Abstract
Raphanus sativus L. (RS) is a cruciferous vegetable that is widely consumed in Korea. The anticancer activity of leaves of RS (RSL) extract has been investigated; however, no studies focused on its anti-inflammatory effects. Therefore, the aim of the current study was to evaluate the anti-inflammatory effects of RSL extract. In brief, RSL powder was fractionated into n-hexane, chloroform, ethyl acetate, n-butanol, and water-soluble fractions. Lipopolysaccharide (LPS)-stimulated RAW264.7 cells were treated with each fraction for initial screening. It was found that the chloroform fraction significantly inhibited nitric oxide release in LPS-stimulated RAW264.7 cells with a half maximal inhibitory concentration value of 196 μg/mL. In addition, the mRNA and protein expression levels of inducible nitric oxide synthase, measured using reverse transcriptase-polymerase chain reaction and western blotting, respectively, were reduced in a concentration-dependent manner. Moreover, the inflammatory cyclooxygenase-2 enzyme expression decreased. Furthermore, the expression of nuclear factor-kappa B (NF-κB), the key regulator of the transcriptional activation of the inflammatory cytokine genes, was reduced by the RSL chloroform fraction. Therefore, the results of our study suggest that RSL exhibits anti-inflammatory effects in LPS-stimulated macrophages via NF-κB inactivation.
Keywords: Raphanus sativus L., nitric oxide, anti-inflammatory, COX-2, NF-κB
INTRODUCTION
Inflammation is a complex host defense mechanism against noxious substances and microorganisms. Inflammatory responses play an important role in the host survival and homeostasis. However, in several cases, excessive inflammatory responses can lead to chronic inflammatory diseases, such as colitis, neuroinflammation, asthma, atopic dermatitis, arthritis, and allergic rhinitis (1). Current therapies for inflammatory diseases generally focus on alleviating pain and suppressing inflammatory cell activation and migration (2,3). However, these treatments result in undesirable side effects and cannot prevent recurrence (4). Recently, natural products with substantial ethnopharmacological activities have received great attention as treatments for inflammatory diseases. Many pharmacologically active compounds isolated from natural products were developed as medicines (5). In particular, food-based compounds with anti-inflammatory activities have gained widespread attention owing to their safety (6–8).
Raphanus sativus L. (RS) is a widely consumed cruciferous vegetable in Korea. Its root and leaves are the main materials for the traditional Korean food, kimchi. RS has been used in traditional medicine to treat digestive related diseases such as indigestion, digestive inflammation, diarrhea, and abdomen illness in East Asia (9). Its extracts are known to show an inhibitory effect on tumor growth. For example, the root of RS extracts showed a pro-apoptotic activity in human colon carcinoma cells and breast cancer cells (10,11). In addition, they inhibited metastasis in melanoma cells (12). Recently, several studies reported that the seeds of RS showed anti-inflammatory effects (9,13,14). However, no studies investigated the anti-inflammatory activity of the leaves of RS (RSL). Therefore, the purpose of this study was to evaluate the anti-inflammatory properties of the RSL by investigating its effects on the inflammatory mediator levels and to elucidate the underlying mechanism. The components of RSL are known as phenolic compounds (caffeic, p-coumaric, ferulic, hydroxycinnamic, p-hydroxybenzoic, vanillic, salicylic, and gentisic acids) and glucosinolates (benzyl isothiocyanate). The roots of RS contain alkaloid, nitrogen compounds (pyrrolidine, phenethylamine, and arabinogalactan proteins), enzyme (isoperoxidases), oil seed (alkenylglucosinolates), and organic acids (oxalic, malic, malonic, and erythorbic acids) (15).
Inflammation is mediated by several factors and cellular signaling molecules that are released from macrophages (6,16). When the macrophages are stimulated by endotoxins, such as lipopolysaccharides (LPS), they release various proinflammatory mediators and cytokines, such as nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 (16). NO is an inflammatory mediator that acts as an important cellular second messenger as well as a regulator of inflammatory responses (17). Cyclooxygenase-2 (COX-2) is a predominant enzyme that is induced by various stimuli, including inflammation, growth factors, and cytokines. Increased expression of inducible NO synthase (iNOS) and COX-2 are related to the activation of the nuclear factor-kappa B (NF-κB) pathway. NF-κB, which is activated in inflammatory responses, is a transcriptional activator of iNOS and COX-2 genes (18,19).
