Abstract
The major trend in the antioxidant market is the growing consumer demand for natural antioxidants. Tea, one of the most widely consumed beverages in the world, is an easy way to obtain antioxidant components from a natural source. Our objective was to develop burdock root tea (BRT) with potent antioxidant activity and good color quality. In order to obtain maximum antioxidant activity and quality, the effect of roasting was determined. The antioxidant capacities and total phenolic contents of BRT increased as roasting increased. The color of BRT became darker with increased roasting, extraction time, and amount of burdock roots. Color of BRT was also positively correlated with total antioxidant capacity. Roasting significantly enhanced the total antioxidant activities and color quality of BRT. These results suggest that roasting BRT increases beneficial antioxidant components from burdock roots.
Keywords: burdock root tea, roasting, total antioxidant capacity, color quality
INTRODUCTION
Burdock (Arctium lappa L.) belongs to the genus of Arctium in the family Asteraceae. It has long been cultivated in Eastern Asian countries, particularly in China, Japan, and Korea (1). It has been used as a root vegetable or a traditional medicinal plant for centuries and remains popular today. Burdock has gradually achieved international recognition for its culinary use due to its nutritional values and health benefits. In traditional medicine, leaves, seeds, and roots of burdock have been used to treat diabetes, inflammatory diseases, and cancer, due to their effects on the immune system and metabolic functions (2–7). Among the parts of the burdock plant, roots are commonly consumed as a side dish and in the form of tea in Korea. Many studies also suggest that burdock roots possess antioxidative, anti-inflammatory, and anti-cancer activities (8–10). These biological activities are attributed to the presence of polyphenols as well as dietary fiber such as inulin and lignan in burdock roots (1,3).
Tea is one of the most popular beverages consumed worldwide and is mainly consumed in Asian and European countries (11). Tea consumption has gradually increased in many countries as reported by the Annual Bulletin of Statistics from International Tea Committee (12). The international trend of increased tea intake is possibly due to the widely reported health benefits of tea (13). Drinking tea has been considered to be health-promoting since ancient times (14,15). In addition, modern medicinal research is continuously providing scientific evidence of the beneficial components in tea (16). Many cohort studies and meta-analyses show that tea intake is positively correlated with lowered risk of diseases such as cancer, cardiovascular disease, hyperlipidemia, diabetes, and obesity (17–20). Polyphenols in tea are responsible for reducing the risk of these diseases via antioxidative, anti-inflammatory, anti-carcinogenic, anti-genotoxic, and cholesterol-lowering activities (21,22). A previous study reported that consumption of over two cups of tea per day resulted in approximately 2.6-fold higher blood polyphenol concentrations than persons consuming less than half cup of tea per day (23). It has also been reported that one cup of black tea contains about 150~200 mg of flavonoids, constituting up to 82% of the total dietary intake of flavonoids (24).
The traditional antioxidant market has shifted toward whole food-based antioxidant use (25). Whole foods containing a combination of multiple polyphenols exhibit strong biological activities due to additive and/or synergistic actions of bioactive components (26). Therefore, it is of interest to identify potent whole food-based antioxidants. Moreover, tea is the easiest way to consume health promoting components from whole foods (27). For this reason, burdock root tea is a promising candidate as whole food-based antioxidant. However, scientific evidence of burdock root tea (BRT) as a whole food-based antioxidant use is lacking.
Recent studies have shown that the roasting of plant-based foods augments biological activates. Heat treatment improves the antioxidant activity because thermal process helps to release bound polyphenols from cell walls (28) and to protect degradation of antioxidant components by the inactivation of endogenous oxidative enzymes (29). In addition, the roasting process produces Maillard reaction products, which are known to possess antioxidant activity (30). Therefore, this study aimed to elucidate the role of roasting in the antioxidant activity of BRT and to determine the optimal conditions for BRT preparation to extract maximum antioxidant activities from burdock roots.
MATERIALS AND METHODS
Materials
Potassium persulfate was purchased from Junsei Chemical (Tokyo, Japan). Pyrocatechol violet (PV) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). All other chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Preparation of roasted burdock roots
Burdock roots were purchased from local markets in Gyeongsan, Korea. Burdock roots were washed and sliced to a thickness of 2 mm. To prevent enzymatic browning of burdock roots, the sliced burdock roots were steamed for 10 min using a home steamer and dried at 60°C for 6 h using a convection oven. Dried burdocks were roasted at 170°C for 5 min and then cooled to room temperature. This roasting procedure was repeated three or six times. Since roasting over six times resulted in burned burdock roots, we decided to roast them up to a maximum of six times. Water content of the dried roasted burdock roots was about 25.3%. Roasted burdocks were stored at 4°C until use.
