Abstract
Lactococcus lactis subsp. lactis NCDO 2118 was recently reported to alleviate colitis symptoms via its anti-inflammatory and immunomodulatory activities, which are exerted by exported proteins that are not produced by L. lactis subsp. lactis IL1403. Here, we used in vitro and in silico approaches to characterize the genomic structure, the safety aspects, and the immunomodulatory activity of this strain. Through comparative genomics, we identified genomic islands, phage regions, bile salt and acid stress resistance genes, bacteriocins, adhesion-related and antibiotic resistance genes, and genes encoding proteins that are putatively secreted, expressed in vitro and absent from IL1403. The high degree of similarity between all Lactococcus suggests that the Symbiotic Islands commonly shared by both NCDO 2118 and KF147 may be responsible for their close relationship and their adaptation to plants. The predicted bacteriocins may play an important role against the invasion of competing strains. The genes related to the acid and bile salt stresses may play important roles in gastrointestinal tract survival, whereas the adhesion proteins are important for persistence in the gut, culminating in the competitive exclusion of other bacteria. Finally, the five secreted and expressed proteins may be important targets for studies of new anti-inflammatory and immunomodulatory proteins. Altogether, the analyses performed here highlight the potential use of this strain as a target for the future development of probiotic foods.
Introduction
The genus Lactococcus is part of the lactic acid bacteria (LAB), one of the most biotechnologically important groups of bacteria, which is composed of Lactococcus, Streptococcus, Lactobacillus, Weissella and others [1]. LAB species share in common the ability to convert sugar (mainly glucose) into lactic acid through specific metabolic pathways. Additionally, these species are facultative anaerobic, catalase negative and non-motile. Moreover, there is a close phylogenetic relationship between the bacteria of this group [2].
Many LAB species are biotechnologically important due to their safety aspects, achieved because they have been used for years in the preservation and maintenance of food [3]. Previous studies highlight the importance of genome sequencing in the discovery of new features related to LAB: genes coding for proteolytic enzymes (which participate in cheese maturation) in Lactobacillus helveticus [4], identification of citrate catabolic pathways in Lactobacillus casei [5], and genes responsible for decarboxylation of alpha-keto acid branched chain in Lactococcus lactis [6; 7].
Genome sequencing studies have also helped in the elucidation of probiotic effects exerted by LAB. For instance, in Lactobacillus reuteri, genome analyses have focused on the capacity to adapt to nutrient availability and environmental conditions of the GI tract, the adhesion mechanisms, the production of antimicrobial compounds, and the mechanisms of immunomodulation, such as the synthesis of pro-inflammatory extracellular polymeric substances (EPS compounds) [8]. Moreover, Lactobacillus rhamnosus and L. casei strains isolated from marketed probiotic products were compared with the well-studied L. rhamnosus GG and L. casei BL23, mainly focusing on pilus gene clusters and metabolic pathways analyses [9]. Interestingly, a new adhesion-associated protein, cwaA, was identified through genome sequencing and comparative genomics analyses of Lactobacillus plantarum NL42. The expression of cwaA in L. lactis has significantly increased its autoaggregation, hydrophobicity and exclusion ability, where the mutant strain was able to inhibit the adhesion of Staphylococcus aureus and Escherichia coli to HT-29 cells [10]. Another study illustrated the mechanisms by which Lactobacillus species from the intestinal niche have adapted to the gastrointestinal tract (GIT) by acquiring traits, such as stress tolerance, carbohydrate absorption, adhesion to epithelial cells and mucus [11].
Additionally, many species of this group are important for their probiotic effects, such as the genus Lactobacillus, which is used in the production of the fermented milk Yakult [12], and Bifidobacteria, widely known for their beneficial effects on the host intestinal tract [13]. However, although several works highlight the probiotic effects of LAB, most focus on Lactobacillus and Bifidobacterium species [14], whereas few studies report the beneficial effects of L. lactis strains. For instance, Lactococcus lactis subsp. cremoris FC has an important anti-inflammatory activity [15]. The probiotic properties of L. lactis subsp. cremoris IBB477 have attracted attention due to their adhesion mechanisms and survival in the intestinal environment [16; 17]. Additionally, it was recently demonstrated, through the evaluation of three L. lactis strains in vitro, that Lactococcus lactis subsp. lactis NCDO 2118 has anti-inflammatory and immunomodulatory activity that can alleviate colitis symptoms [18]. This strain was described as a gamma-aminobutyric acid (GABA) producer [19]. It has been extensively used for heterologous expression [20], and its probiotic effect is associated with exported proteins [18].
Here, we use comparative genomics and in silico analyses to provide insights into the probiotic nature of L. lactis NCDO 2118. The criteria for screening LAB strains before their use as probiotics include assessing functional features, such as the ability to resist environmental conditions found in the digestive tract (low gastric pH and bile salts) and the ability to antagonize or competitively exclude pathogens, which is achieved by secreting antimicrobial substances or competing for nutrients and epithelial adhesion sites. LAB produce different antimicrobial components, such as organic acids, hydrogen peroxide, carbon peroxide, diacetyl, low molecular weight antimicrobial substances, bacteriocins and adhesion inhibitors. The adhesiveness of LAB may involve passive forces, electrostatic interactions, hydrophobic steric forces, lipoteichoic acids, and lectins [21]. The hydrophobic nature of the outermost surface of microorganisms facilitates the adhesion of bacteria to the host epithelium, thereby conferring competitive advantages during the colonization of the GIT [22]. The antimicrobial susceptibility of intestinal microorganisms is an important criterion for the selection of probiotic strains, mainly due to the potential transfer of those genes to pathogenic or commensal bacteria that inhabit the GIT [23]. In the following sections, we present comparative genomic analyses of L. lactis NCDO 2118 and other Lactococcus species and predict genes that putatively code for acid stress resistance proteins, bacteriocins, adhesins and exported proteins.
Results
General features, phylogenomics and synteny analyses
The general genomic features of all genomes used in this work are summarized in Table 1.
Table 1. Complete genomes and genomic features of Lactococcus species and Streptococcus thermophilus used in genomic comparisons.
| Strain | Size (bp) | GC% | Genes | Proteins | Source | Accession Number | Plasmids | Pseudogenes | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Lactococcus lactis subsp. lactis NCDO 2118 | 2,554,693 | 34,86 | 2,471 | 2,386 | Frozen peas | CP009054 | 1 | 52 | [24] |
| Lactococcus lactis subsp. lactis IL1403 | 2,365,589 | 35,30 | 2,406 | 2,277 | Dairy starter | AE005176 | - | 45 | [3] |
| Lactococcus lactis subsp. lactis KF147 | 2,598,144 | 34,86 | 2,662 | 2,473 | Mung Bean | CP001834 | 1 | 93 | [25] |
| Lactococcus lactis subsp. lactis KLDS 40325 | 2,589,250 | 35,39 | 2,732 | 2,593 | Fermented milk | CP006766 | 1 | 56 | [26] |
| Lactococcus lactis subsp. lactis IO-1 | 2,421,471 | 35,10 | 2,318 | 2,224 | Water (drain pit of a kitchen sink) | AP012281 | - | - | [27] |
| Lactococcus lactis subsp. lactis CV56 | 2,399,458 | 35,09 | 2,549 | 2,408 | Healthy woman’s vagina | CP002365 | 5 | 51 | [28] |
| Lactococcus lactis subsp. lactis S0 | 2,488,699 | 35,20 | 2,482 | 2,311 | Fresh raw milk | CP010050 | - | 88 | Unpublished |
| Lactococcus lactis subsp. lactis AI06 | 2,398,091 | 35,04 | 2,320 | 2,178 | Açaí palm | CP009472 | - | 61 | [29] |
| Lactococcus lactis subsp. cremoris A76 | 2,452,616 | 35,88 | 2,845 | 2,769 | Dairy starter | CP003132 | 4 | - | [30] |
| Lactococcus lactis subsp. cremoris KW2 | 2,427,048 | 35,70 | 2,353 | 2,268 | Fermented corn | CP004884 | - | 1 | [31] |
| Lactococcus lactis subsp. cremoris MG1363 | 2,529,478 | 35,70 | 2,597 | 2,434 | Dairy starter | AM406671 | - | 82 | [32] |
| Lactococcus lactis subsp. cremoris NZ9000 | 2,530,294 | 35,70 | 2,594 | 2,510 | Dairy starter | CP002094 | - | - | [33] |
| Lactococcus lactis subsp. cremoris SK11 | 2,438,589 | 35,82 | 2,739 | 2,501 | Dairy starter | CP000425 | 5 | 144 | [34] |
| Lactococcus lactis subsp. cremoris UC5099 | 2,250,427 | 35,76 | 2,401 | 2,109 | Dairy starter | CP003157 | 8 | 188 | [35] |
| Lactococcus garvieae ATCC49156* | 1,950,135 | 38,80 | 2,024 | 1,947 | Fish (Alosa fallax) | AP009332 | - | 0 | [36] |
| Lactococcus garvieae Lg2* | 1,963,964 | 38,80 | 2,045 | 1,968 | Fish (Alosa fallax) | AP009333 | - | 0 | [36] |
| Streptococcus thermophilus LMD-9** | 1,856,368 | 39.08 | 1960 | 1743 | Dairy starter | CP000419 | 2 | 132 | [37] |
* Lactococcus garvieae are fish pathogens
** Streptococcus thermophilus was used as a closely related outgroup in the analyses
Briefly, Lactococcus garvieae strains have the highest G+C content, ~38.80%, whereas the lowest G+C contents, ~34.86%, were from L. lactis NCDO 2118 and L. lactis KF147, both isolated from vegetables. Additionally, the genome sizes of the Lactococcus species range from ~1.95 Mb to ~2.60 Mb, and the two L. garvieae strains have the smallest genomes.
