Abstract
The anti-obesity effects of yeast hydrolysate (YH) supplementation (1.0 g/d) have already been demonstrated. We investigated whether a low dose of YH (0.5 g/d, YH-500) also has the anti-obesity effects. Thirty obese women were randomly assigned to the control or YH-500 groups. After 8 weeks, weight and body mass index were significantly reduced by the YH treatment (0.5 g/d) (P<0.05). The YH-500 group lost a significant amount of body fat after the 8-week treatment: fat mass 25.9 kg (baseline) versus 23.8 kg (8th week), P<0.01; fat mass ratio 38.8% (baseline) versus 36.5% (8th week), P<0.05. The YH-500 group showed a significant reduction in calorie intake during the 8-week treatment (P<0.001). The control group wanted to eat much more food (P<0.05) and sometimes thought about eating more often compared with the YH-500 group (P<0.05). Whereas the control group showed a slightly increased sweet preference, the YH-500 group showed a significant reduction in sweet preference (P<0.05). In conclusion, low dose YH supplementation (0.5 g/d) may induce a reductions in weight and body fat in obese women via the reduction of calorie intake.
Keywords: low dose, obesity, yeast hydrolysate, weight loss
INTRODUCTION
Yeast naturally rich in minerals, vitamins, amino acids, and proteins, plays a fundamental role in human food and nutrition. Yeast extract has high potential as a source of biologically active molecules and functional food ingredients (1). The small proportion of yeast extract (mainly containing peptides below 10 kDa), yeast hydrolysate (YH), has been industrially purified from Saccharomyces cerevisiae by protein hydrolysis (2,3). Recently, YH has gained a large and growing dietary market as a useful anti-obesity supplement (4,5). It was reported that YH could assist in weight loss in obese humans (5–7).
Although the exact mechanisms for the anti-obesity effects of YH are not fully understood, previous studies support the idea that YH might induce weight loss through appetite control (3,5). The anti-obesity effects of YH supplementation (1.0 g/d) have already been demonstrated: YH supplementation (1.0 g/d) significantly induced a reduction in weight and abdominal fat accumulation in obese adults, regardless of sex, via the reduction of calorie intake (5–7). To determine the proper daily amount of YH for this application, we investigated whether a low dose of YH (0.5 g/d) has anti-obesity effects in obese people.
MATERIALS AND METHODS
Yeast hydrolysate
S. cerevisiae IFO 2346 was incubated in a medium containing 2% molasses, 0.6% (NH4)2SO4, 0.1% MgSO4·7H2O, 0.2% KH2PO4, 0.03% K2HPO4, and 0.1% NaCl for 3 days at 30°C. After incubation, the culture was centrifuged at 10,000 g for 20 min. The cells were suspended in 20 mM phosphate buffer (pH 7.0) and hydrolyzed with 1,000 units of bromelain at 30°C for 4 h. The hydrolysate was subsequently centrifuged at 10,000 g for 20 min. The supernatant was then passed through a 10 kDa molecular-weight cutoff membrane (Sartocon®, Sartorius Stedim Biotech GmbH, Göttingen, Germany) and lyophilized.
Participants
Women aged 20 to 60 years with a body mass index (BMI) of at least 25 kg/m2, which is used as a cutoff point for obesity in the Asia-Pacific region (8), were recruited. The study was approved by the Ethical Committee for Human Experimentation of Jeonju University and was conducted in accordance with its rules and regulations (1041042-141204-05). This randomized, placebo-controlled study was carried out for 8 weeks. Subjects were randomly assigned to the control or YH-500 groups. The YH-500 group consumed one capsule (YH/capsule of 0.25 g) with water twice a day, 30 min before breakfast and dinner. The total daily dose of YH was 0.5 g. The control group received only a placebo (100% dextrin), which was given at the same amount and color as YH. All subjects were instructed to continue their regular diet and exercise patterns.