Preparation of burdock root tea and burdock methanol extract
Since methanol is frequently used to extract bioactive compounds from plant materials, and antioxidant activity of burdock methanol extract has been reported, it was of interest to compare antioxidant activates of methanol extracts with those of water extracts. In order to compare total antioxidant capacity, BRT and burdock root methanol extract were produced from burdock roots (1.00 mg/mL) roasted six times. Roasted burdock roots were heated in water for 60 min at 100°C to produce BRT. Roasted burdock roots were extracted in methanol for 24 h at 30°C to prepare burdock methanol extract. Conditions for BRT preparation were varied in terms of roasting number, extraction time, and concentration of roasted burdock roots. As described above, burdock roots were roasted three or six times. Unroasted and roasted burdock roots (1.00 mg/mL) were heated in distilled water for 30 min or 60 min at 100°C. BRT was also produced with various concentrations of burdock roots (0.10, 0.25, 0.50, and 1.00 mg/mL). Burdock roots roasted six times at different concentrations were heated in distilled water for 60 min at 100°C. After BRT was extracted according to the conditions described above, extracts were centrifuged at 1,500 g for 20 min at 4°C. Supernatants were collected, filtered, and used as either burdock methanol extracts or BRT. Yield was calculated based on percent yield [Eq. (1)].
| (1) |
BRT color measurement
The color of tea is one of the most important product qualities for consumers. It is believed that conditions for BRT preparation affect the color of BRT. In order to evaluate the effects of roasting and extraction time on the color of BRT, the color of BRT prepared by changing roasting times (0, 3, and 6 times) and extraction times (30 min and 60 min) was determined by colorimeter. Moreover, the effect of the concentration of burdock roots on the color of BRT was tested only in BRT extracted from six-time roasted burdock roots for 60 min. The color of BRT was measured using a Minolta colorimeter (CR-300, Konica Minolta, Inc., Tokyo, Japan) at room temperature to determine Hunter L*, a*, and b* values. The Hunter L* value is experimentally confirmed psychometric lightness, whereas Hunter a* values indicate greenness and redness, and Hunter b* values determine blueness and yellowness. Hunter L*, a*, and b* values were utilized to estimate total color difference [ΔE: Eq. (2)] and chroma [C value: Eq. (3)]. The standards were BRT extracted from unroasted burdock roots (1.00 mg/mL) for 30 min.
| (2) |
| (3) |
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
The DPPH assay was used to measure the radical scavenging activity of BRT. Briefly, 100 μL of 0.02% DPPH solution in methanol was added to a 96-well plate containing 50 μL of test samples at different concentrations. After a 30 min incubation period in the dark, the absorbance at 510 nm was measured using a microplate spectrophotometer (Epoch, BioTek, Winooski, VT, USA). The results were calculated based on the standard curve of ascorbic acid and expressed as an ascorbic acid equivalent value (mg AA/mL).
ABTS radical scavenging activity
The ability of BRT to scavenge ABTS radical cation was determined using the ABTS assay conducted as described by Re et al. (31). ABTS solution was freshly prepared by mixing 7 mM ABTS stock solution with 2.6 mM potassium persulfate in equal quantities and reacting for 16 h at room temperature in the dark. ABTS solution was then diluted to obtain an absorbance of 0.7±0.02 units at 734 nm using a spectrophotometer. Samples (5 μL) were allowed to react with 295 μL of ABTS solution for 6 min in the dark. The absorbance was then taken at 734 nm using a microplate spectrophotometer (Epoch, BioTek) and converted to Trolox equivalents (TE) values based on the standard curve of Trolox. The standard curve of Trolox was linear between 0 and 1 mM in concentration. Results are expressed in mg TE/mL.
Ferric reducing antioxidant power (FRAP)
The FRAP assay was used since it is widely suitable for antioxidants. In this assay, FRAP is determined by reduction of FeIII+ to FeII+ due to the action of antioxidants (32). The stock solutions consisted of 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) solution in 40 mM HCl, and 20 mM FeCl3·6H2O solution. The fresh working solution was prepared by mixing 25 mL of acetate buffer, 2.5 mL of TPTZ solution, and 2.5 mL of FeCl3·6H2O solution, followed by incubation at 37°C before use. Extracts (10 μL) were allowed to react with 100 μL of FRAP solution for 4 min in the dark. The colored product, ferrous tripyridyltriazine complex, was read at 593 nm using a microplate spectrophotometer (Epoch, BioTek). Ferrous sulfate was used as a positive control.
Copper chelating activity
Copper (II) ion chelating activity was determined using a modified Megías method (33). Briefly, 30 μL of extract, 290 μL of 50 mM sodium acetate buffer (pH 6.0), 10 μL of copper sulfate (2 mg/mL), and 4 mM PV were loaded into a 96-well plate. The absorbance was measured at 632 nm using a microplate spectrophotometer (Epoch, BioTek). Copper chelating activity was calculated based on the following equation [Eq. (4)].
| (4) |
Determination of total phenol content
Total phenolic content was determined by the Folin-Ciocalteu method as described by Singleton et al. (34). Extract (10 μL) was mixed with 20 μL of 10% (v/v) Folin-Ciocalteu reagent in a 96-well plate. The mixture was allowed to react for 6 min, after which 80 μL of 700 mM Na2CO3 was added to the mixture. After 60 min of incubation at room temperature in the dark, absorbance of the reaction mixture was measured at 750 nm using a spectrophotometer. The results were expressed in gallic acid equivalents (GAE) using the standard curve of gallic acid (0~1 mg/mL).
Measurement of flavonoid content
The flavonoid content was measured by the aluminum chloride colorimetric method. A mixture of 25 μL of extract, 125 μL of distilled water, and 10 μL of 5% sodium nitrite solution was incubated for 6 min, and 15 μL 10% (w/v) aluminum chloride was added. After 5 min of incubation, 50 μL of 1 M sodium hydroxide and 125 μL of distilled water were added to the mixture. The absorbance was measured at 510 nm using a multilevel plate reader (VICTORTM X, PerkinElmer, Inc., San Jose, CA, USA), and the results were expressed in catechin equivalents (CE) using the standard curve of catechin (0~1 mg/mL).