In this work, the only species harboring plasmids were L. lactis NCDO 2118, L. lactis KF147, Lactococcus lactis subsp. lactis KLDS 40325 and Lactococcus lactis subsp. lactis CV56 strains, Lactococcus lactis subsp. cremoris A76, Lactococcus lactis subsp. cremoris SK11 and Lactococcus lactis subsp. cremoris UC5099 (L. cremoris UC5099) strains, where the latter harbored the greatest number of plasmids (Table 1).
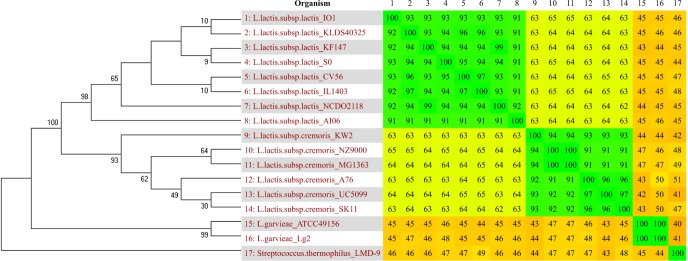
From the heatmap created with Gegenees (Fig 1), it is possible to visualize a high similarity between the subspecies of Lactococcus, with nucleotide similarities ranging from 40% to 100%. Additionally, the species and subspecies clustered separately, creating 3 green blocks of strains at the chart, represented by L. lactis subsp. lactis and L. lactis subsp. cremoris, with similarities ranging from 91% to 100%, and L. garvieae, in which the two strains of this species were 100% similar to each other.
Fig 1. 16S phylogenetic tree and genomic heatmap of Lactococcus genus.
The Streptococcus thermophilus LMD-9 (position 17) was added to root the tree. The species in comparison are distributed from 1 to 17 in the same order, both vertically and horizontally. The numbers in the heatmap show the percentage of similarity between the species, varying from yellow (low similarity) to green (high similarity), or from 40% to 100%, respectively. The heatmap and the phylogenetic tree were created with the software Gegenees and Mega (Neighbor-Joining method with 1000 bootstraps replicates), respectively.
On the phylogenetic tree created using 16S, the species and subspecies also clustered together, forming two main clades corresponding to the best similarity among L. lactis subsp. lactis and L. lactis subsp. cremoris (Fig 1). Additionally, L. garvieae strains appeared in an outside node compared to L. lactis species and are the two most distinct and distant species of Lactococcus on the heatmap and phylogenetic tree. Briefly, on the heatmap, the degree of intraspecies similarity varies from 91% to 100%, whereas interspecies similarity varies from 40% to 65%.
From the genome synteny analysis (S1 Fig), all strains from L. lactis subsp. lactis presented a high degree of synteny, where the most conserved genome compared to L. lactis NCDO 2118 (chosen as reference genome) was L. lactis KF147. Additionally, we performed a comparison with the plasmids of L. lactis NCDO 2118 and L. lactis KF147 strains. However, we verified a high degree of similarity from the beginning to the end of each plasmid sequence, meaning that they possibly harbored the same plasmid (data not shown).
Metabolic pathways prediction
To identify conserved or non-conserved metabolic pathways, we used three different datasets, consisting of (1) the closely related L. lactis NCDO 2118, L. lactis KF147 and L. lactis IL1403, (2) all strains from L. lactis subsp. lactis and L. lactis subsp. cremoris (non-pathogenic dataset), and (3) all strains from this study (including L. garviae). The number of metabolic pathways harbored by each genome varies from 148 to 206, with a general mean of ~183 pathways. Both L. garvieae strains contained 148 metabolic pathways, L. lactis subsp. lactis showed an average of ~192 metabolic pathways, and L. lactis subsp. cremoris showed ~186 pathways.
The main differences were that the strain L. lactis NCDO 2118 contains more peptidoglycan biosynthesis pathways than L. lactis KF147 and L. lactis IL1403 strains. Other exclusive metabolic features of L. lactis NCDO 2118 in this context were complete anaerobic respiration pathways, fermentation of pyruvate to acetate, fermentation of fumarate, complete heterolactic fermentation, valine degradation, L-serine degradation, ammonia assimilation to glutamate, complete superpathway of acetate utilization and formation, protein degradation, initial pathway of sucrose degradation I, valine degradation, lysine degradation I and acyl-ACP thioesterase pathway (S1 Table).
Genome plasticity
We identified 5 prophages in L. lactis NCDO 2118, of which 2 were incomplete, and 3 were considered intact (Table 2). The three intact phages harbored important genes such as rusA, arsC1, arsC3, amtB, rpmE2, carA, pyrB, pyrP and pepT.
Table 2. Intact and incomplete phages predicted in L. lactis subsp. lactis NCDO 2118.
| Phages | Genes | Proteins |
|---|---|---|
| Region 1 –Intact phage | rusA e arsC1 | Integrase, Prophage, Phage antirepressor, Transcriptional regulator, Recombinase, Endodeoxyribonuclease, Aminotransferase, Phage terminase small subunit, Peptidase, Bacteriophage lysine, Arsenate reductase |
| Region 2 –Intact phage | amtB, kinA, llra, rpmE2, arsC3, carA, pyrB, pyrP | Ammonium transporter, Sensor protein kinase, Two-component system regulator, 50S ribosomal protein L31 type B, Universal stress protein, Arsenate reductase, Bacteriophage lysine, Phage tail protein, Head-tail joining protein, Capsid protein, Phage ATP-dependent endopeptidase, Phage terminase small subunit, Endonuclease, Terminase, Replisome organizer, BRO-like protein, DNA binding protein, Phage integrase, Carbamoyl-phosphate synthase small chain, Aspartate carbamoyltransferase, Uracil transporter |
| Region 3 –Intact phage | pepT, ppaC, pflA, ysiA, ysiB | Amino Acid permease, Peptidase T, Manganese-dependent inorganic pyrophosphatase, Pyruvate-formate lyase activating enzyme, Permease, Phage protein, Integrase |
| Region 4 –Incomplete phage | ardA, ecfA1, ecfA2, ecfT, dapH, yciA | Peptidoglycan hydrolase, Antirestriction protein, Integrase, ATPase, Energy-coupling factor transporter, Thiol-disulfide isomerase, N-acetyldiaminopimelate deacetylase |
| Region 5 –Incomplete phage | glnA | Integrase, Bacteriocin, DNA primase, Glutamine synthetase |
Phage locations were predicted using the software PHAST.
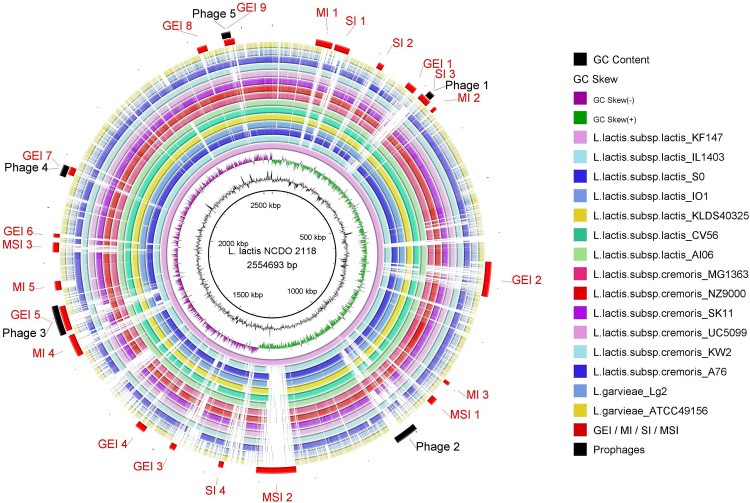
Additionally, we used BRIG to visualize the plasticity events from phage sequences (Fig 2). According to the BRIG analyses, phage 1 was incomplete in all species, except for the reference genome L. lactis NCDO 2118 and L. lactis KF147. Both phages 2 and 3, predicted as intact in the reference, were also present in L. lactis KF147, L. lactis IL1403, Lactococcus lactis subsp. cremoris NZ9000 and L. cremoris MG1363, whereas the former phage was also found in Lactococcus lactis subsp. cremoris KW2. Phage 4, also intact in the reference genome, was present in all other species. Phage 5, predicted as incomplete in the reference genome, was absent in L. lactis IO-1, L. cremoris KW2, L. cremoris UC5099 and partially present in both L. garvieae strains.
Fig 2. Circular comparison of the Lactococcus genus using L. Lactis NCDO 2118 as a reference.
Each ring of the circle corresponds to a specific complete genome represented in the legend on the right. The similarity between species is represented by the intensity of the color. Darker colors represent higher similarities than bright ones. Deleted regions are represented by blank spaces inside the circles. (GEI = Genomic Island; MI = Metabolic Island; SI = Symbiotic Island; MSI = Miscellaneous Island, harboring both metabolic and symbiotic factors). Genomic islands and phage sequences were predicted with GIPSy and PHAST, respectively. The circular genomic comparisons were created with BRIG.
In the GIPSy predictions, we identified 9 Genomic Islands (GEIs), 5 Metabolic Islands (MIs), 4 Symbiotic Islands (SIs) and 3 Miscellaneous Islands (MSIs), which were predicted as harboring both metabolic and symbiotic related factors. The GEIs are listed in S2 Table.
All SIs were only partially present in the other strains, except for SI4, which was absent from all L. garviae strains, L. lactis subsp. cremoris strains and L. lactis IL 1403 (Fig 2). Additionally, all MIs presented regions of deletions in the pathogenic species L. garviae. The most prominent GEIs were MI3, which was only present in the two strains isolated from plants (L. lactis NCDO 2118 and L. lactis KF147), and MSI 2, which presented the biggest region of deletion in all Lactococcus, except for L. lactis NCDO 2118 and L. lactis KF147.