Study protocol
The subjects recorded all the food and beverages that they consumed three times a week (twice on weekdays and once on the weekend). Food intake records were analyzed by Can-Pro 3.0 (Korean Nutrition Society, Seoul, Korea). All subjects completed a side-effect questionnaire weekly. After 8 weeks, they were also asked about the change of appetite based on General Food Cravings Questionnaire-Trait (9). Responses were scored using a 4-point scale. Anthropometric measurements were performed at a screening visit (baseline) and subsequently at the 4th and 8th weeks. Weight was measured to the nearest 0.1 kg with a standard balance beam scale (Giant-150N, Hana Co., Seoul, Korea). Body fat was measured with a body impedance assessment (In Body 720, Bio-space Co., Seoul, Korea).
Taste preference for sweetness was measured with a 5-point scale. Taste detection threshold for sweetness was also measured with a whole-mouth gustatory test method at baseline and at the 8th week. A spray-and-swallow method was used to stimulate oral taste receptors. For this test, all subjects were instructed in the use of magnitude estimation to rate the intensity of a stimulator solution. Thirteen concentration levels (in 1/2 steps) of 40% sucrose solution were prepared in a 1 mL sample. The subjects were then asked to identify the sweetness and intensity of the test solution.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences ver. 12.0 (SPSS Inc., Chicago, IL, USA). Differences between the two groups (control versus YH-500) were statistically evaluated by a t-test. A repeated measures ANOVA, followed by Bonferroni-adjusted pairwise comparisons, was used to assess the differences in change from baseline to each week within the groups. All data were reported as the mean±standard error of the mean (SEM).
RESULTS
Thirty individuals were selected for participation in this study. All subjects completed the study requirements, and each group ultimately consisted of 15 subjects. Table 1 shows the changes in weight and body composition from baseline to end point. At baseline, the control and YH-500 groups had mean weights of 65.3 and 66.2 kg, BMI values of 26.3 and 26.5 kg/m2, and body fat masses of 25.1 and 25.9 kg, respectively. The initial values of weight and body composition did not significantly differ between the two groups. Weight and BMI were significantly reduced in the YH-500 group: weight 66.2 kg (baseline) versus 64.6 kg (8th week), P<0.05; BMI 26.5 kg/m2 (baseline) versus 25.8 kg/m2 (8th week), P<0.05. Fat mass and fat mass ratio in control group were not significantly changed during the experiment. The YH-500 group lost a significant amount of body fat after the 8-week treatment: fat mass 25.9 kg (baseline) versus 23.8 kg (8th week), P<0.01; fat mass ratio 38.8% (baseline) versus 36.5% (8th week), P<0.05. Reductions in weight and body composition between baseline and the 8th week in the YH-500 group were significantly greater than those in the control group: weight 0.09 kg (control) versus −1.61 kg (YH-500), P<0.05; BMI 0.03 kg/m2 (control) versus −0.64 kg/m2, P<0.05; fat mass 0.04 kg (control) versus −2.02 kg (YH-500), P<0.001; fat mass ratio −0.02% (control) versus −2.26% (YH-500), P<0.01. Neither group experienced a significant change from baseline in fat free mass; despite the increased loss in weight and fat mass in the YH-500 group, fat free mass did not significantly differ between the control and YH-500 groups.
Table 1.
Changes in weight and body composition after the 8-week treatment with yeast hydrolysate (YH)
| Control (n=15) | YH-500 (n=15) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Week 0 | Week 4 | Week 8 | Week 0 | Week 4 | Week 8 | |
| Weight (kg) | 65.3±1.6 | 65.4±1.7 | 65.4±1.9 | 66.2±2.0 | 65.3±2.3† | 64.6±2.4†* |
| BMI (kg/m2) | 26.3±0.7 | 26.3±0.7 | 26.4±2.8 | 26.5±0.6 | 26.1±0.7† | 25.8±0.8†* |
| Fat mass (kg) | 25.1±1.4 | 25.3±1.3 | 25.1±1.5 | 25.9±1.3 | 25.4±1.6 | 23.8±1.5††*** |
| Fat mass ratio (%) | 38.5±2.0 | 38.8±7.8 | 38.4±8.2 | 38.8±1.0 | 38.5±1.8 | 36.5±1.3†** |
Data are mean±SEM.