Statistical analysis
All data were collected from six independent experiments performed in triplicate and expressed as the mean±standard deviation (SD). Statistically significant differences among treatments were obtained using the SAS program (version 9.4, SAS Institute, Cary, NC, USA). Statistical comparisons were performed via one-way analysis of variance, followed by Bonferroni’s multiple comparison test. Differences at P<0.05 were considered to be significant. Correlations between the evaluated parameters were obtained using Pearson’s correlation coefficient (r).
RESULTS AND DISCUSSION
Comparison of antioxidant activities of burdock root tea and burdock root methanol extracts
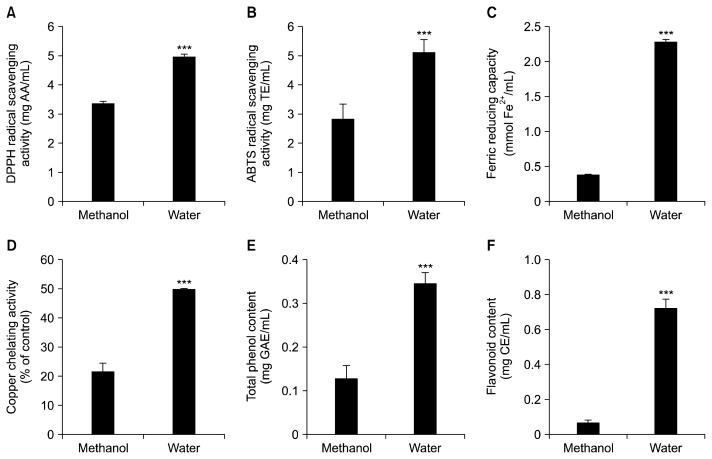
Although the antioxidant activity of burdock roots has been reported before, most studies tested the antioxidant activity of burdock root methanol extracts. Since methanol extracts cannot be consumed, it is useful to elucidate the antioxidant ability of BRT, which is a whole food directly consumed by humans. Therefore, the antioxidant activity of BRT was compared with that of burdock root methanol extract. Results from DPPH and ABTS radical scavenging activity assays show that BRT had approximately 1.5-fold higher antioxidant capacities compared with methanol extract of burdock roots (Fig. 1A and 1B). Ferric reducing and copper chelating activities of BRT were about 5-fold and 2.5-fold higher than those of burdock methanol extracts, respectively (Fig. 1C and 1D). Consistent with these data on antioxidant activities, total phenol and flavonoid contents of BRT were significantly higher than those of burdock root methanol extract (Fig. 1E and 1F). Overall, BRT showed strong total antioxidant capacities compared with methanol extract of burdock roots.
Fig. 1.
Comparison of antioxidant activities of burdock root tea (BRT) and burdock methanol extract. BRT was produced by boiling burdock roots (1.00 mg/mL) roasted six times in water at 100°C for 60 min. Methanol extract of burdock roots was prepared by extracting burdock roots (1.00 mg/mL) roasted six times in methanol at 30°C for 24 h. Burdock root extracts were subjected to total antioxidant capacity assays, including 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity (A), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity (B), ferric reducing capacity (C), copper chelating activity (D), total polyphenol contents (E), and flavonoid contents (F). Values are expressed as the mean±standard deviation (n=6). ***P<0.001 versus methanol extract. AA, ascorbic acid equivalent; TE, Trolox equivalents; GAE, gallic acid equivalents; CE, catechin equivalents.
Our data indicate that hot water extraction for BRT preparation may be a more effective method to obtain bioactive components from burdock roots than methanol extraction. The higher total phenol and flavonoid contents of BRT compared to methanol extract contributed stronger antioxidant activities. High temperature increases the liberating of polyphenols and flavonoids, the major bioactive components exhibiting antioxidant activities (35,36). Overall, these results suggest that BRT consumption is a good whole food-based antioxidant dietary approach.
Yield of BRT
The yield of BRT under various preparation conditions is shown in Table 1. Conditions of BRT preparation varied in terms of roasting number, extraction time, and ratio between water and burdock roots. Extraction time was a major parameter affecting the yield of BRT. When BRT was extracted for 30 min, yields of BRT were approximately 70%. The yields of BRT decreased with increasing extraction time, and the yields of BRT extracted for 60 min ranged from 36.63±1.40% to 59.00±8.49%.
Table 1.
Extraction yield of burdock root tea using various conditions
| Number of roasting | Extraction time (min) | Concentration (mg/mL) | Yield (%) |
|---|---|---|---|
| 0 | 30 | 1.00 | 71.78±7.70 |
| 3 | 30 | 1.00 | 68.81±10.50 |
| 6 | 30 | 1.00 | 68.32±8.40 |
| 0 | 60 | 1.00 | 44.55±0.10 |
| 3 | 60 | 1.00 | 36.63±1.40 |
| 6 | 60 | 1.00 | 40.97±0.74 |
| 6 | 60 | 0.10 | 59.00±8.49 |
| 6 | 60 | 0.25 | 42.50±16.26 |
| 6 | 60 | 0.50 | 51.50±2.12 |
Values are mean±SD of six separate experiments.