Antibiotic resistance
LAB that are widely used as probiotics or in starter cultures have the potential to host antibiotic resistance genes, thereby presenting a risk of transferring such genes to many lactic acid bacteria and other pathogenic bacteria [23]. In the antibiogram assay, L. lactis NCDO 2118 was susceptible to ceftriaxone, erythromycin, tetracycline, ampicillin, penicillin and chloramphenicol and resistant to vancomycin, oxacillin and amikacin antibiotics (Table 3). Additionally, we tried to correlate the antibiogram profile with the genome content of L. lactis NCDO 2118, which presented 22 antibiotic resistance-related genes putatively coding for a VanZ family protein (NCDO2218_1094), penicillin-binding proteins (NCDO2118_0402, NCDO2118_0445, NCDO2118_0526, NCDO2118_0880 and NCDO2118_2216), and multidrug efflux pump proteins (Table 4). Additionally, no antibiotic resistance related gene presented deviation in its genomic signature.
Table 3. Antibiotic susceptibility of L. lactis NCDO 2118.
| Antibiotic susceptibility assay | |||
|---|---|---|---|
| Antibiotic | Concentration | Inhibition zone diameter (mm) | Susceptibility* |
| Ceftriaxone | 30 μg | 31 | S |
| Erythromycin | 10 μg | 31 | S |
| Tetracycline | 30 μg | 25 | S |
| Ampicillin | 30 μg | 35 | S |
| Vancomycin | 10 U | 0 | R |
| Penicillin | 30 μg | 35 | S |
| Amikacin | 30 μg | 15 | R |
| Chloramphenicol | 30 μg | 28 | S |
| Oxacillin | 1 μg | 14 | R |
* R = resistant, S = susceptible.
Table 4. Genes putatively coding for antibiotic resistance-related proteins.
| Query ID | Product | Gene | G+C Content | Codon Usage |
|---|---|---|---|---|
| NCDO2118_0089 | Multidrug resistance protein | sugE | NORMAL | NORMAL |
| NCDO2118_0090 | Multidrug efflux transporter | blt | NORMAL | NORMAL |
| NCDO2118_0108 | Multidrug resistance efflux pump | pmrB | NORMAL | NORMAL |
| NCDO2118_0144 | MFS transporter | ybfD | NORMAL | NORMAL |
| NCDO2118_0258 | Multidrug resistance ABC transporter | - | NORMAL | NORMAL |
| NCDO2118_0259 | Multidrug ABC transporter ATP-binding protein | - | NORMAL | NORMAL |
| NCDO2118_0363 | MFS transporter | napC | NORMAL | NORMAL |
| NCDO2118_0369 | Multidrug ABC transporter ATP-binding protein | lmrC | NORMAL | NORMAL |
| NCDO2118_0370 | Multidrug transporter | lmrD | NORMAL | NORMAL |
| NCDO2118_0402 | Penicillin-binding protein 2B | pbp2B | NORMAL | NORMAL |
| NCDO2118_0445 | Penicillin-binding protein 1B | pbp1B | NORMAL | NORMAL |
| NCDO2118_0526 | Penicillin-binding protein 1A | ponA | NORMAL | NORMAL |
| NCDO2118_0593 | Multidrug transporter | - | NORMAL | NORMAL |
| NCDO2118_0645 | Multi-drug resistance efflux pump | pmrA | NORMAL | NORMAL |
| NCDO2118_0726 | Multidrug resistance ABC transporter ATP-binding and permease protein | lmrA | NORMAL | NORMAL |
| NCDO2118_0880 | Penicillin-binding protein 2X | pbpX | NORMAL | NORMAL |
| NCDO2118_0930 | Multidrug resistance protein B | - | NORMAL | NORMAL |
| NCDO2118_1094 | VanZ family protein | - | NORMAL | NORMAL |
| NCDO2118_1401 | Multidrug MFS transporter | - | NORMAL | NORMAL |
| NCDO2118_1736 | Multidrug transporter | yqiA | NORMAL | NORMAL |
| NCDO2118_1995 | MFS transporter permease | yteD | NORMAL | NORMAL |
| NCDO2118_2216 | Penicillin-binding protein 2a | pbp2A | NORMAL | NORMAL |
G+C content and codon usage information were retrieved from GIPSy analyses.
Identification of genes involved in acid stress and bile salt resistance
We searched the genome sequence of L. lactis NCDO 2118 for genes previously shown to be differentially expressed on cells cultivated under low and optimum pH (5.1 and 6.5, respectively) in L. cremoris MG1363 [38] (Table 5). Additionally, we also searched for genes differentially regulated by bile exposure in Bifidobacterium animalis and Bifidobacterium longum NCIMB 8809 [39; 40] and/or identified on the total proteome and surfome of Lactobacillus rhamnosus GG using proteomics analyses (Table 5). Here, we identified some genes in L. lactis NCDO 2118 that were previously reported to be involved in the acid stress response, including genes coding for chaperones (dnaK) and stringent response. Additionally, DnaK and Enolase are plasminogen receptors involved in bile modulation during intestinal colonization.
Table 5. Genes coding for proteins involved in acid stress and bile salt resistance.
| Locus_tag | EC Number | Gene | Product | Stress response |
|---|---|---|---|---|
| NCDO2118_1870 | - | atpC | ATP synthase epsilon chain | Acid stress |
| NCDO2118_1871 | 3.6.3.14 | atpD | ATP synthase subunit beta | Acid stress |
| NCDO2118_1872 | - | atpG | ATP synthase gamma chain | Acid stress |
| NCDO2118_1873 | 3.6.3.14 | atpA | ATP synthase subunit alpha | Acid stress |
| NCDO2118_1874 | - | atpH | ATP synthase subunit delta | Acid stress |
| NCDO2118_1875 | - | atpF | ATP synthase subunit b | Acid stress |
| NCDO2118_1876 | - | atpB | ATP synthase subunit a | Acid stress |
| NCDO2118_1877 | - | atpE | ATP synthase subunit C | Acid stress |
| NCDO2118_1384 | 1.1.1.27 | ldh | L-lactate dehydrogenase | Acid stress |
| NCDO2118_0475 | - | ptcC | PTS system, cellobiose-specific IIC component | Acid stress |
| NCDO2118_0542 | 1.2.1.12 | gapA | Glyceraldehyde-3-phosphate dehydrogenase | Acid stress |
| NCDO2118_0399 | 5.4.2.11 | gpmA | 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase | Acid stress/bile resistance |
| NCDO2118_2272 | 5.3.1.9 | pgi | Glucose-6-phosphate isomerase | Acid stress |
| NCDO2118_0096 | 2.7.1.40 | pyk1 | Pyruvate kinase | Acid stress |
| NCDO2118_1385 | 2.7.1.40 | pyk2 | Pyruvate kinase | Acid stress |
| NCDO2118_0240 | 2.7.2.3 | pgk | Phosphoglycerate kinase | Acid stress/bile resistance |
| NCDO2118_0417 | - | recA1 | Protein RecA | Acid stress |
| NCDO2118_1251 | - | recA2 | Protein RecA | Acid stress |
| NCDO2118_0540 | - | clpE | ATP-dependent Clp protease ATP-binding subunit | Acid stress |
| NCDO2118_0453 | - | groL | 60 kDa chaperonin | Acid stress |
| NCDO2118_1545 | - | clpB | Chaperone protein | Acid stress |
| NCDO2118_0467 | 1.15.1.1 | sodA | Superoxide dismutase | Acid stress |
| NCDO2118_0073 | 2.7.6.5 | relA | GTP pyrophosphokinase | Acid stress |
| NCDO2118_0637 | 4.2.1.11 | eno | Enolase | Acid stress/bile resistance |
| NCDO2118_1019 | - | dnaK | Chaperone protein | Acid stress/bile resistance |
| NCDO2118_1594 | 3.5.99.6 | nagB | Glucosamine-6-phosphate deaminase/isomerase | Bile resistance |
| NCDO2118_1909 | 3.4.24.- | pepO | Endopeptidase O | Bile resistance |
| NCDO2118_0941 | 5.4.99.9 | glf | UDP-galactopyranose mutase | Bile resistance |
| NCDO2118_0500 | 6.3.4.2 | pyrG | CTP synthase | Bile resistance |
| NCDO2118_0035 | 1.8.1.4 | pdhd | Pyruvate dehydrogenase | Bile resistance |
| NCDO2118_2145 | 6.1.1.19 | argS | Arginyl-tRNA synthetase | Bile resistance |
| NCDO2118_1958 | - | oppA | Oligopeptide-binding protein | Bile resistance |
| NCDO2118_2203 | - | rpsC | 30S ribosomal protein S3 | Bile resistance |
| NCDO2118_2191 | - | rpsE | 30S ribosomal protein S5 | Bile resistance |
| NCDO2118_2208 | - | rplD | 50S ribosomal protein L4 | Bile resistance |
| NCDO2118_2197 | - | rplE | 50S ribosomal protein L5 | Bile resistance |
| NCDO2118_2193 | - | rplF | 50S ribosomal protein L6 | Bile resistance |
Additionally, we assayed L. lactis NCDO 2118 to see how it responds to the challenges of acid pH and bile salt secretion in the gastrointestinal tract. When in contact with artificial gastric juice, 48% of the L. lactis NCDO 2118 was not inhibited and was able to grow after acid pH challenge, whereas the contact with bile salts inhibited 95% of the bacteria growth, showing a high sensibility, as a result of three independent experiments (S2 Fig).
Identification of genes coding for adhesins and adhesion-related proteins
Based on literature data, we predicted proteins involved in the adhesion mechanisms of L. lactis NCDO 2118, shown in Table 6. L. lactis NCDO 2118 harbors 19 genes putatively coding for adhesion-related proteins, such as the gene chiA (NCDO2118_2053) and the genes coding for the Chitin binding protein (CBP–NCDO2118_2054) and the laminin-binding protein (NCDO2118_1446).
