi: 10.1172/JCI101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr. 1998;17:109–115. doi: 10.1080/07315724.1998.10718735. [DOI] [PubMed] [Google Scholar]
- 24.Tallman DL, Taylor CG. Potential interactions of zinc in the neuroendocrine-endocrine disturbances of diabetes mellitus type 2. Can J Physiol Pharmacol. 1999;77:919–933. doi: 10.1139/y99-111. [DOI] [PubMed] [Google Scholar]
- 25.Maret W. Zinc and diabetes. Biometals. 2005;18:293–294. doi: 10.1007/s10534-005-3684-z. [DOI] [Google Scholar]
- 26.Suckale J, Solimena M. The insulin secretory granule as a signaling hub. Trends Endocrinol Metab. 2010;21:599–609. doi: 10.1016/j.tem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Schvartz D, Brunner Y, Couté Y, Foti M, Wollheim CB, Sanchez JC. Improved characterization of the insulin secretory granule proteomes. J Proteomics. 2012;75:4620–4631. doi: 10.1016/j.jprot.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Hutton JC, Penn EJ, Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983;210:297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunn MF. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer–a review. Biometals. 2005;18:295–303. doi: 10.1007/s10534-005-3685-y. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara H, Wollheim CB. Is zinc an intra-islet regulator of glucagon secretion? Diabetol Int. 2016;7:106–110. doi: 10.1007/s13340-016-0259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamaki M, Fujitani Y, Hara A, Uchida T, Tamura Y, Takeno K, Kawaguchi M, Watanabe T, Ogihara T, Fukunaka A, Shimizu T, Mita T, Kanazawa A, Imaizumi MO, Abe T, Kiyonari H, Hojyo S, Fukada T, Kawauchi T, Nagamatsu S, Hirano T, Kawamori R, Watada H. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest. 2013;123:4513–4524. doi: 10.1172/JCI68807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gavrilova J, Tõugu V, Palumaa P. Affinity of zinc and copper ions for insulin monomers. Metallomics. 2014;6:1296–1300. doi: 10.1039/C4MT00059E. [DOI] [PubMed] [Google Scholar]
- 33.Keltner Z, Meyer JA, Johnson EM, Palumbo AM, Spence DM, Reid GE. Mass spectrometric characterization and activity of zinc-activated proinsulin C-peptide and C-peptide mutants. Analyst. 2010;135:278–288. doi: 10.1039/B917600D. [DOI] [PubMed] [Google Scholar]
- 34.Brender JR, Hartman K, Nanga RPR, Popovych N, de la Salud Bea R, Vivekanandan S, Marsh ENG, Ramamoorthy A. Role of zinc in human islet amyloid polypeptide aggregation. J Am Chem Soc. 2010;132:8973–8983. doi: 10.1021/ja1007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wineman-Fisher V, Miller Y. Effect of Zn2+ ions on the assembly of amylin oligomers: insight into the molecular mechanisms. Phys Chem Chem Phys. 2016;18:21590–21599. doi: 10.1039/C6CP04105A. [DOI] [PubMed] [Google Scholar]
- 36.Landreh M, Alvelius G, Johansson J, Jörnvall H. Insulin, islet amyloid polypeptide and C-peptide interactions evaluated by mass spectrometric analysis. Rapid Commun Mass Spectrom. 2014;28:178–184. doi: 10.1002/rcm.6772. [DOI] [PubMed] [Google Scholar]
- 37.Egefjord L, Bak AM, Petersen AB, Rungby J. Zinc, alpha cells and glucagon secretion. Curr Diabetes Rev. 2010;6:52–57. doi: 10.2174/157339910790442655. [DOI] [PubMed] [Google Scholar]