Effects of roasting, extraction time, and concentration of burdock roots on color quality of BRT
In order to elucidate the effects of tea preparation parameters on the color of BRT, BRT was prepared according to different roasting numbers and tea extraction conditions, such as extraction temperature, time, and concentration, and then subjected to Hunter’s colorimetry. The roasting number, extraction time, and amount of burdock roots influenced color quality of BRT (Table 2). A higher roasting number and/or longer extraction time of BRT were associated with a darker color. BRT from burdock roots roasted six times for 30 min and 60 min showed ΔE values of 3.31±1.39 and 11.38±1.07, respectively. This indicates that the ΔE between BRT made from unroasted burdock roots and burdock roots roasted six times is clearly distinguishable since the human eye can conceive noticeable differences in color when ΔE values are over 2.3 (37). Moreover, Hunter b* values of BRT increased in a dose-dependent manner. Extraction temperature of BRT also impacted the color quality. ΔE Hunter values of BRT were temperature-dependent (data not shown).
Table 2.
Color characteristics of burdock root tea prepared by different conditions of extraction
| Number of roasting | Extraction time (min) | Concentration (mg/mL) | L* | a* | b* | ΔE | C |
|---|---|---|---|---|---|---|---|
| 0 | 30 | 1.00 | 55.53±0.20ns | 0.54±0.06a | −1.44±0.43c | 0±0.59g | 1.60±0.33d |
| 3 | 30 | 1.00 | 55.42±0.08 | 0.35±0.16bc | −0.95±0.05c | 0.55±0.16f | 1.01±0.07f |
| 6 | 30 | 1.00 | 53.98±1.06 | −0.09±0.01d | 4.33±1.30b | 3.31±1.39c | 4.71±1.13c |
| 0 | 60 | 1.00 | 55.20±0.47 | 0.12±0.03d | −0.26±0.13c | 1.29±0.24d | 0.29±0.08g |
| 3 | 60 | 1.00 | 54.70±0.74 | 0.33±0.04c | 1.55±0.47c | 0.87±0.54e | 1.53±0.34e |
| 6 | 60 | 1.00 | 50.73±0.06 | 0.45±0.12ab | 11.76±0.70a | 11.38±1.07a | 11.53±0.63a |
| 6 | 60 | 0.10 | 55.22±0.08 | 0.17±0.17c | −0.29±0.01c | 1.24±0.64d | 0.58±0.40g |
| 6 | 60 | 0.25 | 55.51±0.16 | 0.29±0.06bc | 0.97±1.19c | 0.53±1.13f | 1.13±0.80f |
| 6 | 60 | 0.50 | 54.34±1.03 | 0.12±0.01d | 5.45±0.18b | 4.21±0.56b | 5.59±0.28b |
Values are mean±SD of six separate experiments.
Means sharing the same letter (a–g) are not significantly different at the 5% level.
L*, lightness; a*, redness; b*, yellowness; ΔE, color difference C, chroma.
Not significant.
Maillard browning is a chemical process that produces a brown color in foods without enzyme activity. Heat treatment such as roasting increases the non-enzymatic browning reaction, consequently enhancing color development (38). Since the color of tea is one of the major factors determining tea quality, the Maillard reaction is a key phenomenon in the tea industry (39). It is believed that the brown color development of BRT is due to the non-enzymatic browning reaction and improves the quality of tea.
Effects of tea preparation conditions on antioxidant activity of BRT
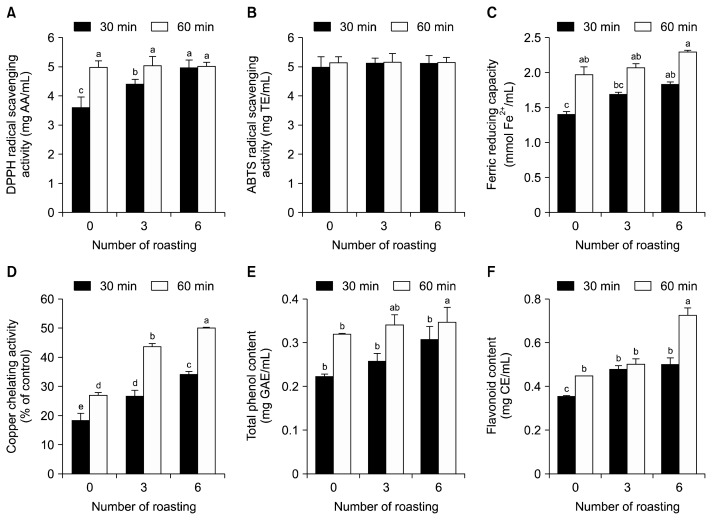
In order to investigate the effects of tea preparation conditions on antioxidant activity, the total antioxidant capacities of BRT produced with different roasting numbers and extraction times were determined. DPPH radical scavenging activity increased with higher roasting number in BRT extracted for 30 min but not in BRT extracted for 60 min (Fig. 2A). However, ABTS radical scavenging activities of all BRT groups were approximately 5 mg TE/mL, regardless of roasting number and extraction time (Fig. 2B). Ferric reducing capacity tended to elevate with increasing roasting number and extraction time of burdock roots (Fig. 2C). Copper chelating activity was positively associated with roasting number and extraction time (Fig. 2D). While roasting time did not affect radical scavenging activities and ferric reducing capacity of BRT extracting for 60 min, roasting time was important for copper chelating activity. BRT with an extraction time of 60 min showed significantly higher total phenolic content than that extracted for 30 min (Fig. 2E). In BRT boiled for 30 min, only BRT from burdock roots roasted six times had a statistically significant amount of total phenolics. However, roasting number did not influence total phenol content in BRT boiled for 60 min. On the other hand, flavonoid content was affected by roasted number and extraction time (Fig. 2F).