Table 6. Proteins potentially involved in the adhesion mechanisms of L. lactis.
| Locus_tag | Gene | Product |
|---|---|---|
| NCDO2118_0315 | Hypothetical protein | |
| NCDO2118_0552 | Hypothetical protein | |
| NCDO2118_0647 | pycA | Pyruvate carboxylase |
| NCDO2118_0684 | ChW repeat-/cell adhesion domain-containing transglutaminase-like protease | |
| NCDO2118_0727 | Hypothetical protein | |
| NCDO2118_0774 | Flagellar hook-length control protein FliK | |
| NCDO2118_0776 | Hypothetical protein | |
| NCDO2118_0806 | exoA | Exodeoxyribonuclease |
| NCDO2118_0857 | Hypothetical protein | |
| NCDO2118_1205 | Hypothetical protein | |
| NCDO2118_1365 | Hypothetical protein | |
| NCDO2118_1446 | bmpA | Basic membrane protein A (laminin-binding protein) |
| NCDO2118_1515 | ypdD | Sugar hydrolase |
| NCDO2118_1627 | Hypothetical protein | |
| NCDO2118_2053 | chiA | Chitinase |
| NCDO2118_2054 | Chitin binding protein | |
| NCDO2118_2211 | Hypothetical protein | |
| NCDO2118_2278 | Fibronectin-binding protein | |
| NCDO2118_2284 | Hypothetical protein |
To determine whether L. lactis NCDO 2118 exhibited adhesive ability, corroborating the in silico data, we performed microbial adhesion to solvents (MATS) experiments, which demonstrated a moderate cell surface hydrophobicity, as suggested by Nader-Macías et al., (2008) [41], with 52% association with xylene.
Bacteriocins and other competitive exclusion mechanisms
To predict putative bacteriocins, we used the software BAGEL [42]. In addition to identification, BAGEL also classifies the bacteriocins into three classes: (i) lanthionine-containing bacteriocins/lantibiotics, (ii) non-lanthionine-containing bacteriocins and (iii) bacteriolysins/non-bacteriocin lytic proteins [43].
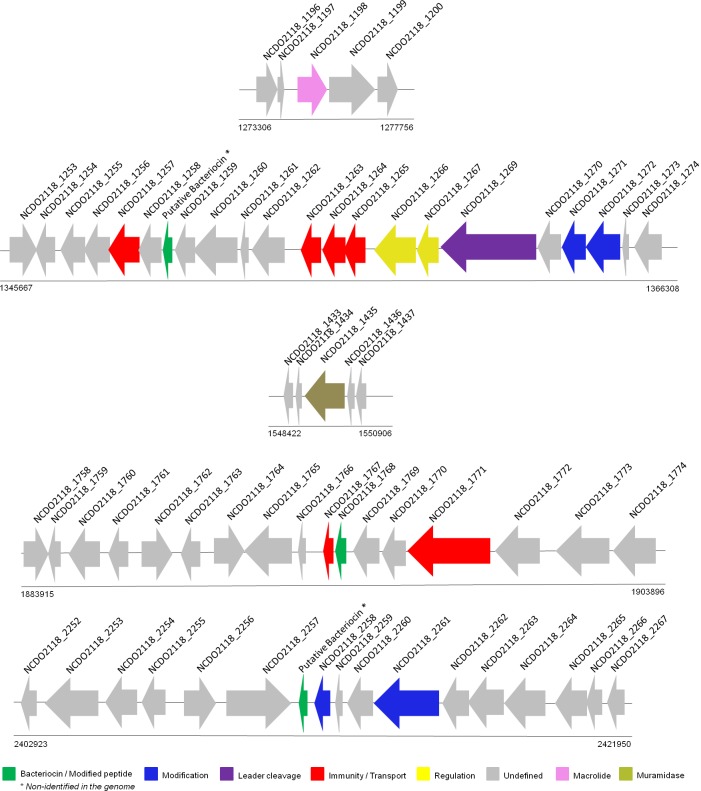
In L. lactis NCDO 2118, BAGEL predicted one bacteriocin for each of the three classes (Fig 3): a lanthipeptide (class I), NCDO2118_1768 (putative Bacteriocin-lactococcin-A—class II) and a putative bacteriocin (class III), located between NCDO2118_2257 and NCDO2118_2258. The class III putative bacteriocin was not described in the L. lactis NCDO 2118 genome, possibly because the gene-finding methodology failed to predict it. The bacteriocin of class I is a lantibiotic Nisin coded by the nisZ gene (NCDO2118_1272), a natural variant of nisA [44]. Briefly, Nisin is commonly produced by strains of L. lactis, and the cluster of genes coding for the nisin biosynthesis proteins consists of 11 genes: nisABTCIP (biosynthesis and immunity), nisFEG (immunity) and the two-component regulatory system nisRK [45]. L. lactis NCDO 2118 harbors a nisBCIP operon (where nisP is a pseudogene), a nisRK two-component system and a nisFEG operon. Additionally, BAGEL has predicted the presence of another putative bacteriocin between NCDO2118_1258 and NCDO2118_1259 that is located close to the class I cluster of genes. However, the amino acid sequence predicted from this region only presents similarity to a hypothetical protein. Lactococcin A is a class IId, non-pediocin-like, single-peptide bacteriocin normally produced by strains of L. lactis. Four genes are responsible for the biosynthesis of lactococcin: the lactococcin-A coding gene, one immunity gene and the dedicated ABC transporter system along with its accessory protein. L. lactis NCDO 2118 harbors an immunity protein (NCDO2118_1767) and lactococcin-A (NCDO2118_1768). As for the class III prediction, the predicted putative bacteriocin is located upstream of two hypothetical proteins (NCDO2118_2258 and NCDO2118_2259); however, little is known about the organization of the gene cluster of class III bacteriocins [45], and the putative bacteriocin predicted by BAGEL only presents similarity to hypothetical proteins in GENBANK.
Fig 3. Regions of bacteriocins predicted with BAGEL in L. lactis NCDO 2118.
BAGEL predicted three putative bacteriocins, one of each class. (A) Putative bacteriocin/Class I predicted on orf010 (pseudogene) and nisZ was found with manual curation. (B) Putative bacteriocin/Class II predicted on orf027 (pseudogene). (C) Putative bacteriocin/Sactipeptidase predicted on orf011 (this region was not previously characterized in the L. lactis subsp. lactis NCDO 2118 genome). All putative bacteriocins were also identified in Bactibase.
Moreover, an additional bacteriocin-coding gene was harbored by GEI 9 (S2 Table), which was not predicted by BAGEL. Through blast analyses, we found a significant amino acid similarity, with identities varying from 76% to 98%, between this gene and a bacteriocin-coding gene from other L. lactis in the UNIPROT and NCBI BLAST databases. However, many of the genes were also described as hypothetical proteins. In addition, we also searched for other genes that could possibly play a role in the competitive exclusion of other bacteria. A Lyzozyme M1 and a Macrolide biosynthetic protein encoding genes were also included in S3 Table after manual curation in the L. lactis NCDO 2118 genome.
In the present study, a deferred agar spot assay was used for the initial determination of antagonistic activity via diffusible compound(s) produced by L. lactis NCDO 2118. To assay whether L. lactis NCDO 2118 could affect the growth of pathogenic bacteria, we used an approach to measure its antagonistic activity against the strains Salmonella enterica ATCC 14028, Escherichia coli ATCC 25723, Staphylococcus aureus 29213, Bacillus cereus ATCC 11778, Listeria monocytogenes ATCC 15313, Enterococcus faecalis ATCC 19433 and Pseudomonas aeruginosa ATCC 5853. L. lactis NCDO 2118 showed no effect on the growth of the abovementioned pathogenic strains.
In silico identification of putatively secreted proteins
Here, we strove to predict genes encoding secreted proteins from L. lactis NCDO 2118 that are absent from the genomes of the strains L. lactis IL1403 and L. cremoris MG1363, as the secreted proteins of L. lactis NCDO 2118 are possibly responsible for the immunomodulatory effects of this transient bacterium inside the host [18].
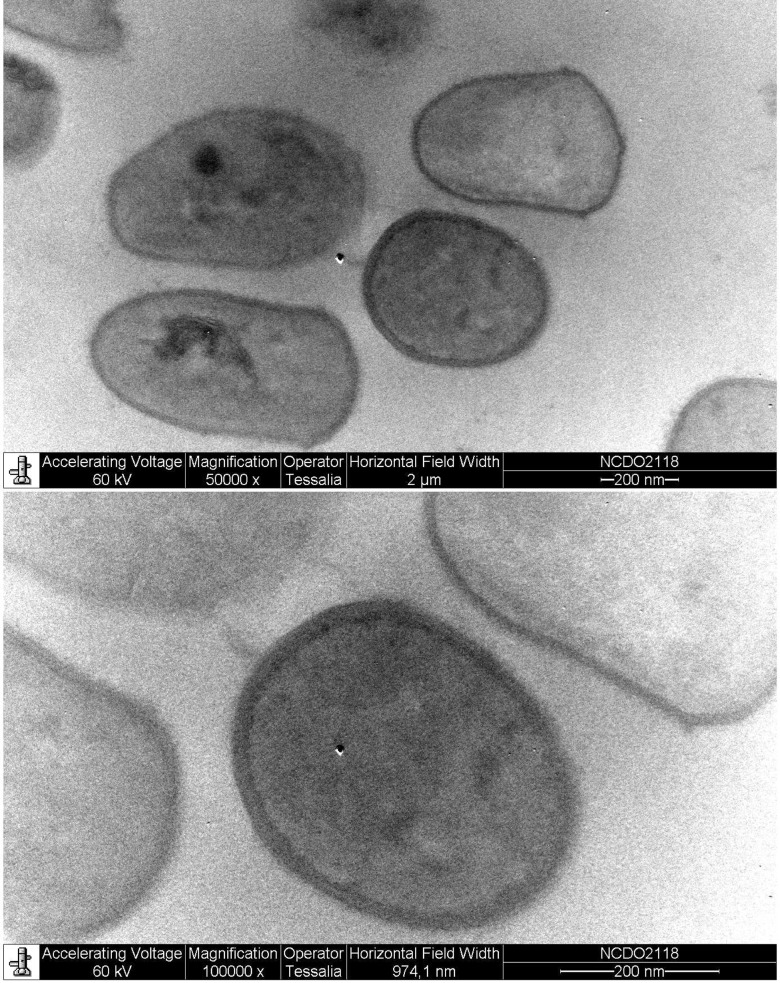
To predict the secreted proteins, we used the software SurfG+, which classifies the proteins using an identification approach based on the presence/absence of signal peptides, signal retention and transmembrane helix [46], which are correlated with the cell wall thickness of the bacteria. To determine the cell wall thickness, we made photomicrographs of L. lactis NCDO 2118 (Fig 4); the cell wall was measured more than 270 times, showing an average size of ~20 nm, and this value was used to determine the motifs. If none of the motifs were found in the protein sequence, SurfG+ characterized the protein as cytoplasmic (CYT) [47]. Using SurfG+, we predicted 94 secreted proteins in L. lactis NCDO 2118.