- 38.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a β-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–2337. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 39.Solomou A, Meur G, Bellomo E, Hodson DJ, Tomas A, Li SM, Philippe E, Herrera PL, Magnan C, Rutter GA. The zinc transporter Slc30a8/ZnT8 is required in a subpopulation of pancreatic α-cells for hypoglycemia-induced glucagon secretion. J Biol Chem. 2015;290:21432–21442. doi: 10.1074/jbc.M115.645291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson HW, Wenzlau JM, O’Brien RM. Zinc transporter 8 (ZnT8) and β cell function. Trends Endocrinol Metab. 2014;25:415–424. doi: 10.1016/j.tem.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flannick J, Thorleifsson G, Beer NL, Jacobs SB, Grarup N, Burtt NP, Mahajan A, Fuchsberger C, Atzmon G, Benediktsson R, Blangero J, Bowden DW, Brandslund I, Brosnan J, Burslem F, Chambers J, Cho YS, Christensen C, Douglas DA, Duggirala R, Dymek Z, Farjoun Y, Fennell T, Fontanillas P, Forsén T, Gabriel S, Glaser B, Gudbjartsson DF, Hanis C, Hansen T, Hreidarsson AB, Hveem K, Ingelsson E, Isomaa B, Johansson S, Jørgensen T, Jørgensen ME, Kathiresan S, Kong A, Kooner J, Kravic J, Laakso M, Lee JY, Lind L, Lindgren CM, Linneberg A, Masson G, Meitinger T, Mohlke KL, Molven A, Morris AP, Potluri S, Rauramaa R, Ribel-Madsen R, Richard AM, Rolph T, Salomaa V, Segrè AV, Skärstrand H, Steinthorsdottir V, Stringham HM, Sulem P, Tai ES, Teo YY, Teslovich T, Thorsteinsdottir U, Trimmer JK, Tuomi T, Tuomilehto J, Vaziri-Sani F, Voight BF, Wilson JG, Boehnke M, McCarthy MI, Njølstad PR, Pedersen O Go-T2D Consortium; T2D-GENES Consortium. Groop L, Cox DR, Stefansson K, Altshuler D. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet. 2014;46:357–363. doi: 10.1038/ng.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merriman C, Huang Q, Rutter GA, Fu D. Lipid-tuned zinc transport activity of human ZnT8 protein correlates with risk for type-2 diabetes. J Biol Chem. 2016;291:26950–26957. doi: 10.1074/jbc.M116.764605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutter GA, Chimienti F. SLC30A8 mutations in type 2 diabetes. Diabetologia. 2015;58:31–36. doi: 10.1007/s00125-014-3405-7. [DOI] [PubMed] [Google Scholar]
- 45.Quarterman J, Mills CF, Humphries WR. The reduced secretion of, and sensitivity to insulin in zinc-deficient rats. Biochem Biophys Res Commun. 1966;25:354–358. doi: 10.1016/0006-291X(66)90785-6. [DOI] [PubMed] [Google Scholar]
- 46.Coulston L, Dandona P. Insulin-like effect of zinc on adipocytes. Diabetes. 1980;29:665–667. doi: 10.2337/diab.29.8.665. [DOI] [PubMed] [Google Scholar]
- 47.Wong VV, Nissom PM, Sim SL, Yeo JH, Chuah SH, Yap MGS. Zinc as an insulin replacement in hybridoma cultures. Biotechnol Bioeng. 2005;93:553–563. doi: 10.1002/bit.20746. [DOI] [PubMed] [Google Scholar]
- 48.Haase H, Maret W. Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin- like growth factor-1 signaling. Exp Cell Res. 2003;291:289–298. doi: 10.1016/S0014-4827(03)00406-3. [DOI] [PubMed] [Google Scholar]
- 49.Haase H, Maret W. Protein tyrosine phosphatases as targets of the combined insulinomimetic effects of zinc and oxidants. Biometals. 2005;18:333–338. doi: 10.1007/s10534-005-3707-9. [DOI] [PubMed] [Google Scholar]
- 50.Bellomo E, Massarotti A, Hogstrand C, Maret W. Zinc ions modulate protein tyrosine phosphatase 1B activity. Metallomics. 2014;6:1229–1239. doi: 10.1039/C4MT00086B. [DOI] [PubMed] [Google Scholar]
- 51.Bellomo E, Singh KB, Massarotti A, Hogstrand C, Maret W. The metal face of protein tyrosine phosphatase 1B. Coord Chem Rev. 2016;327–328:70–83. doi: 10.1016/j.ccr.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang X, Shay NF. Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. J Nutr. 2001;131:1414–1420. doi: 10.1093/jn/131.5.1414. [DOI] [PubMed] [Google Scholar]
- 53.Taylor KM, Hiscox S, Nicholson RI, Hogstrand C, Kille P. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci Signal. 2012;5:ra11. doi: 10.1126/scisignal.2002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 56.Gerber PA, Rutter GA. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 58.Maret W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid Redox Signal. 2006;8:1419–1441. doi: 10.1089/ars.2006.8.1419. [DOI] [PubMed] [Google Scholar]