This is the first study to report the anti-inflammatory effects of the RSL in LPS-activated macrophages. The anti-inflammatory activity was investigated via determination of the levels of pro-inflammatory cytokines, as well as iNOS, COX-2, and NF-κB proteins levels.
MATERIALS AND METHODS
Radish extracts preparation
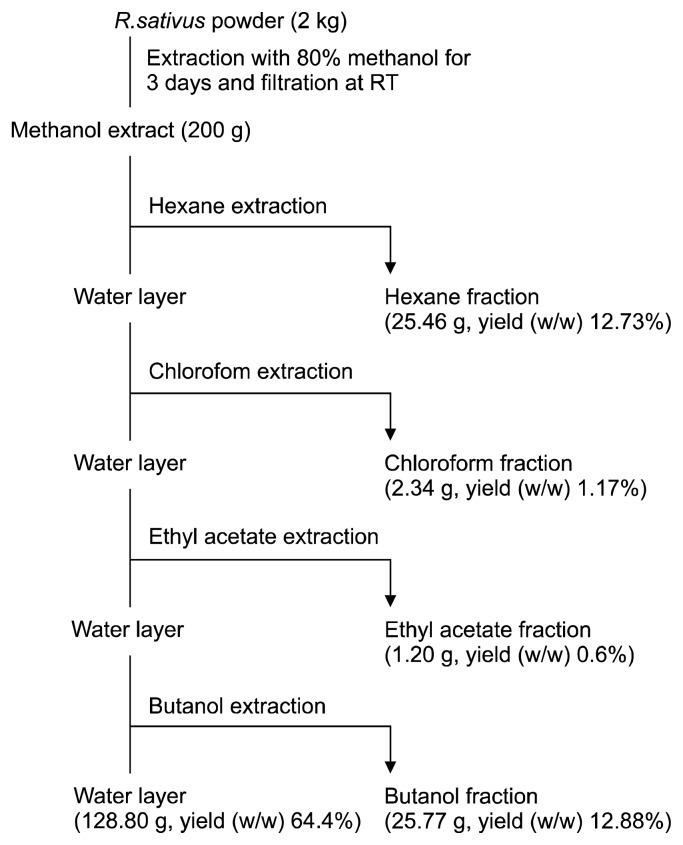
RSL was purchased from Kyungdong pharmaceutics (Seoul, Korea) and freeze-dried using liquid nitrogen. A voucher specimen (kucari 201210) was deposited at the herbarium of the Plant Extract Bank at Gachon University. A dried sample (2 kg) was ground and dissolved in methanol (6 L, 3 times a day for 3 days) at 24°C in a dark room. After filtration, the methanol extracts were concentrated under reduced pressure using a rotor evaporator (EYELA, Tokyo, Japan), and the extract (644 g) was collected. The methanol extract (200 g) was re-suspended in distilled water (1 L), and further fractionated with n-hexane, chloroform, ethyl acetate, n-butanol, and water based on its polarity. The dried weights and ratios of the n-hexane, chloroform, ethyl acetate, n-butanol, and water fractions were 25.46 g (12.73%), 2.34 g (1.17%), 1.20 g (0.60%), 25.77 g (12.88%), and 128.80 g (64.40%), respectively (Fig. 1).
Fig. 1.
The extraction procedure of leaves of Raphanus sativus L. (n-hexane, chloroform, ethyl acetate, n-butanol, and water) fractions.
Macrophage culture and cell viability by CCK-8 assay
RAW264.7 macrophages were purchased from American Type Culture Collection (Rockville, MD, USA) and cultured in the media consisting of Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA), 10% fetal bovine serum (FBS; Invitrogen), and 100 U/mL penicillin-streptomycin (Sigma, St. Louis, MO, USA). RAW264.7 cell viability in the presence of different concentrations of RSL was measured with the cell counting kit-8 (CCK-8) assay (Daelillab Co., Seoul, Korea). Cells were placed onto 96-well plates (5×103 cells/well) and treated with RSL (0, 1, 10, 100, and 200 μg/mL) for 48 h. CCK-8 solutions were added, and viable cells were detected using a microplate reader at 450 nm (Model550; Bio-Rad Laboratories, Hercules, CA, USA).