Fig 4. Photomicrograph of L. lactis NCDO 2118.
The measurements of the membrane wall were performed with ImageJ software using images generated with electron microscopy with EM10A equipment (Zeiss). Top: magnification of 50,000 times; bottom: magnification of 100,000 times.
From this data, the secreted proteins of L. lactis NCDO 2118 were compared to the proteins identified in L. lactis IL1403 using OrthoMCL [48]. In this comparison, 26 of the secreted proteins were exclusive from L. lactis NCDO 2118. Because the probiotic effect was searched using secreted proteins previously expressed in vitro, we searched for proteins that were expressed in L. lactis NCDO 2118 in vitro using proteomics analyses. Five proteins were both present in the 26 secreted proteins that were exclusive from L. lactis NCDO 2118 and in the 867 expressed proteins from proteomic analyses (Table 7). The complete lists of genes identified in proteomic analyses, in the prediction of subcellular location and the exclusive proteins of L. lactis NCDO 2118 are described in S4 Table.
Table 7. Prediction of exclusive secreted proteins of L. lactis NCDO 2118.
| Locus tag | Gene | Start | Stop | Product | Orthology/Subcellular Location/Proteome |
|---|---|---|---|---|---|
| NCDO2118_0052 | NCDO2118_0052 | 57803 | 58270 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_0128 | epsX | 133945 | 134712 | Polysaccharide biosynthesis protein | Exclusive/ Secreted |
| NCDO2118_0139 | epsK | 144750 | 145652 | Polysaccharide biosynthesis protein | Exclusive/ Secreted/ Expressed |
| NCDO2118_0140 | epsL | 145677 | 146600 | Transcriptional regulator | Exclusive/ Secreted/ Expressed |
| NCDO2118_0212 | NCDO2118_0212 | 214606 | 215988 | Hypothetical protein | Exclusive/ Secreted/ Expressed |
| NCDO2118_0256 | NCDO2118_0256 | 255719 | 256297 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_0291 | NCDO2118_0291 | 285998 | 287113 | Endoglucanase | Exclusive/ Secreted |
| NCDO2118_0294 | NCDO2118_0294 | 288612 | 289397 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_0483 | NCDO2118_0483 | 478392 | 479351 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_0533 | NCDO2118_0533 | 527774 | 527965 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_0683 | NCDO2118_0683 | 697697 | 698158 | Hypothetical protein | Exclusive/ Secreted/ Expressed |
| NCDO2118_0684 | NCDO2118_0684 | 698177 | 701176 | ChW repeat-/cell adhesion domain-containing transglutaminase-like protease | Exclusive/ Secreted |
| NCDO2118_0882 | NCDO2118_0882 | 918428 | 918706 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_0904 | NCDO2118_0904 | 939391 | 940704 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_0942 | NCDO2118_0942 | 985414 | 986700 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_0991 | NCDO2118_0991 | 1034860 | 1035321 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_1361 | NCDO2118_1361 | 1468537 | 1469364 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_1363 | NCDO2118_1363 | 1474372 | 1475115 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_1364 | NCDO2118_1364 | 1475137 | 1475901 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_1420 | NCDO2118_1420 | 1537567 | 1538400 | Hypothetical protein | Exclusive/ Secreted/ Expressed |
| NCDO2118_1459 | NCDO2118_1459 | 1569307 | 1569477 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_1795 | NCDO2118_1795 | 1927992 | 1929140 | Transcriptional regulator | Exclusive/ Secreted |
| NCDO2118_2077 | NCDO2118_2077 | 2227730 | 2228593 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_2151 | NCDO2118_2151 | 2304776 | 2305051 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_2232 | NCDO2118_2232 | 2371307 | 2372062 | Hypothetical protein | Exclusive/ Secreted |
| NCDO2118_2330 | NCDO2118_2330 | 2482143 | 2482712 | Hypothetical protein | Exclusive/ Secreted |
Exclusive, secreted and expressed proteins were predicted using OrthoMCL, SurfG+ and proteomic analyses, respectively.
Discussion
Genomic characterization of L. lactis NCDO 2118 and comparison with other species
The genomic lengths of the Lactococcus species analyzed here are highly variable (from ~1.95 Mb to ~2.60 Mb). However, the finding that L. garvieae strains have the smallest genomes compared to L. lactis strains is in agreement with the lifestyle of L. garvieae, isolated from diseased fish. Because pathogenic bacteria may scavenge compounds from the host for their own metabolism, they tend to lose genes involved in biosynthetic pathways, thus, presenting smaller genomes [49].
The high similarity at the subspecies level may be related with some specific characteristics already described in literature, such as the propensity of L. lactis subsp lactis to form longer chains. Besides, L. lactis subsp. lactis are able to produce GABA, ammonia from arginine, carbon dioxide and diacetyl formation from citrate as opposing to L. lactis subsp. cremoris subspecies. Additionally, analyses using southern hybridization, PFGE, 16 rRNA and housekeeping genes (atpA, rpoA, pheS, pepN, bcaT, pepX) showed two separate clusters formed by L. lactis subsp. lactis and L. lactis subsp. cremoris with a low degree of similarity between them [50–52].
From the genome synteny analyses, we have found a high degree of synteny between L. lactis KF147 and L. lactis IL1403, which was already reported in a previous work [25]. However, there was no other genome sequence of any Lactococcus species correlated with plants available at the time the work was performed. Here, we found that the most conserved genome compared to L. lactis NCDO 2118 was L. lactis KF147. The material of fermented plant covers a highly variable niche according to some characteristics as: chemical composition and physical conditions. Thus, plant-related strains posses a great metabolic diversity that certainly extrapolates that from dairy strains [53].
Finally, although L. lactis NCDO 2118 shares several pathways in common with L. lactis KF147 and L. lactis IL1403, it presents several exclusive metabolic features that may be explored for future utilization in industry.
Evaluation of safety aspects in the use of L. lactis NCDO 2118 by genome plasticity and antibiotic resistance approaches
Plasmid-linked antibiotic resistance is not very common among LAB, but it does occur, and safety implications should be taken into consideration. Strains harboring resistance plasmids should not be used as human or animal probiotics. Checking the ability of a proposed probiotic strain to act as a donor for conjugative antibiotic resistance genes may be a sensible precaution in some instances [54].
To provide a better understanding of the putative plasticity of L. lactis NCDO 2118, we have predicted putative phage and genomic islands of this species. The presence of phage regions may contribute to the acquirement of antibiotic resistance, the ability to survive in a new environment, the improvement of adhesion ability, or even to turning the bacteria pathogenic [55]. Here, we found 5 phages; the 3 intact phages harbored important genes such as rusA, arsC1, arsC3, amtB, rpmE2, carA, pyrB, pyrP and pepT. The rusA gene is associated with the prophage sequences of several genera of bacteria, including Bacillus, Streptococcus, Staphylococcus, and Enterococcus, and it is also present in Lactococcus lactis phage r1t [56]. The arsC1 gene is related to arsenate resistance in Corynebacterium glutamicum [57]. arsC3 codes for a thioredoxin-dependent arsenate reductase of the Mycobacterium sp. A33 [58]. amtB is a gene of the ammonia transporter family, which is found in eubacteria, archaea, fungus, plants and animals, whereas in prokaryotes, its homologue is co-transcribed with a PII paralogue, GlnK, in response to nitrogen limitation [59]. The rpmE2 gene codes for a L31 ribosomal protein. The genes carA, pyrB and pyrP are organized as an operon in L. cremoris MG1363, where pyrP encodes a membrane-bound protein with high affinity to uracil permease and pyrimidines, and pyrB and carA encode pyrimidine biosynthetic enzymes [60]. Finally, the gene pepT encodes for a tripeptidase.
Additionally, we predicted 9 GEIs, 5 MIs, 4 SIs and 3 MSIs in the genome sequence of L. lactis NCDO 2118. Interestingly, all MIs present deletions in the pathogenic species L. garvieae, which is a common feature of pathogenic bacteria that adapted to scavenge nutrients from the host [61]. Additionally, MI3 is only present in the L. lactis NCDO 2118 and L. lactis KF147 and may be important for the adaptation of those strains to plants.
We have also assayed L. lactis NCDO 2118, aiming to characterize its antibiotic resistance profile. L. lactis NCDO 2118 is susceptible to most of the antibiotics assayed here. Although L. lactis NCDO 2118 presented resistance to oxacillin and susceptibility to penicillin, it only harbored genes coding for a VanZ family protein, which may be related to the vancomycin resistance, penicillin-binding proteins, and multidrug efflux pump proteins.