Fig. 2.
Effects of methods of burdock root tea (BRT) preparation on antioxidant activities. In order to elucidate the effects of BRT preparation conditions on antioxidant activity, roasting number and tea extraction time were varied. Burdock roots were roasted 0, 3, or 6 times, and BRT was produced by boiling these roasted burdock roots (1.00 mg/mL) for 30 or 60 min at 100°C. Total antioxidant capacities of BRT were determined based on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity (A), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity (B), ferric reducing capacity (C), copper chelating activity (D), total polyphenol contents (E), and flavonoid contents (F). Results are expressed as the mean±standard deviation (n=6). Mean sharing the same letter (a–e) are not significantly different at the 5% level. AA, ascorbic acid equivalent; TE, Trolox equivalents; GAE, gallic acid equivalents; CE, catechin equivalents.
It has been reported that roasting number and extraction time influence antioxidant activities. The roasting process reinforces antioxidant capacity in coffee, wheat germ, hazelnuts, and sweet almonds (40,41). Extraction time of tea also affects antioxidant capacity and polyphenol compounds. In a previous study, total phenolic compounds of black tea reached a maximum concentration at 40 min, whereas extraction up to 120 min reduced polyphenols (42). Consistent with previous studies, roasting number and extraction time of BRT were closely linked to antioxidant activity and polyphenol contents as shown in Fig. 2. However, data on DPPH radical scavenging activity, ferric reducing capacity, and total phenol contents show that roasting number did not affect BRT boiled for 60 min. It is believed that an extraction time of 60 min is sufficient to extract all antioxidant components from burdock roots, thus maximizing the antioxidant activities of BRT regardless of roasting number. Our data suggest that BRT is useful as a whole food-based antioxidant when brewed from burdock roots roasted six times for 60 min.
Effects of concentration of roasted burdock roots on antioxidant activity of BRT
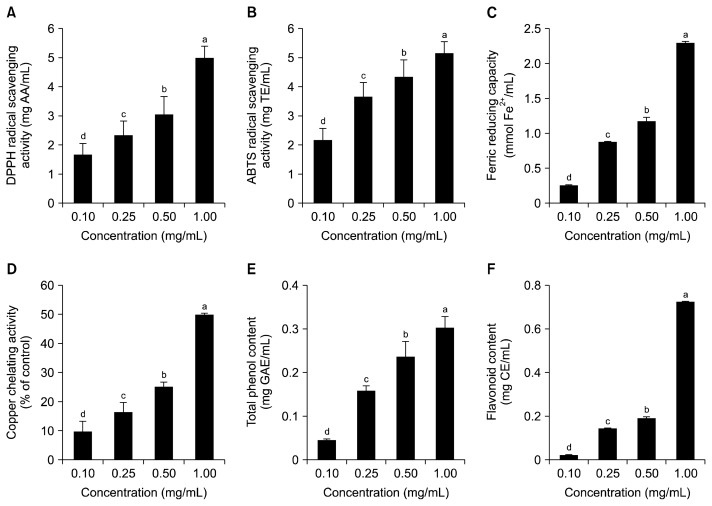
The effect of ratio of water to roasted burdock roots on antioxidant ability of BRT was examined (Fig. 3). Different concentrations of burdock roots (0.10, 0.25, 0.50, and 1.00 mg/mL) were used to produce BRT. Since the highest antioxidant ability of BRT was observed in BRT prepared from burdock roots roasted six times for 60 min, BRT extraction conditions were maintained as before. DPPH and ABTS radical scavenging activities of BRT at a concentration of 1.00 mg/mL were approximately 2.5-fold higher than BRT at a concentration of 0.10 mg/mL (Fig. 3A and 3B). Ferric reducing and copper chelating capacities also dose-dependently increased (Fig. 3C and 3D). Copper chelating activity of BRT at a concentration of 1.00 mg/mL was approximately 5-fold higher than BRT at a concentration of 0.10 mg/mL (Fig. 3D). Moreover, total phenol and flavonoid contents of BRT were elevated in a dose-dependent manner (Fig. 3E and 3F). Total phenol and flavonoid contents of BRT at a concentration of 1.00 mg/mL were 0.30 mg GAE/mL and 0.73 mg CE/mL, respectively.
Fig. 3.
Effects of amount of burdock root tea (BRT) on antioxidant activities. In order to examine the effect of concentration, BRT was prepared by boiling burdock roots roasted six times at various concentrations (0.10, 0.25, 0.50, and 1.00 mg/mL) for 60 min. Total antioxidant capacities of BRT were tested based on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity (A), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity (B), ferric reducing capacity (C), copper chelating activity (D), total polyphenol contents (E), and flavonoid contents (F). Data are expressed as the mean±standard deviation (n=6). Mean sharing the same letter (a–d) are not significantly different at the 5% level. AA, ascorbic acid equivalent; TE, Trolox equivalents; GAE, gallic acid equivalents; CE, catechin equivalents.
It is believed that BRT prepared from burdock roots (1.00 mg/mL) had the highest total antioxidant capacity due to its high amounts of total phenols and flavonoids. The bioactive components of burdock roots have been reported before. Ferracane et al. (43) reported that burdock roots contain chlorogenic acids, ester of caffeic acid, and quinic acid. Further, Liu et al. (44) showed that caffeoylquinic acids and lignans in burdock roots are responsible for the antioxidant activity of burdock roots. Among those bioactive components, chlorogenic acids are the predominant polyphenols in burdock roots. Thus, the antioxidant activity of BRT may be derived from mainly chlorogenic acids and a combination of other bioactive components present in burdock roots.