NO and PGE2 assay
Cells were plated and treated with each RSL fraction (n-hexane, chloroform, ethyl acetate, n-butanol, and water) for 1 h followed by LPS (1 μg/mL; Sigma). After 18 h incubation, the supernatants were collected. For the NO assay, the supernatants were reacted with Griess solution (1% sulfanilamide in 5% phosphoric acid, 0.1% N-1-naphthyl ethylenediamine in water) to measure nitrite levels, and the absorbance was determined at 540 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader (BioTek Instruments Inc., Winooski, VT, USA). PGE2 production was measured with ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA), and final absorbance was determined at 470 nm.
RNA isolation and real-time RT-PCR
For quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) analysis, cells were plated onto 6-well plate at 5×105 cells/well and treated with the RSL chloroform fraction (0, 1, 10, and 100 μg/mL) followed by a 1 h LPS stimulation. After 4 h, the total RNA was isolated using an RNA extraction kit (Toyobo Co., Ltd., Osaka, Japan). qPCR was performed using an ABI7500 thermal cycler (Applied Biosystems, Foster City, CA, USA). Specific primers for iNOS (QT00100275), TNF-α (QT00104006), IL-6 (QT00098875), and GAPDH (QT01658692) were purchased from Qiagen (Venlo, the Netherlands). The relative iNOS mRNA levels were normalized to those of GAPDH and calculated using the 2ΔΔCt method.
Western blot analysis
Cells were plated and treated with each RSL chloroform fraction for 1 h and LPS (1 μg/mL; Sigma) was added. After 18 h incubation, cells were lysed with ice-cold lysis buffer with protease inhibitors (Sigma). The protein concentration was measured with the Bradford protein assay reagent (Bio-Rad Laboratories). Equal amounts of proteins were loaded to each well and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blocked in 5% non-fat milk. Samples were probed with the following primary antibodies: mouse anti-iNOS (Abcam, Cambridge, MA, USA), rabbit anti-COX-2 (Cell Signaling Technology, Inc., Danvers, MA, USA), mouse anti-NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and mouse anti-β-actin (Santa Cruz Biotechnology) antibodies. The secondary antibodies used were either goat anti-mouse IgG or anti-rabbit IgG-peroxidase conjugates (Amersham Biosciences, Uppsala, Sweden). The membranes were incubated with horseradish peroxidase-labeled secondary antibody (Santa Cruz Biotechnology).