The efflux pumps are membrane transporter proteins responsible for the extrusion of relevant antibiotics, which are found in both Gram-positive and Gram-negative bacteria [62; 63]. Penicillin-binding proteins are transpeptidases or caboxypeptidases that harbor specific motifs that limit the active site serine penicillin-recognizing enzyme family, including class A and C β-lactamases [64]. Vancomycin is a glycopeptide antibiotic used in severe infections. Some species used in the food industry or found naturally in raw food material present an intrinsic resistance to vancomycin, including L. rhamnosus, L. casei, Lactobacillus plantarum, and Leuconostoc lactis [65].
Finally, although L. lactis NCDO 2118 does present genes putatively coding for antibiotic resistance-related proteins, none of those genes present anomalous G+C or codon usage deviation, nor are they harbored by the putative horizontally acquired regions predicted by GIPSy or PHAST. More interestingly, no Resistance Island was identified in L. lactis NCDO 2118, corroborating its safety aspects [66].
In vitro and in silico analyses of survival, exclusion mechanisms and probiotic properties of L. lactis NCDO 2118
Susceptibility of L. lactis NCDO 2118 to acid stress and bile salts
Concerning the acid stress, lowering the intracellular pH reduces the transmembrane pH difference and the activity of acid-sensitive enzymes and damages proteins and DNA [67].The first mechanism used by L. lactis species to cope with acid stress is to maintain a low intracellular pH (pHi) by using membrane ATPase FoF1 [68; 69] and the generation of alkaline substances through the catabolism of amino acids (deamination, for example) [70; 71]. Bile salts, on the other hand, are surface-active, amphipathic molecules with a potent antimicrobial activity, and they act as detergents that disrupt biological membranes [67]. The percentage of resistance to bile salts also tends to vary among LAB and even between strains of the same species [72].
Here, we have identified 25 and 16 genes previously shown to be involved in acid stress and bile resistance in other species, respectively. In an in vitro assay, however, only 48% of L. lactis NCDO 2118 was able to grow after pH challenge, and 95% of bacteria was inhibited by bile salts. Other authors have already found that bacteria with an intestinal origin tend to be more resistant to stomach acids [73]. Therefore, this finding corroborates our results because L. lactis NCDO 2118 was isolated from frozen peas. Most of the genes found in L. cremoris MG1363 were also identified in L. lactis NCDO 2118. Additionally, a work using proteomics analyses identified some genes related to acid response and they are present in L. lactis NCDO 2118 genome (clpEP, ahpC, tig, hpr and luxS) [74] showing that other approaches may better elucidate the mechanism of survival to acid stress on this strain.
The high susceptibility of L. lactis NCDO 2118 to bile salts, on the other hand, must be further explored in vitro and in vivo using transcriptomics analyses to determine the expression rates of the described genes.
Competitive exclusion mechanisms of L. lactis NCDO 2118
There are several mechanisms used by bacteria to competitively exclude other species, such as bacteriocin production, space competition through the use of adhesins or receptors that bind to specific surface features, predation and even rapid growth [75].
Adhesins are responsible for the recognition and colonization of host tissues through specific binding. This process may activate the innate host cells or the expression of new genes. Adhesins may be characterized as hair-like attachments named pili or fimbriae or in other cases, named non-pilus adhesin, related to the microbial cell surface [76].
In L. lactis NCDO 2118, we have identified the gene chiA (NCDO2118_2053) and the genes coding for the Chitin binding protein (CBP–NCDO2118_2054) and the laminin-binding protein (NCDO2118_1446), which are normally related to adhesion in other bacteria. Chitin is degraded by chitinases that belong to members of the glycoside hydrolase of family 18 [77]. One example of bacteria that produces chitinase is Serratia marcescens, one of the most efficient organisms in chitin degradation [78]. When E. coli was cloned with a chitin-binding protein of Serratia marcescens, there was a significant increase in its ability to adhere to human colon cells [77].
Chitin-binding encoding genes are broadly distributed in many microorganisms. The L. lactis IL1403 genome, for example, harbors chitinolytic machinery represented by one family 33 CBP (yucG; referred as LlCBP33A), one family 18 chitinase (chiA, referred as LlChi18A) and one family 20 N-acetylhexosaminidase [3; 79]. Another example of bacteria that present a high adhesion degree is Borrelia burgdorferi, which is able to bind to mammalian laminin, an important extracellular matrix (ECM) component [80]. A laminin-binding protein has also been identified in L. lactis NCDO 2118.
Additionally, we have found using MATS experiments that L. lactis NCDO 2118 presents a 52% of association to xylene, which supports the presence of genes coding for adhesion-related proteins in this strain. The hydrophobicity is directly related to the capacity of strains to adhere to surfaces. This capacity is determined by hydrophobic components present in the outer membrane of microorganisms, and it is known that hydrophobic interactions have an important role in the adhesion of bacteria to the epithelium. The application of MATS experiments facilitates a qualitative assessment of the polarity or non-polarity of the bacterial surface, which is important because it indicates the potential for probiotic adhesion to apolar surfaces in the intestinal and vaginal epithelia. However, this test is only a primary indicator of the adherence of microorganisms [81; 82].
The other bacterial competitive exclusion mechanism assayed here was the production of exclusion antimicrobial peptides, named bacteriocins. Bacteriocins produced by a bacterium may be activated against others, even ones from the same species, while the producer is immune to its own peptides [43]. This exclusion mechanism is very important for probioses, as it renders probiotic organisms able to compete with and kill pathogenic ones, promoting a health benefit to the host [2]. We have predicted one bacteriocin for each of the three classes in L. lactis NCDO 2118 (class I-III), which may be important for exclusion mechanisms of this bacteria. However, the lack of nisT and the pseudogenization of nisP on the class I gene cluster, the lack of ABC-transporters in the class II cluster and, also, the lack of information regarding the product of the putative bacteriocin in the class III cluster have to be further studied using in vitro analyses to elucidate whether those bacteriocins are produced and present antimicrobial activity or not.
We have also performed a deferred agar spot assay for the initial determination of antagonistic activity produced by L. lactis NCDO 2118. This test indicates the activity against various Gram-positive and -negative bacteria. This inhibitory effect may be due to H2O2, lactic acid, bacteriocins, antibiotic-like substances, or a combination of these compounds [83]. However, L. lactis NCDO 2118 showed no effect on the growth of the pathogenic strains assayed here.
Secreted proteins and immunomodulatory effects
According to Luerce et al., (2014), the secreted proteins of L. lactis NCDO 2118 are possibly responsible for the immunomodulatory effects of this transient bacterium inside the host. In a comparison of the anti-inflammatory effects between L. lactis NCDO 2118, L. lactis IL1403 and L. cremoris MG1363 strains, only the L. lactis NCDO 2118 supernatant was able to decrease the IL-8 production (45%), showing its immunomodulatory ability against inflammation [18].
Here, we predicted 5 proteins that are present in the 26 secreted proteins exclusive from L. lactis NCDO 2118 and in the 867 expressed proteins from proteomic analyses and may thus be related to the probiotic effect of this strain (Table 7). From those 5 exclusive, secreted and expressed genes of L. lactis NCDO 2118, epsK and epsL are part of the operon epsABCDEFGHIJKLX, whereas there is an epsR gene located in another genomic region.
The EPSs are a type of biopolymer able to facilitate intense interactions of biofilm cells through adhesion, aggregation of bacterial cells, cohesion of biofilms, protective barriers, and cell component export [84]. Through microarray and electron microscopy analyses, Denou et al., 2008 found an eps cluster of genes exclusive from a probiotic Lactobacillus strain compared to a type strain and they have shown that deletion of this cluster from the probiotic strain results in lack of the fuzzy layer on the outside of the cell wall [85].
Altogether, the lack of further knowledge of the eps cluster of genes and the presence of three other genes coding hypothetical exclusive/secreted/expressed proteins highlight the need for additional studies to better elucidate the underlying mechanisms involved in the anti-inflammatory and immunomodulatory activities of this strain.
Materials and methods
Genome sequences
The genome sequences of L. lactis NCDO 2118 [24] and 15 other strains of Lactococcus were retrieved from the GENBANK dataset of NCBI (Table 1). Briefly, the dataset is composed of 8 strains of Lactococcus lactis subsp. lactis, 2 of which were isolated from legumes (L. lactis NCDO 2118 and Lactococcus lactis subsp. lactis KF147), 6 Lactococcus lactis subsp. cremoris isolated from dairy or other fermented foods, and 2 Lactococcus garvieae isolated from diseased fish. L. garvieae was added to the analyses because it is a closely related pathogenic species. S. thermophilus LMD-9 was used as an outgroup to root the phylogenetic tree. Only complete genomes were used to avoid bias.
In silico analyses
Heatmap of genome similarities and 16S phylogenetic tree
The heatmap analyses of the 17 strains were performed with Gegenees [86]. The input files consisted of complete genomes in.fna format. Streptococcus thermophilus LMD-9, a closely related species, was used as an outgroup to root the tree. The analyses were performed with default parameters for comparative analyses using the alignment method BLASTn. Gegenees performs an all-versus-all alignment process of the fragments generated from the 17 genomes. The result was exported from Gegenees as a heatplot image. Additionally, a phylogenetic tree was made using the 16S sequences from all genomes as identified by RNAmmer [87]. After that, they were aligned in MUSCLE [88], and the phylogenetic tree was inferred using the Neighbor-Joining method with 1000 bootstrap replicates.
Genome synteny
The genome synteny analyses were performed using Mauve, with the "progressiveMauve" option and all genome sequences in the.fna format. Mauve predicts gene synteny by merging locally collinear blocks of conserved genome orthologous regions and ordering them according to a reference genome [89].