Relationship between color and antioxidant activity of BRT
The relationship between color and antioxidant capacity of BRT was investigated since color development in foods influences antioxidant activity (45). Pearson’s correlation coefficient values between parameters related to color and antioxidant activities of BRT were determined and are presented in Table 3. A strong positive correlation between ΔE and DPPH radical scavenging activity was detected (r=0.978). In addition, the correlation value between ΔE and flavonoid content was 0.953. Our data suggest that there is a strong positive relationship between ΔE and total antioxidant capacities. Thus, the color of BRT can be a useful indicator for estimating the antioxidant activity of BRT.
Table 3.
Pearson’s correlation between color difference (ΔE) and total antioxidant capacities
| ΔE | DPPH | ABTS | FRAP | Copper chelating activity | Total phenol content | Flavonoid content | |
|---|---|---|---|---|---|---|---|
| ΔE | 1.000 | ||||||
| DPPH | 0.978*** | 1.000 | |||||
| ABTS | 0.832*** | 0.874*** | 1.000 | ||||
| FRAP | 0.965*** | 0.986*** | 0.882*** | 1.000 | |||
| Copper chelating activity | 0.971*** | 0.971*** | 0.857*** | 0.960*** | 1.000 | ||
| Total phenol content | 0.864*** | 0.913*** | 0.960*** | 0.911*** | 0.890*** | 1.000 | |
| Flavonoid content | 0.953*** | 0.963*** | 0.774*** | 0.955*** | 0.948*** | 0.821*** | 1.000 |
DPPH, 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity; FRAP, ferric reducing antioxidant power.
Correlation is significant at the 0.001 level.
A positive relationship between ΔE and total antioxidant capacity or polyphenol concentration has been well documented. For example, darker color was shown to be associated with increased antioxidant activities and polyphenol contents in honey samples (46). Moreover, the higher antioxidant activity in highly colored malts was shown to be related to higher contents of reductones and melanoidins, which are produced during the roasting process (47). Reductones and melanoidins, collectively known as Maillard reaction products (MRPs), contribute to color development in foods as well as antioxidant activities (47). In a previous study, MRPs generated from the reaction between histidine and glucose at 120°C for 30 min exhibited peroxyl radical scavenging activity (48). In brewed coffee, radical scavenging activity of the non-phenolic fraction was shown to increase upon roasting together with accumulation of brown-colored MRPs (49). Thus, there is a possibility that MRPs could be partially responsible for the antioxidant activity and color of BRT, and further study to determine the role of MRPs in BRT quality is needed.
In order to satisfy recent consumer demands, development and identification of whole food-based natural antioxidants have gained attention. This study demonstrated that roasting treatment improves the total antioxidant capacity of BRT. Total antioxidant capacity of BRT was stronger than that of burdock root methanol extract. Interestingly, the total antioxidant capacity of BRT was positively associated with color, an important tea quality criterion. Our data support a correlation between darker color and stronger antioxidant capacity in BRT, and thus the color of BRT can serve as an indicator of total antioxidant capacity. Color and total antioxidant capacity of BRT were dependent on BRT preparation parameters such as roasting number, tea extraction time, and ratio between water and burdock roots. Higher roasting number, extraction time, and amount of burdock roots enhanced total antioxidant capacity and color development. This study proposes the practical use of BRT as a whole food-based antioxidant. Further studies are required to confirm the antioxidant capacity of BRT for health benefits in an in vivo system.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A01058195).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Maruta Y, Kawabata J, Niki R. Antioxidative caffeoylquinic acid derivatives in the roots of burdock (Arctium lappa L.) J Agric Food Chem. 1995;43:2592–2595. doi: 10.1021/jf00058a007. [DOI] [Google Scholar]
- 2.Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12:553–564. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 3.Chan YS, Cheng LN, Wu JH, Chan E, Kwan YW, Lee SMY, Leung GPH, Yu PHF, Chan SW. A review of the pharmacological effects of Arctium lappa (burdock) Inflammopharmacology. 2011;19:245–254. doi: 10.1007/s10787-010-0062-4. [DOI] [PubMed] [Google Scholar]
- 4.Yu BS, Yan XP, Xiong J, Xin Q. Simultaneous determination of chlorogenic acid, forsythin and arctiin in Chinese traditional medicines preparation by reversed phase-HPLC. Chem Pharm Bull. 2003;51:421–424. doi: 10.1248/cpb.51.421. [DOI] [PubMed] [Google Scholar]
- 5.Xu Z, Wang X, Zhou M, Ma L, Deng Y, Zhang H, Zhao A, Zhang Y, Jia W. The antidiabetic activity of total lignan from Fructus Arctii against alloxan-induced diabetes in mice and rats. Phytother Res. 2008;22:97–101. doi: 10.1002/ptr.2273. [DOI] [PubMed] [Google Scholar]