Statistical analysis
All data were expressed as the mean±standard deviation. The significance of the differences was analyzed using analysis of variance (ANOVA) (version 18, SPSS, IBM, New York, NY, USA). Differences at P<0.05 were considered statistically significant.
RESULTS AND DISCUSSION
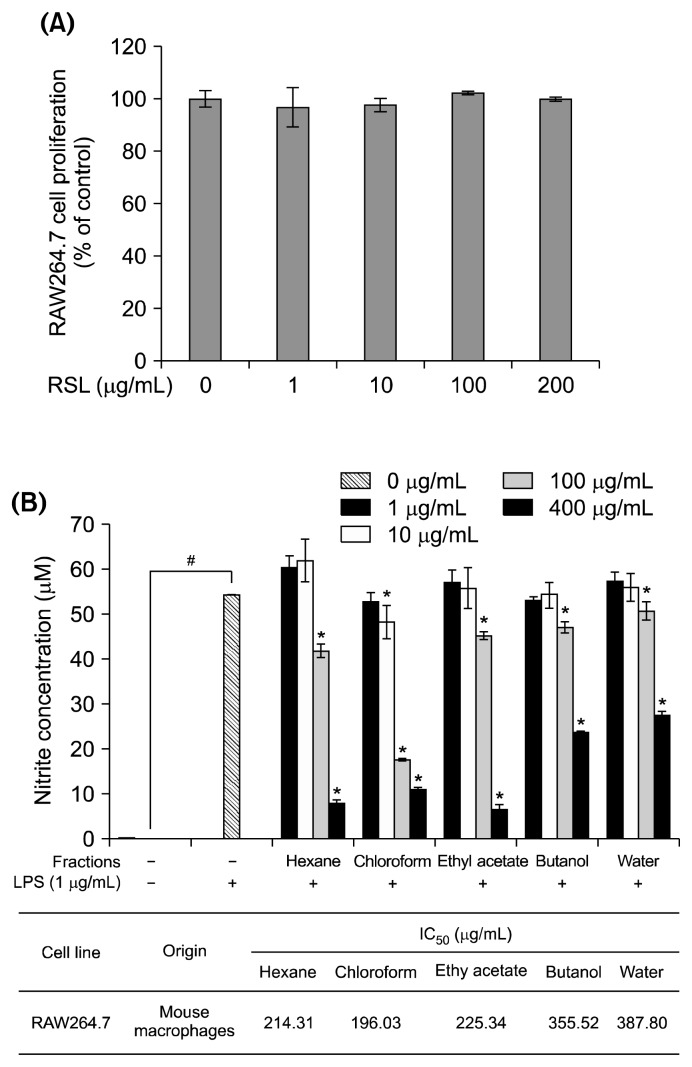
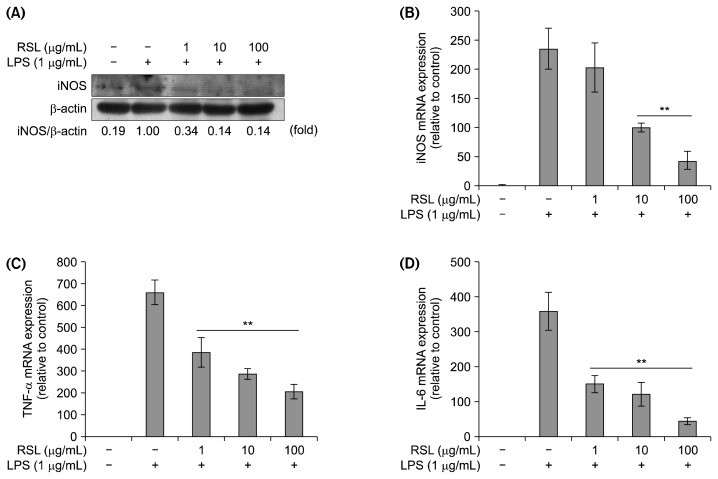
The effect of RSL on RAW 264.7 cell viability was investigated using the CCK-8 assay. Treatment with different concentrations of RSL extract (0, 1, 10, 100, and 200 mg/mL) did not change cell viability (Fig. 2A). As shown in Fig. 2B, NO production significantly increased upon LPS stimulation (55 μM) compared to the control group (0 μM). Chloroform, ethyl acetate, and water RSL fractions significantly inhibited the LPS-induced NO production in a concentration-dependent manner. The half-maximal inhibitory concentration (IC50) values were 214.31 μg/mL, 196.03 μg/mL, and 387.8 μg/mL in n-hexane, chloroform, and water fraction-treated cells, respectively. Among these fractions, the chloroform fraction showed the strongest inhibitory effects, and thus, it was selected for further studies. NO is an inflammatory mediator that acts as an important cellular second messenger as well as a regulator of inflammatory responses (3,7). The iNOS protein expression decreased, which confirmed the suppressive effect of the RSL chloroform fraction on NO production (Fig. 3A). Activation of iNOS can promote NO production. As shown in Fig. 3B, the RSL chloroform fraction suppressed iNOS mRNA expression in a concentration-dependent manner. The blocking effect of the RSL chloroform fraction in LPS-stimulated RAW264.7 macrophages might have resulted from transcriptional inhibition of the iNOS gene. In addition, mRNA expression of inflammatory related cytokines such as TNF-α and IL-6 was suppressed by RSL in a concentration-dependent manner (Fig. 3C and 3D).
Fig. 2.