The cross indicates a significant difference (†P<0.05 and ††P<0.01) between baseline and at each week by a repeated measures ANOVA followed by Bonferroni-adjusted pairwise comparisons within groups. The asterisks indicate a significant difference (*P<0.05, **P<0.01, and ***P<0.001) of the change at each week between the two groups (control group versus YH-500 group) by t-test.
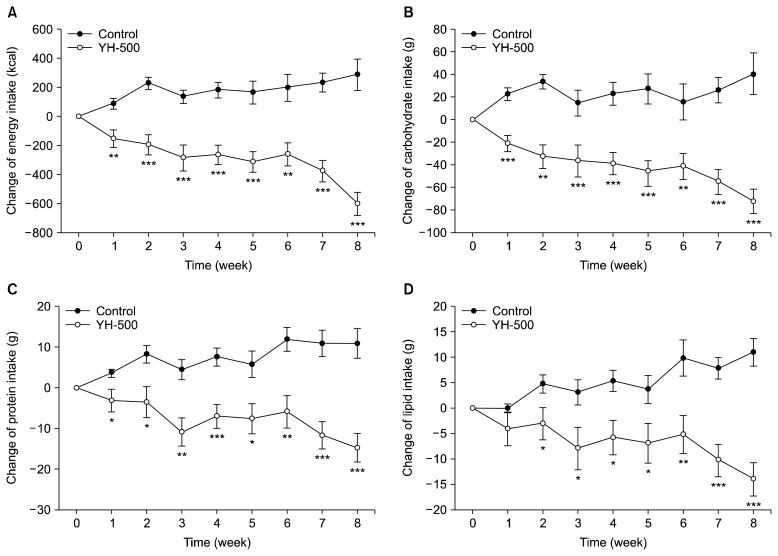
Fig. 1 compares the effect of YH on energy intake for 8 weeks. Reduction of calorie intake including carbohydrate, protein, and lipid intake in the YH-500 group were significantly greater than those in the control group during the 8-week treatment: YH-500 group showed a significant reduction in calorie intake including carbohydrate, protein, and lipid intake during the 8-week treatment (at 8th week, P<0.001), whereas the control group showed a slight increase. Subjects’ physical activity did not significantly differ during the treatment compared with baseline in either group (data not shown).
Fig. 1.
Changes in energy intake during the 8-week treatment with yeast hydrolysate (YH). Data are mean±SEM. The asterisks indicate a significant difference (*P<0.05, **P<0.01, and ***P<0.001) of the change between the two groups (control group versus YH-500 group) by t-test at each week.
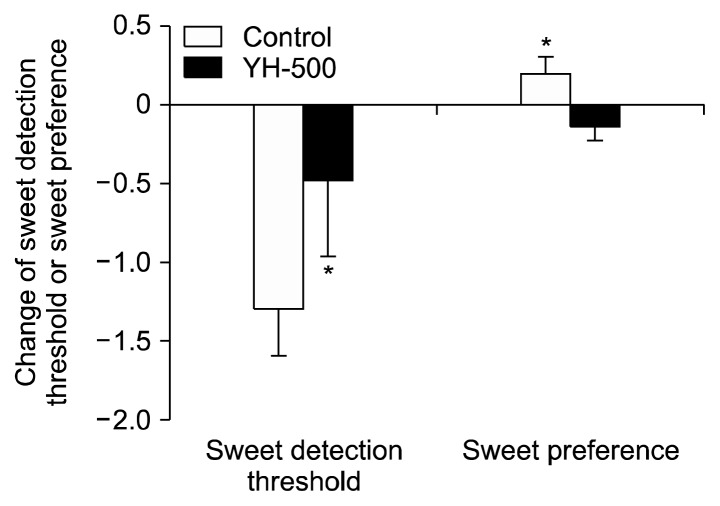
Table 2 shows changes in appetite after the 8-week treatment. The control group wanted to eat much more food (P<0.05) and some thought about eating more often compared with the YH-500 group (P<0.05). As shown in Fig. 2, both groups reduced their sweetness detection threshold after the 8-week treatment. Reduction of sweetness detection threshold in the YH-500 group from baseline was less than in control group (control group versus YH-500 group: P=0.022). Whereas the control group showed a slightly increased sweet preference, the YH-500 group showed a significant reduction in sweet preference: the difference in sweet preference between the two groups was significant after the 8-week treatment (control group versus YH-500 group: P= 0.025).