Genome plasticity
The genome plasticity analyses were performed by searching for horizontally acquired regions such as genomic islands and phage sequences. The genomic islands were searched using the software GIPSy: Genomic Island Prediction Software [90], which updates the methodology of the software PIPS: Pathogenicity Island Prediction Software. Briefly, GIPSy performs the prediction of four different classes of genomic islands: Pathogenicity Islands, Resistance Islands, Metabolic Islands and Symbiotic Islands. In this work, we searched for metabolic and symbiotic islands in the genome of L. lactis NCDO 2118 using Lactococcus lactis subsp. cremoris MG1363 and Lactococcus garviae Lg2 genomes as subjects. After, we consolidated and manually curated the results. The choice of metabolic and symbiotic islands was made based on the lifestyle of L. lactis NCDO 2118, a strain isolated from vegetables, and its metabolic importance.
All the analyses were performed using GENBANK files and default parameters. The results were exported in tabulated format and used in BRIG (Blast Ring Image Generator) to generate circular genome comparative views [91]. Finally, the prophage prediction was performed using the GENBANK file and the software Phast [92], and the results were exported in table format and used as input in BRIG.
Bacteriocin prediction
The bacteriocin prediction was performed in BAGEL software using the.fna file from L. lactis NCDO 2118. Briefly, the software works with a curated dataset of bacteriocins, in which the input data are evaluated based on a Hidden Markov Model. The genetic information is analyzed based on combinations of PFAM domains [42]. For the putative bacteriocin predicted on L. lactis NCDO 2118 (NCDO2118_1768), we used the Transporter Classification Database (TCDB) [93] with an e-value of e-07.
Circular comparison map of genomic sequences
To create circular genome comparisons, we used the software BRIG and all genome sequences in the.fna format; we created the figure with L. lactis NCDO 2118 as reference strain. Additionally, we added the coordinates of the genomic islands and phage regions to the figure to visualize genome plasticity events. Finally, all genomes underwent BLAST analyses against the reference strain to create the circular comparison map.
Metabolic pathway prediction
A genome sequence in.fasta and a genome annotation in the.gbk format were used for reconstructing the Lactococcus species metabolic pathways. Posteriorly, the Pathway/Genome Databases (PGDB) for each of the 16 strains were computationally predicted using Pathway Tools software version 16.5 [94], developed by SRI International. The MetaCyc, a highly curated and non-redundant reference database of small-molecule metabolism, was used as a reference database for the PathoLogic component of the Pathway Tools software [95]. The metabolic pathways of L. lactis NCDO 2118 were used as a reference for the comparative analysis using the following comparisons: i) L. lactis NCDO 2118, L. lactis KF147 and Lactococcus lactis subsp. lactis IL1403, ii) non-pathogenic strains of L. lactis (L. lactis subsp. lactis and cremoris), and iii) all strains in this study.
Identification of the secretome
The prediction of the putative subcellular localizations of L. lactis NCDO 2118 proteins was performed in silico using SurfG+. This software contains such tools as SignalP, LipoP and TMHMM for the identification of motifs [46]. Interestingly, SurfG+ uses the size of the membrane wall to better differentiate the membrane (MEM) and potentially surface exposed (PSE) proteins. Here, the measurements of the membrane wall were performed with electron microscopy with EM10A equipment (Zeiss), as previously described [96].
L. lactis NCDO 2118 was grown at 30°C for 18 h in M17 medium (Difco) containing 0.5% glucose [18] and then centrifuged. The resulting precipitate (~500 mL) was placed in an Eppendorf tube, fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 6 h at 8°C and washed three times with 0.1 M sodium cacodylate buffer (pH 7.2). After washing, the sample was post-fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.2) + 1.5% potassium ferrocyanide for 90 minutes, washed with 0.1 M with sodium cacodylate buffer (pH 7.2), dehydrated in a graduated ethanol series (50% EtOH, 70% EtOH, 95% EtOH, and 100% EtOH), and incorporated in Eponate–Araldite resin. Ultrathin sections were obtained using uranyl acetate and lead citrate and then verified by Zeiss-EM-10A [97]. The micrograph was obtained by one CCD Mega View camera. The thickness of the L. lactis NCDO 2118 wall was determined from the image analysis micrograph in ImageJ software (available at imagej.nih.gov/ij/).
To measure the wall, we used at least five micrographs of L. lactis NCDO 2118 with magnifications of 50,000 and 100,000 times. We calculated the mean size of the cell walls, and the average number of amino acids for the obtained wall thickness was ~55 amino acids. This value was added to the SurfG+ software together with the.fasta sequence of amino acids (.faa) exported from the strain of interest.
After this process, we used OrthoMCL tool to predict the orthologous and paralogous genes between L. lactis NCDO 2118 and L. lactis IL1403.
In vitro analyses
Bacterial strains and growth conditions
For in vitro analyses, we used the probiotic strain L. lactis NCDO 2118 [18] and the pathogenic strains Salmonella enterica serovar Typhimurium ATCC 14028, Escherichia coli ATCC 25723, Staphylococcus aureus ATCC 29213, Bacillus cereus ATCC 11778, Listeria monocytogenes ATCC 15313, Enterococcus faecalis ATCC 19433, and Pseudomonas aeruginosa ATCC 25853.
L. lactis NCDO 2118 was grown at 37°C in MRS medium (Difco) without agitation for 18 hours. L. monocytogenes was cultured in TSB-YE for 24 hours at 28–30°C. The pathogenic strains were grown in BHI medium (BD) for 24 hours at 37°C. To prepare the solid and semi-solid culture media, we added 1.5% and 0.2–0.75% of agar, respectively.
L. lactis gastric juice susceptibility
L. lactis NCDO 2118 stationary phase cells were suspended in either 0.9% saline solution (pH 7) or simulated gastric juice (NaCl 2 g/L, pepsin 3.2 g/L, adjusted to pH 2.5 with concentrated HCl) and incubated at 37°C for 3 h. Solutions were centrifuged, the supernatant was discarded, and the pellets were suspended in MRS broth. Bacterial growth was evaluated by inoculating MRS broth with 2% v/v of control cells in saline and artificial gastric juice-treated cells onto microplates in triplicate, before incubating them in a Microplate Spectrophotometer System SpectraMax 340 (Molecular Devices Inc., Sunnyvale, CA, USA) at 37°C for 18 h. The OD620nm (optic density) was recorded at 30 min intervals. The percentage of growth inhibition was calculated as (1 –areaAGJ/areaCT) x 100, where areaAGJ and areaCT are the areas under the growth curve for the simulated gastric juice and control, respectively. The total area under the curve was calculated by definite integration using the OriginPro 8.5 program (OriginLab Corporation, Northampton, MA, USA). The results were based on the average of three independent assays.
Susceptibility to bile salts
The susceptibility of L. lactis NCDO 2118 to bile salts was evaluated according to the method of Silva et al., (2013) [98]. For this, the L. lactis NCDO 2118 strain was grown in MRS medium at optical density of 0.6 and transferred (2% v/v) to MRS medium supplemented or not with 0.3% of Oxgall (Oxoid Ltd., Basingstoke, UK). The OD620nm was recorded at 30 min intervals while incubating at 37°C for 18 h in a microplate reader. The percentage of growth inhibition was calculated as (1 –areaBS/areaCT) x 100, where areaBS and areaCT are the areas under the growth curve for bile salt and control cells, respectively. The percentage of bacterial viability was determined in a Microplate Spectrophotometer System SpectraMax 340 (Molecular Devices Inc., Sunnyvale, CA, USA) in the same manner as described above. The results were based on an average of three independent assays.
Cell surface hydrophobicity
MATS was measured to evaluate the bacterial cell surface hydrophobicity [99]. Measurement of the cell surface hydrophobicity of L. lactis NCDO 2118 was performed with xylene using the MATS method. Bacterial stationary phase cultures were centrifuged, washed twice and adjusted to an OD600nm of 0.6 with 0.1 M KNO3, pH 6.2 (A0). Then, xylene was added in suspension 16% (v/v) and maintained for 10 minutes at room temperature. The tube was agitated vigorously, and after 30 minutes, the aqueous phase was collected for optical density OD600nm measurement. The reduction percentage of optical density was calculated. The results were based on the average of three independent assays.
Antagonistic activity
Bacterial isolates were cultured in MRS broth for 24 h at 37°C within an anaerobic chamber. A 5 μL aliquot of the culture was then spotted onto MRS agar. After incubation at 37°C for 48 h under anaerobic conditions, the cells were killed by exposure to chloroform for 20 min. Residual chloroform was allowed to evaporate, and Petri dishes were overlaid with 3.5 mL of a soft agar containing brain heart infusion (Acumedia, Neogen Co., Lansing, MI, USA), tryptone soy broth (Difco) supplemented with 0.5% yeast extract (Acumedia), or Ellinghausen–McCullogh–Johnson–Harris with Leptospira enrichment EMJH (Difco) inoculated with 0.2 mL of a 24 h culture of Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 19433, Pseudomonas aeruginosa ATCC 25853, Bacillus cereus ATCC 11778, Escherichia coli ATCC 25723, Salmonella enterica serovar Typhimurium ATCC 14028, Leptospira interrogans serovar Icterohaemorrhagiae, or Listeria monocytogenes ATCC 15313. After incubating at 37°C for 24 h under aerobic or anaerobic conditions, depending on the indicator strain, the antagonistic activity was determined based on the presence of a growth inhibition zone, using the method of Tagg as modified by Branco et al., (2010) [100].
Antibiotic susceptibility
L. lactis NCDO 2118 antibiotic susceptibility was determined using antibiotic diffusion discs (Oxoid, England) on MRS plates. Bacteria were inoculated in MRS broth and incubated overnight at 37°C. Solutions of 108 viable cells (McFarland scale) were prepared from the colonies in 3.5 mL of 0.9% buffered saline. The diluted culture (100 μL) was streaked onto MRS agar, and antibiotic discs were applied to the surface using an antibiotic disc dispenser. The discs included amikacin (30 μg), ampicillin (30 μg), ceftriaxone (30 μg), chloramphenicol (30 μg), erythromycin (10 μg), oxacillin (1 μg), penicillin G (10 U), tetracycline (30 μg) and vancomycin (30 μg). The results were interpreted according to Charteris et al., (1998) [101].