(A) Cytotoxicity of leaves of Raphanus sativus L. (RSL) at different concentrations (0, 1, 10, 100, and 200 μg/mL). (B) Effect of RSL fractions (n-hexane, chloroform, ethyl acetate, n-butanol, and water) on lipopolysaccharide (LPS)-stimulated nitrite production in RAW264.7 macrophages and IC50 values. Each value represents the mean±SD. Nitrite concentration in the absence and presence of LPS was compared and statistical difference was denoted as #P<0.001. Nitrite concentration under each fraction treatment was compared to LPS stimulated control and statistical difference was denoted as *P<0.05. All experiments were performed in triplicate.
Fig. 3.
(A) iNOS protein expression by western blot on leaves of Raphanus sativus L. (RSL) chloroform fraction. Relative mRNA expression of iNOS (B), TNF-α (C), and IL-6 (D). Each value represents the mean±SD. mRNA level in the absence and presence of RSL was compared and expressed as **P<0.01. All experiments were performed in triplicate.
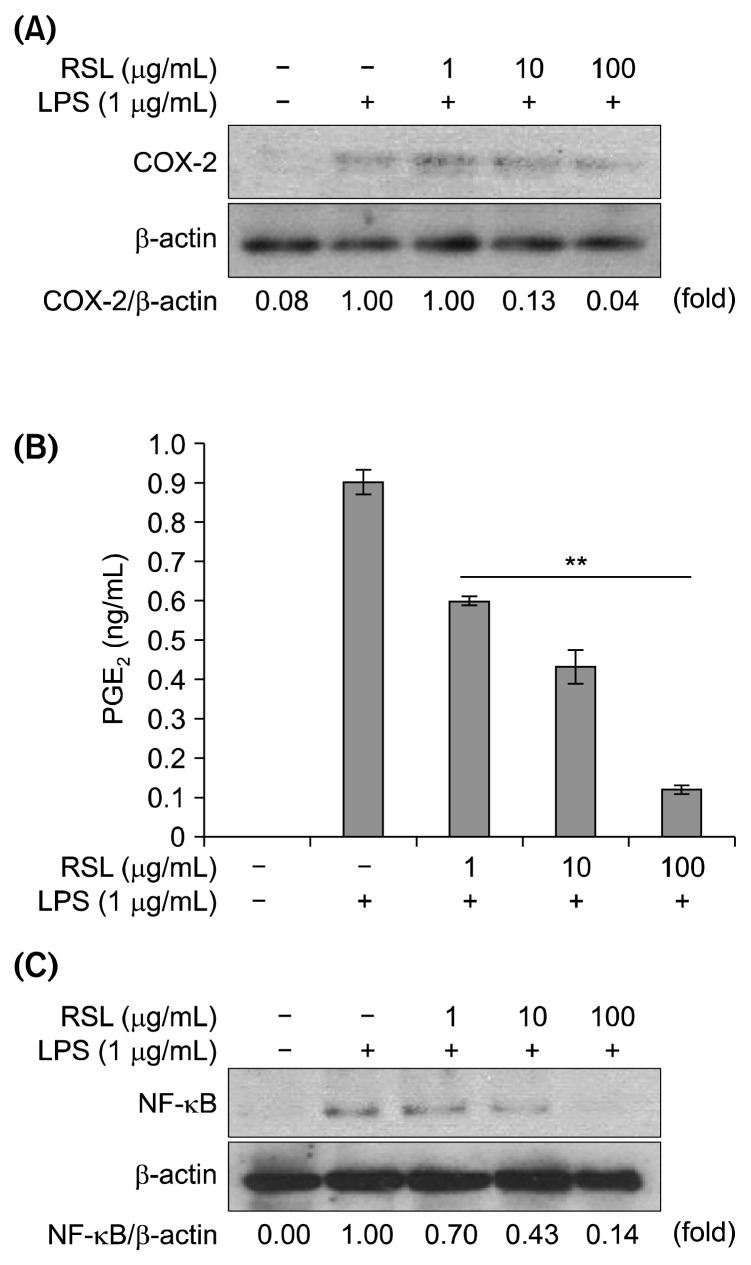
The effects of the RSL chloroform fraction on COX-2 protein expression were investigated. As shown in Fig. 4A, LPS treatment significantly increased COX-2 protein expression; however, it was suppressed by the RSL chloroform fraction treatment in a concentration-dependent manner. In inflammatory responses, PGE2 is an important inflammatory mediator, which contributes to tissue edema, increased blood flow, hyperalgesia, and pain sensitization at the sites of inflammation (20). PGE2 is mainly produced by the action of COX-2 on arachidonic acid. In this study, it was found that RSL significantly inhibited COX-2 protein expression in a concentration-dependent manner, which reduced PGE2 production as well (Fig. 4B). Thus, the anti-inflammatory effects of RSL might be attributed to its inhibitory effects on PGE2 production via inhibition of COX-2 protein expression.