Table 2.
Changes in appetite after the 8-week treatment with yeast hydrolysate (YH)
| Control (n=15) | YH-500 (n=15) | |
|---|---|---|
| I want to eat much more | 2.2±0.36 | 1.8±0.36* |
| I think about eating more often | 2.0±0.37 | 1.5±0.27* |
| I eat at night much more | 1.3±0.15 | 1.2±0.13 |
Data are mean±SEM.
The asterisk indicates a significant difference (P<0.05) of the change between the two groups (control group versus YH-500 group) by t-test.
Fig. 2.
Changes of sweet detection threshold and sweet preference after the 8-week treatment with yeast hydrolysate (YH). Data are mean±SEM. The asterisk indicates a significant difference (P<0.05) of the change between the two groups (control group versus YH-500 group) by t-test.
DISCUSSION
In the present study, we evaluated the anti-obesity effects of YH (0.5 g/d) in obese women. The results showed that YH (0.5 g/d) supplementation significantly decreased weight and body fat. These results coincide with the results from the previous studies, which showed that obese people who received YH (1.0 g/d) showed greater reductions in weight and body fat compared with a placebo (5–7). It was reported that YH might suppress body fat accumulation by modulating lipogenesis via the activities of hepatic lipid-regulating enzymes: hepatic glucose-6-phosphate dehydrogenase and malic enzyme activities, which provide the reduced nicotinamide adenine dinucleotide phosphate required for fatty acid synthesis, have been reportedly inhibited by YH (3,4).
Reduction of calorie intake in the YH-500 group was significantly greater than in the control group. Results of this study on calorie intake also agreed with previous clinical studies showing that YH (1.0 g/d) treatment significantly reduced calorie intake compared with the control group (5–7). Moreover, YH alters appetite-related neurotransmitters such as neuropeptide Y, nitric oxide synthase, and vasoactive intestinal peptide in the central nervous system. In an animal test, YH significantly inhibited ghrelin secretion relative to that of a placebo (10, 11). The satietogenic mechanism of YH could be related to the reduction in ghrelin, which is known to be an orexigenic hormone that stimulates appetite (3).
A relationship between weight loss and changes in sweet taste was suggested (12). Scruggs et al. (13) reported that weight loss following gastric bypass surgery was associated with up-regulated sweet taste acuity in morbidly obese people. Burge et al. (14) also observed that mean recognition thresholds for sucrose in obese people fell significantly after weight loss. Umabiki et al. (12) demonstrated that a decrease in serum leptin levels was significantly associated with a decrease in sweet taste threshold during weight loss in obese females. In this study, reduction of sweet detection threshold in the YH-500 group from baseline was less than in the control group. Weight loss can improve sweet taste, and YH treatment may influence sweet taste sensitivity and preference.