- 6.Weber V, Rubat C, Duroux E, Lartigue C, Madesclaire M, Coudert P. New 3- and 4-hydroxyfuranones as antioxidants and anti-inflammatory agents. Bioorg Med Chem. 2005;13:4552–4564. doi: 10.1016/j.bmc.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 7.Bhoo-Pathy N, Peeters PH, Uiterwaal CS, Bueno-de-Mesquita HB, Bulgiba AM, Bech BH, Overvad K, Tjønneland A, Olsen A, Clavel-Chapelon F, Fagherazzi G, Perquier F, Teucher B, Kaaks R, Schütze M, Boeing H, Lagiou P, Orfanos P, Trichopoulou A, Agnoli C, Mattiello A, Palli D, Tumino R, Sacerdote C, van Duijnhoven FJ, Braaten T, Lund E, Skeie G, Redondo ML, Buckland G, Pérez MJ, Chirlaque MD, Ardanaz E, Amiano P, Wirfält E, Wallström P, Johansson I, Nilsson LM, Khaw KT, Wareham N, Allen NE, Key TJ, Rinaldi S, Romieu I, Gallo V, Riboli E, van Gils CH. Coffee and tea consumption and risk of pre- and postmenopausal breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Breast Cancer Res. 2015;17:15. doi: 10.1186/s13058-015-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira JV, Bergamo DCB, Pereira JO, França SC, Pietro RCLR, Silva-Sousa YTC. Antimicrobial activity of Arctium lappa constituents against microorganisms commonly found in endodontic infections. Braz Dent J. 2005;16:192–196. doi: 10.1590/S0103-64402005000300004. [DOI] [PubMed] [Google Scholar]
- 9.Lin CC, Lu JM, Yang JJ, Chuang SC, Ujiie T. Anti-inflammatory and radical scavenge effects of Arctium lappa. Am J Chin Med. 1996;24:127–137. doi: 10.1142/S0192415X96000177. [DOI] [PubMed] [Google Scholar]
- 10.Predes FS, Ruiz AL, Carvalho JE, Foglio MA, Dolder H. Antioxidative and in vitro antiproliferative activity of Arctium lappa root extracts. BMC Complement Altern Med. 2011;11:25. doi: 10.1186/1472-6882-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grigg D. The worlds of tea and coffee: patterns of consumption. GeoJournal. 2002;57:283–294. doi: 10.1023/B:GEJO.0000007249.91153.c3. [DOI] [Google Scholar]
- 12.Dufrene B. Global tea consumption remains robust: world tea consumption continues to grow with strong trends towards more green tea, more premium leaf and more convenience cups. Tea Coffee Trade J. 2012;22:24–30. [Google Scholar]
- 13.Wierzejska R. Tea and health–a review of the current state of knowledge. Przegl Epidemiol. 2014;68:501–506. 595–599. [PubMed] [Google Scholar]
- 14.Pan T, Jankovic J, Le W. Potential therapeutic properties of green tea polyphenols in Parkinson’s disease. Drugs Aging. 2003;20:711–721. doi: 10.2165/00002512-200320100-00001. [DOI] [PubMed] [Google Scholar]
- 15.Zhong X, Zhang T, Liu Y, Wei X, Zhang X, Qin Y, Jin Z, Chen Q, Ma X, Wang R, He J. Short-term weight-centric effects of tea or tea extract in patients with metabolic syndrome: a meta-analysis of randomized controlled trials. Nutr Diabetes. 2015;5:e160. doi: 10.1038/nutd.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan N, Mukhtar H. Tea and health: studies in humans. Curr Pharm Des. 2013;19:6141–6147. doi: 10.2174/1381612811319340008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang J, Zhang Z, Zheng TZ, Bassig BA, Mao C, Liu X, Zhu Y, Shi K, Ge J, Yang YJ, Huang D, Bai M, Peng Y. Green tea consumption and risk of cardiovascular and ischemic related diseases: a meta-analysis. Int J Cardiol. 2016;202:967–974. doi: 10.1016/j.ijcard.2014.12.176. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe N, Suzuki H, Aizawa Y, Seki N. Consumption of green and roasted teas and the risk of stroke incidence: results from the Tokamachi-Nakasato cohort study in Japan. Int J Epidemiol. 2008;37:1030–1040. doi: 10.1093/ije/dyn211. [DOI] [PubMed] [Google Scholar]
- 19.Agudelo-Ochoa GM, Pulgarín-Zapata IC, Velásquez-Rodriguez CM, Duque-Ramírez M, Naranjo-Cano M, Quintero-Ortiz MM, Lara-Guzmán OJ, Muñoz-Durango K. Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J Nutr. 2016;146:524–531. doi: 10.3945/jn.115.224774. [DOI] [PubMed] [Google Scholar]
- 20.Yu F, Jin Z, Jiang H, Xiang C, Tang J, Li T, He J. Tea consumption and the risk of five major cancers: a dose-response meta-analysis of prospective studies. BMC Cancer. 2014;14:197. doi: 10.1186/1471-2407-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayat K, Iqbal H, Malik U, Bilal U, Mushtaq S. Tea and its consumption: benefits and risks. Crit Rev Food Sci Nutr. 2015;55:939–954. doi: 10.1080/10408398.2012.678949. [DOI] [PubMed] [Google Scholar]
- 23.Grosso G, Stepaniak U, Topor-Mądry R, Szafraniec K, Pająk A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition. 2014;30:1398–1403. doi: 10.1016/j.nut.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somerset SM, Johannot L. Dietary flavonoid sources in Australian adults. Nutr Cancer. 2008;60:442–449. doi: 10.1080/01635580802143836. [DOI] [PubMed] [Google Scholar]