Fig. 4.
The effect of leaves of Raphanus sativus L. (RSL) chloroform fraction on LPS-stimulated RAW264.7 cells at (A) COX-2 protein expression, (B) PGE2 level on different RSL concentration. PGE2 levels in the absence and presence of RSL was compared and expressed as **P<0.01. (C) NF-κB protein expression when treated on different concentrations (0, 1, 10, and 100 μg/mL).
The release of various inflammatory cytokines and mediators by LPS-stimulated macrophages is related to NF-κB (21–24). NF-κB is activated in inflammatory responses, where it is involved in the transcriptional activation of iNOS, TNF-α, IL-6, and COX-2 expressing genes (12). Because the RSL chloroform fraction showed suppressive effects of those genes as shown in Fig. 3, the expression level of NF-κB was investigated. As seen in Fig. 4C, the result showed that LPS-induced NF-κB activation in RAW264.7 cells was suppressed by RSL treatment. The chloroform fraction of RSL inhibited NF-κB expression, and subsequently inhibited NF-κB nuclear translocation. Treatment with the RSL chloroform fraction might lead to transcriptional suppression of iNOS-and COX-2-encoding genes because NF-κB is the key regulator of the transcriptional activation of these genes. To our knowledge, this is the first study to show that the RSL chloroform fraction inhibits inflammatory responses in macrophages via inhibition of NF-κB signaling pathways.
In conclusion, we showed that the RSL chloroform fraction suppressed LPS-induced production of pro-inflammatory mediators in RAW264.7 macrophages via modulation of the signal transduction cascades. The chloroform fraction of RSL inhibited the release of several inflammatory mediators from the macrophages, including iNOS, COX-2, and pro-inflammatory cytokines. These findings suggest that the RSL extract can be used as a potential cost-effective alternative for treatment and/or prevention of inflammatory diseases. However, further studies on inflammatory disease animal models and more mechanism studies are needed to provide a better understanding of the preventive effects of RSL in inflammatory disorders.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NO. 2013R1A1A1075992 and 2015R1C1 A2A01051880), the Industrial Strategic technology development program (No. N039200089) funded by the Ministry of Trade, Industry & Energy (MI, Korea), and the Gachon University research fund of 2014 (GCU-2014-0115).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp Neurol. 2007;205:295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 3.Rainsford KD. Anti-inflammatory drugs in the 21st century. Subcell Biochem. 2007;42:3–27. doi: 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- 4.Hawkey CJ, Langman MJS. Non-steroidal anti-inflammatory drugs: overall risks and management. Complementary roles for COX-2 inhibitors and proton pump inhibitors. Gut. 2003;52:600–608. doi: 10.1136/gut.52.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan K, Shaw D, Simmonds MS, Leon CJ, Xu Q, Lu A, Sutherland I, Ignatova S, Zhu YP, Verpoorte R, Williamson EM, Duez P. Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional Chinese medicine and Chinese materia medica. J Ethnopharmacol. 2012;140:469–475. doi: 10.1016/j.jep.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Goh AR, Youn GS, Yoo KY, Won MH, Han SZ, Lim SS, Lee KW, Choi SY, Park J. Aronia melanocarpa concentrate ameliorates pro-inflammatory responses in HaCaT keratinocytes and 12-O-tetradecanoylphorbol-13-acetate-induced ear edema in mice. J Med Food. 2016;19:654–662. doi: 10.1089/jmf.2015.3624. [DOI] [PubMed] [Google Scholar]