- 59.Maret W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp Gerontol. 2008;43:363–369. doi: 10.1016/j.exger.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Oteiza PI. Zinc and the modulation of redox homeostasis. Free Radic Biol Med. 2012;53:1748–1759. doi: 10.1016/j.freeradbiomed.2012.08.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayaz M, Turan B. Selenium prevents diabetes-induced alterations in [Zn2+]i and metallothionein level of rat heart via restoration of cell redox cycle. Am J Physiol Heart Circ Physiol. 2006;290:H1071–H1080. doi: 10.1152/ajpheart.00754.2005. [DOI] [PubMed] [Google Scholar]
- 62.Gerber PA, Bellomo EA, Hodson DJ, Meur G, Solomou A, Mitchell RK, Hollinshead M, Chimienti F, Bosco D, Hughes SJ, Johnson PR, Rutter GA. Hypoxia lowers SLC30A8/ ZnT8 expression and free cytosolic Zn2+ in pancreatic beta cells. Diabetologia. 2014;57:1635–1644. doi: 10.1007/s00125-014-3266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chabosseau P, Rutter GA. Zinc and diabetes. Arch Biochem Biophys. 2016;611:79–85. doi: 10.1016/j.abb.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 64.Hao Q, Maret W. Aldehydes release zinc from proteins. A pathway from oxidative stress/lipid peroxidation to cellular functions of zinc. FEBS J. 2006;273:4300–4310. doi: 10.1111/j.1742-4658.2006.05428.x. [DOI] [PubMed] [Google Scholar]
- 65.Maret W. Redox biochemistry of mammalian metallothioneins. J Biol Inorg Chem. 2011;16:1079–1086. doi: 10.1007/s00775-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 66.Li X, Cai L, Feng W. Diabetes and metallothionein. Mini Rev Med Chem. 2007;7:761–768. doi: 10.2174/138955707781024490. [DOI] [PubMed] [Google Scholar]
- 67.Giacconi R, Bonfigli AR, Testa R, Sirolla C, Cipriano C, Marra M, Muti E, Malavolta M, Costarelli L, Piacenza F, Tesei S, Mocchegiani E. +647 A/C and +1245 MT1A polymorphisms in the susceptibility of diabetes mellitus and cardiovascular complications. Mol Genet Metab. 2008;94:98–104. doi: 10.1016/j.ymgme.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 68.Pauling L. Orthomolecular psychiatry. Varying the concentrations of substances normally present in the human body may control mental disease. Science. 1968;160:265–271. doi: 10.1126/science.160.3825.265. [DOI] [PubMed] [Google Scholar]
- 69.Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 70.Sakurai H, Adachi Y. The pharmacology of the insulinomimetic effect of zinc complexes. Biometals. 2005;18:319–323. doi: 10.1007/s10534-005-3688-8. [DOI] [PubMed] [Google Scholar]
- 71.Vardatsikos G, Pandey NR, Srivastava AK. Insulinomimetic and anti-diabetic effects of zinc. J Inorg Biochem. 2013;120:8–17. doi: 10.1016/j.jinorgbio.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Bao B, Prasad AS, Beck FW, Fitzgerald JT, Snell D, Bao GW, Singh T, Cardozo LJ. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am J Clin Nutr. 2010;91:1634–1641. doi: 10.3945/ajcn.2009.28836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El Dib R, Gameiro OL, Ogata MS, Módolo NS, Braz LG, Jorge EC, do Nascimento P, Jr, Beletate V. Zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance. Cochrane Database Syst Rev. 2015;5:CD005525. doi: 10.1002/14651858.CD005525.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chu A, Foster M, Samman S. Zinc status and risk of cardiovascular diseases and type 2 diabetes mellitus–a systematic review of prospective cohort studies. Nutrients. 2016;8:707. doi: 10.3390/nu8110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruz M, Carrasco F, Sánchez A, Perez A, Rojas P. Does zinc really “metal” with diabetes? The epidemiologic evidence. Curr Diab Rep. 2016;16:111. doi: 10.1007/s11892-016-0803-x. [DOI] [PubMed] [Google Scholar]