In conclusion, YH (0.5 g/d) supplementation can induce a reduction in weight and body fat without adverse effects on fat free mass in obese women via the reduction of calorie intake like YH 1.0 g/d supplementation’s effects. These results suggest that YH (0.5 g/d) can possibly be used to reduce weight and body fat. This study has some limitations, including a small sample size and a short period of the study. A larger sample size with long-term follow-up are needed to confirm the effectiveness of YH. Further studies of YH will help to elucidate the main components and mechanisms responsible for its anti-obesity effects.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Catiau L, Nedjar-Arroume N, Guillochon D, Ravallec R. In vitro and in vivo satietogenic effect of yeast extracts and control of food intake in rats. Eur Food Res Technol. 2011;233:525. doi: 10.1007/s00217-011-1545-2. [DOI] [Google Scholar]
- 2.Faipoux R, Tomé D, Bensaid A, Morens C, Oriol E, Bonnano LM, Fromentin G. Yeast proteins enhance satiety in rats. J Nutr. 2006;136:2350–2356. doi: 10.1093/jn/136.9.2350. [DOI] [PubMed] [Google Scholar]
- 3.Hong KB, Jung EY, Kim JH, Chang UJ, Suh HJ. Yeast hydrolysate as a functional anti-obesity ingredient: appetite suppressive effects of yeast hydrolysate in food-deprived mice. Prog Nutr. 2015;17:262–264. [Google Scholar]
- 4.Jung EY, Hong YH, Kim JH, Park Y, Bae SH, Chang UJ, Suh HJ. Effects of yeast hydrolysate on hepatic lipid metabolism in high-fat-diet-induced obese mice: yeast hydrolysate suppresses body fat accumulation by attenuating fatty acid synthesis. Ann Nutr Metab. 2012;61:89–94. doi: 10.1159/000338441. [DOI] [PubMed] [Google Scholar]
- 5.Jung EY, Cho MK, Hong YH, Kim JH, Park Y, Chang UJ, Suh HJ. Yeast hydrolysate can reduce body weight and abdominal fat accumulation in obese adults. Nutrition. 2014;30:25–32. doi: 10.1016/j.nut.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Jung EY, Kim SY, Bae SH, Chang UJ, Choi JW, Suh HJ. Weight reduction effects of yeast hydrolysate below 10 kDa on obese young women. J Food Biochem. 2011;35:337–350. doi: 10.1111/j.1745-4514.2010.00385.x. [DOI] [Google Scholar]
- 7.Jung EY, Son HS, Suh HJ. The weight reduction effect of yeast hydrolysate-SR101 on female college students. J Food Sci Nutr. 2009;14:123–128. [Google Scholar]
- 8.Asia Pacific Cohort Studies Collaboration. Ni Mhurchu C, Parag V, Nakamura M, Patel A, Rodgers A, Lam TH. Body mass index and risk of diabetes mellitus in the Asia- Pacific region. Asia Pac J Clin Nutr. 2006;15:127–133. [PubMed] [Google Scholar]
- 9.Noh J, Kim J, Nam H, Lim M, Lee D, Hong K. Validation of the Korean version of the General Food Cravings Questionnaire- Trait (G-FCQ-T) Korean J Clin Psychol. 2008;27:1039–1051. doi: 10.15842/kjcp.2008.27.4.015. [DOI] [Google Scholar]
- 10.Jung EY, Suh HJ, Kim SY, Hong YH, Kim MJ, Chang UJ. Appetite suppressive effects of yeast hydrolysate on nitric oxide synthase (NOS) expression and vasoactive intestinal peptide (VIP) immunoreactivity in hypothalamus. Phytother Res. 2008;22:1417–1422. doi: 10.1002/ptr.2264. [DOI] [PubMed] [Google Scholar]
- 11.Jung EY, Kang DH, Suh HJ, Chang UJ. Effects of yeast hydrolysate on neuropeptide Y (NPY) and tryptophan hydroxylase (TPH) immunoreactivity in rats. Phytother Res. 2009;23:619–623. doi: 10.1002/ptr.2668. [DOI] [PubMed] [Google Scholar]
- 12.Umabiki M, Tsuzaki K, Kotani K, Nagai N, Sano Y, Matsuoka Y, Kitaoka K, Okami Y, Sakane N, Higashi A. The improvement of sweet taste sensitivity with decrease in serum leptin levels during weight loss in obese females. Tohoku J Exp Med. 2010;220:267–271. doi: 10.1620/tjem.220.267. [DOI] [PubMed] [Google Scholar]
- 13.Scruggs DM, Buffington C, Cowan GS., Jr Taste acuity of the morbidly obese before and after gastric bypass surgery. Obes Surg. 1994;4:24–28. doi: 10.1381/096089294765558854. [DOI] [PubMed] [Google Scholar]
- 14.Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients’ taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc. 1995;95:666–670. doi: 10.1016/S0002-8223(95)00182-4. [DOI] [PubMed] [Google Scholar]