- 7.Lee AK, Sung SH, Kim YC, Kim SG. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-α and COX-2 expression by sauchinone effects on I-κBα phosphorylation, C/EBP and AP-1 activation. Br J Pharmacol. 2003;139:11–20. doi: 10.1038/sj.bjp.0705231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saba E, Jeon BR, Jeong DH, Lee K, Goo YK, Kwak D, Kim S, Roh SS, Kim SD, Nah SY, Rhee MH. A novel Korean red ginseng compound gintonin inhibited inflammation by MAPK and NF-κB pathways and recovered the levels of mir-34a and mir-93 in RAW 264.7 cells. Evid Based Complement Alternat Med. 2015;2015:624132. doi: 10.1155/2015/624132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi KC, Cho SW, Kook SH, Chun SR, Bhattarai G, Poudel SB, Kim MK, Lee KY, Lee JC. Intestinal anti-inflammatory activity of the seeds of Raphanus sativus L. in experimental ulcerative colitis models. J Ethnopharmacol. 2016;179:55–65. doi: 10.1016/j.jep.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 10.Barillari J, Iori R, Papi A, Orlandi M, Bartolini G, Gabbanini S, Pedulli GF, Valgimigli L. Kaiware Daikon (Raphanus sativus L.) extract: a naturally multipotent chemopreventive agent. J Agric Food Chem. 2008;56:7823–7830. doi: 10.1021/jf8011213. [DOI] [PubMed] [Google Scholar]
- 11.Hanlon PR, Webber DM, Barnes DM. Aqueous extract from Spanish black radish (Raphanus sativus L. Var. niger) induces detoxification enzymes in the HepG2 human hepatoma cell line. J Agric Food Chem. 2007;55:6439–6446. doi: 10.1021/jf070530f. [DOI] [PubMed] [Google Scholar]
- 12.Kim WK, Kim JH, Jeong DH, Chun YH, Kim SH, Cho KJ, Chang MJ. Radish (Raphanus sativus L. leaf) ethanol extract inhibits protein and mRNA expression of ErbB2 and ErbB3 in MDA-MB-231 human breast cancer cells. Nutr Res Pract. 2011;5:288–293. doi: 10.4162/nrp.2011.5.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KH, Moon E, Kim SY, Choi SU, Lee JH, Lee KR. 4-Methylthio-butanyl derivatives from the seeds of Raphanus sativus and their biological evaluation on anti-inflammatory and antitumor activities. J Ethnopharmacol. 2014;151:503–508. doi: 10.1016/j.jep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Kook SH, Choi KC, Lee YH, Cho HK, Lee JC. Raphanus sativus L. seeds prevent LPS-stimulated inflammatory response through negative regulation of the p38 MAPK-NF-κB pathway. Int Immunopharmacol. 2014;23:726–734. doi: 10.1016/j.intimp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Gutiérrez R, Perez R. Raphanus sativus (radish): their chemistry and biology. Sci World J. 2004;4:811–837. doi: 10.1100/tsw.2004.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laroux FS, Pavlick KP, Hines IN, Kawachi S, Harada H, Bharwani S, Hoffman JM, Grisham MB. Role of nitric oxide in inflammation. Acta Physiol. 2001;173:113–118. doi: 10.1046/j.1365-201X.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-κB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 19.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF- κB activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/S0027-5107(01)00183-X. [DOI] [PubMed] [Google Scholar]
- 20.Giuliano F, Warner TD. Origins of prostaglandin E2: involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. J Pharmacol Exp Ther. 2002;303:1001–1006. doi: 10.1124/jpet.102.041244. [DOI] [PubMed] [Google Scholar]
- 21.Herlaar E, Brown Z. p38 MAPK signalling cascades in inflammatory disease. Mol Med Today. 1999;5:439–447. doi: 10.1016/S1357-4310(99)01544-0. [DOI] [PubMed] [Google Scholar]
- 22.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy–from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Keys JR, Landvatter SW, Strickler JE, McLaughlin MM, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 24.Kim MS, Bang JH, Lee J, Han JS, Kang HW, Jeon WK. Fructus mume ethanol extract prevents inflammation and normalizes the septohippocampal cholinergic system in a rat model of chronic cerebral hypoperfusion. J Med Food. 2016;19:196–204. doi: 10.1089/jmf.2015.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]