- 25.Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C, Barikmo I, Berhe N, Willett WC, Phillips KM, Jacobs DR, Jr, Blomhoff R. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010;9:3. doi: 10.1186/1475-2891-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burton-Freeman BM, Sesso HD. Whole food versus supplement: comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv Nutr. 2014;5:457–485. doi: 10.3945/an.114.005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee A, Saluja M, Agarwal G, Alam M. Green tea: a boon for periodontal and general health. J Indian Soc Periodontol. 2012;16:161–167. doi: 10.4103/0972-124X.99256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peleg H, Naim M, Rouseff RL, Zehavi U. Distribution of bound and free phenolic acids in oranges (Citrus sinensis) and grapefruits (Citrus paradisi) J Sci Food Agric. 1991;57:417–426. doi: 10.1002/jsfa.2740570312. [DOI] [Google Scholar]
- 29.Schweiggert U, Schieber A, Carle R. Inactivation of peroxidase, polyphenoloxidase, and lipoxygenase in paprika and chili powder after immediate thermal treatment of the plant material. Innovative Food Sci Emerging Technol. 2005;6:403–411. doi: 10.1016/j.ifset.2005.05.001. [DOI] [Google Scholar]
- 30.Vignoli JA, Viegas MC, Bassoli DG, Benassi MT. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res Int. 2014;61:279–285. doi: 10.1016/j.foodres.2013.06.006. [DOI] [Google Scholar]
- 31.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 32.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 33.Megías C, Pastor-Cavada E, Torres-Fuentes C, Girón-Calle J, Alaiz M, Juan R, Pastor J, Vioque J. Chelating, antioxidant and antiproliferative activity of Vicia sativa polyphenol extracts. Eur Food Res Technol. 2009;230:353–359. doi: 10.1007/s00217-009-1178-x. [DOI] [Google Scholar]
- 34.Singleton VL, Orthofer R, Lamuela-Raventós RM. Methods in Enzymology. Vol. 299. Academic Press; New York, NY, USA: 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent; pp. 152–178. [DOI] [Google Scholar]
- 35.Sánchez-Moreno C, Larrauri JA, Saura-Calixto F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int. 1999;32:407–412. doi: 10.1016/S0963-9969(99)00097-6. [DOI] [Google Scholar]
- 36.Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC, Ahn DU, Lee SC. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J Agric Food Chem. 2004;52:3389–3393. doi: 10.1021/jf049899k. [DOI] [PubMed] [Google Scholar]
- 37.Mahy M, Van Eycken L, Oosterlinck A. Evaluation of uniform color spaces developed after the adoption of CIELAB and CIELUV. Color Res Appl. 1994;19:105–121. [Google Scholar]
- 38.Garza S, Ibarz A, Pagán J, Giner J. Non-enzymatic browning in peach puree during heating. Food Res Int. 1999;32:335–343. doi: 10.1016/S0963-9969(99)00094-0. [DOI] [Google Scholar]
- 39.Chaturvedula VSP, Prakash I. The aroma, taste, color and bioactive constituents of tea. J Med Plants Res. 2011;5:2110–2124. [Google Scholar]
- 40.Krings U, Berger RG. Antioxidant activity of some roasted foods. Food Chem. 2001;72:223–229. doi: 10.1016/S0308-8146(00)00226-0. [DOI] [Google Scholar]
- 41.del Castillo MD, Ames JM, Gordon MH. Effect of roasting on the antioxidant activity of coffee brews. J Agric Food Chem. 2002;50:3698–3703. doi: 10.1021/jf011702q. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad M, Baba WN, Gani A, Wani TA, Gani A, Masoodi FA. Effect of extraction time on antioxidants and bioactive volatile components of green tea (Camellia sinensis), using GC/MS. Cogent Food Agric. 2015;1:1106387. [Google Scholar]
- 43.Ferracane R, Graziani G, Gallo M, Fogliano V, Ritieni A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J Pharm Biomed Anal. 2010;51:399–404. doi: 10.1016/j.jpba.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Cai YZ, Wong RN, Lee CK, Tang SC, Sze SC, Tong Y, Zhang Y. Comparative analysis of caffeoylquinic acids and lignans in roots and seeds among various burdock (Arctium lappa) genotypes with high antioxidant activity. J Agric Food Chem. 2012;60:4067–4075. doi: 10.1021/jf2050697. [DOI] [PubMed] [Google Scholar]
- 45.Manzocco L, Calligaris S, Mastrocola D, Nicoli MC, Lerici CR. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci Technol. 2000;11:340–346. doi: 10.1016/S0924-2244(01)00014-0. [DOI] [Google Scholar]
- 46.Pontis JA, da Costa LAMA, da Silva SJR, Flach A. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci Technol. 2014;34:69–73. [Google Scholar]
- 47.Woffenden HM, Ames JM, Chandra S. Relationships between antioxidant activity, color, and flavor compounds of crystal malt extracts. J Agric Food Chem. 2001;49:5524–5530. doi: 10.1021/jf010583b. [DOI] [PubMed] [Google Scholar]
- 48.Yilmaz Y, Toledo R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005;93:273–278. doi: 10.1016/j.foodchem.2004.09.043. [DOI] [Google Scholar]
- 49.Sacchetti G, Di Mattia C, Pittia P, Mastrocola D. Effect of roasting degree, equivalent thermal effect and coffee type on the radical scavenging activity of coffee brews and their phenolic fraction. J Food Eng. 2009;90:74–80. doi: 10.1016/j.jfoodeng.2008.06.005. [DOI] [Google Scholar]