Bacterial strain, growth conditions and preparation of proteins from culture filtrates for proteomic analysis
L. lactis NCDO 2118 and L. lactis IL1403 were pre-inoculated in M17 medium (Difco, New Jersey, USA) and incubated at 30°C for 16 h. The precultures were then inoculated (1:100) in fresh M17 medium supplemented with 0.5% (w/v) glucose (M17Glc) at 30°C until reaching an OD600 = 0.8 (three independent experiments). The cultures were then centrifuged for 20 min at 2,700 x g. The supernatants were filtered using 0.22-μm filters, 30% (w/v) ammonium sulfate was added to the samples, and the pH of the mixtures was adjusted to 4.0. Next, 20 mL of N-butanol was added to each sample. The samples were centrifuged for 10 min at 1,350 x g and 4°C. The interfacial precipitate was collected and resuspended in 1 mL of 20 mM Tris-HCl pH 7.2 [102]. To perform label-free proteomic analysis, the protein extract was concentrated using a spin column with a 10 kDa threshold (Millipore, Billerica, MA, USA). The protein was denatured (0.1% RapiGEST SF at 60°C for 15 min) (Waters, Milford, CA, USA), reduced (10 mM DTT), alkylated (10 mM iodoacetamide) and enzymatically digested with trypsin (Promega, Sequencing Grade Modified Trypsin, Madison, WI, USA).
Proteomic analysis
Qualitative and quantitative nanoUPLC tandem nanoESI-HDMSE (Nano Electrospray High Definition Mass Spectrometry) experiments were performed using both a 1 h reversed phase gradient from 7% to 40% (v/v) acetonitrile (0.1% v/v formic acid) at 500 nL min-1 and a nanoACQUITY UPLC 2D RPxRP Technology system [103]. A nanoACQUITY UPLC HSS T3 1.8 μm, 75 μm × 15 cm column (pH 3) was used with an RP XBridge BEH130 C18 5 μm 300 μm x 50 mm nanoflow column (pH 10). Typical on-column sample loads were 250 ng of the total protein digests for each of the 5 fractions (250 ng/fraction/load). All analyses were performed using nano-electrospray ionization in the positive ion mode nanoESI (+) and a NanoLockSpray (Waters, Manchester, UK) ionization source. The mass spectrometer was calibrated using a MS/MS spectrum of [Glu1]-Fibrinopeptide B human (Glu-Fib) solution (100 fmol.μL-1) delivered through the NanoLockSpray source reference sprayer. The multiplexed data-independent (DIA) scanning with additional specificity and selectivity for non-linear ‘T-wave’ ion mobility (HDMSE) experiments were performed using a Synapt G2-S HDMS mass spectrometer (Waters, Manchester, UK).
Following the identification of proteins, the quantitative data were packaged using dedicated algorithms [104; 105] and searching against a database with default parameters to account for ions [106]. The databases used were reversed “on-the fly” during the database queries and appended to the original database to assess the false positive rate during identification. For proper spectra processing and database searching conditions, the ProteinLynxGlobalServer v.2.5.2 (PLGS) with IdentityE and ExpressionE informatics v.2.5.2 (Waters) was used. UniProtKB with manually reviewed annotations was used, and the search conditions were based on taxonomy (L. lactis). The maximum allowed missed cleavages by trypsin were up to one, variable modifications by carbamidomethyl (C), acetyl N-terminal, phosphoryl (STY) and oxidation (M) were allowed, and a peptide mass tolerance value of 10 ppm was used [107]. The collected proteins were organized by the PLGS ExpressionE tool algorithm into a statistically significant list that corresponded to higher or lower regulation ratios among the different groups. For protein quantification, the PLGS v2.5.2 software was used with the IdentityE algorithm using the Hi3 methodology. The search threshold to accept each spectrum was the default value in the program with a false positive value of 4%. The quantitative values were averaged over all samples, and the standard deviations at p < 0.05 were determined using the Expression software [107].
Conclusions
Although L. lactis NCDO 2118 presented a high similarity to the other L. lactis strains, it presents an SI that is commonly shared with L. lactis KF147, along with high genomic synteny conservation with this strain. Additionally, the antibiotic resistance of this strain to vancomycin, amikacin and oxacillin could be an obstacle for its use as a probiotic. However, the absence of resistance-related genes in regions acquired by HGT and the absence of RIs in the genome sequence corroborates its safety aspects and supports its use as a probiotic strain. Moreover, the high susceptibility of L. lactis NCDO 2118 to acid and bile salts stresses have to be further evaluated in a complete digestion simulation, using transcriptomics and proteomics analyses, to elucidate whether the identified genes are differentially expressed in those environmental conditions.
Interestingly, the adhesion of L. lactis NCDO 2118 to xylene and the putative production of three classes of bacteriocins are important indicators of the exclusion mechanisms used by this strain. However, the in vitro analyses have not shown any sign of an antagonistic effect against the assayed pathogenic bacteria. Future works could also take advantage of combined transcriptomics and proteomics analyses of L. lactis NCDO 2118 in vitro before and after intestinal passage to evaluate the expression of the identified genes. Additionally, the identification of the EPS cluster of genes putatively associated with the probiotic effect of L. lactis NCDO 2118 could be further explored in 16S metagenomics analyses of gut microbiota, after expression, purification and administration of EPS proteins. Finally, through the analyses of the safety, survival and probiotic aspects of L. lactis NCDO 2118, we highlight here the potential use of this strain as a target for the future development of probiotic foods.
Supporting information
L. lactis subsp. lactis NCDO 2118 (top) was used as a reference for the comparison analyses. The genomes are represented according to the nucleotide conservation and synteny. Low similarity regions are represented as white regions inside the blocks, highlighted by a red (*). Regions of deletions are represented as blank spaces between the blocks, letter (A). Insertion regions are highlighted with the letter (B), and inversion regions are represented by the letter (C). To perform the genome synteny analysis, we used the software Mauve, which compares the genomes by identifying and clustering homologous genes between the genomes into large collinear blocks of genes [89]. The most conserved genome compared to L. lactis NCDO 2118 was L. lactis KF147. Between these two strains, it is possible to see some regions of: deletion; insertion; inversion and specific areas with low or no similarity with the reference genome. The comparison of those features with other strains shows: a deletion on the genome position 1,200,000 of Lactococcus lactis subsp. lactis IO-1; a big inversion region in Lactococcus lactis subsp. lactis AI06 in the range from 800,000 to 1,600,000; a small insertion near the genome position 200,000 of L. lactis KLDS 40325 (in green); and a block on Lactococcus lactis subsp. lactis S0 (2,000,000 position) with low similarity to the reference genome.
(TIF)
(A) L. lactis subsp. lactis NCDO 2118 growth under acid stress conditions. Blue: (LL) L. lactis without acid contact. Red: (LLAT) L. lactis under acid treatment. (B) L. lactis growth under intestinal conditions. Blue: (LL) L. lactis without salt contact salt. Red: (LLOG) L. lactis growth with 0.3% ox gall.
(TIF)
The metabolic pathways were predicted using the software Pathway Tools.
(XLS)
(XLSX)
Bacteriocin regions were predicted using BAGEL.
(XLS)
The exclusive, secreted and expressed proteins were predicted using the software OrthoMCL, SurfG+ and proteomics analyses, respectively.
(XLS)
Acknowledgments
We thank the Institute of Biological Sciences of the Federal University of Pará and the Official Laboratory of Fisheries Ministry for the sequencing and assembly of L. lactis NCDO 2118. We also thank the Laboratory of Cellular and Molecular Genetics for the manual curation of the genome annotation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Support was provided by: National Counsel of Technological and Scientific Development [http://cnpq.br/]; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [https://www.capes.gov.br/]; Fundação de Amparo à Pesquisa de Minas Gerais [http://www.fapemig.br/en/].
References
- 1.Khalid K. An overview of lactic acid bacteria. International Journal of Biosciences. 2011;1: 1–13. [Google Scholar]
- 2.Pfeiler EA, Klaenhammer TR. The genomics of lactic acid bacteria. Trends Microbiol. 2007;15: 546–553. doi: 10.1016/j.tim.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 3.Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, et al. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001;11: 731–753. doi: 10.1101/gr.169701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smeianov V, Wechter P, Broadbent J, Hughes J, Rodríguez B, Christensen TK, et al. Comparative high-density microarray analysis of gene expression during growth of Lactobacillus helveticus in milk versus rich culture medium. Appl. Environ. Microbiol. 2007;73: 2661–2672. doi: 10.1128/AEM.00005-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Díaz-Muñiz I, Banavara DS, Budinich MF, Rankin SA, Dudley EG, Steele JL, et al. Lactobacillus casei metabolic potential to utilize citrate as an energy source in ripening cheese: a bioinformatics approach. J Appl Microbiol. 2006;101: 872–882. doi: 10.1111/j.1365-2672.2006.02965.x [DOI] [PubMed] [Google Scholar]
- 6.Smit BA, van Hylckama Vlieg JET, Engels WJM, Meijer L, Wouters JTM, Smit G. Identification, cloning, and characterization of a branched-chain alpha-keto acid decarboxylase involved in flavor formation. Appl Environ Microbiol. 2005;71: 303–311. doi: 10.1128/AEM.71.1.303-311.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Plaza M, Fernández de Palencia P, Peláez C, Requena T. Biochemical and molecular characterization of alpha-ketoisovalerate decarboxylase, an enzyme involved in the formation of aldehydes from amino acids by Lactococcus lactis. FEMS Microbiol Lett. 2004;238: 367–374. doi: 10.1016/j.femsle.2004.07.057 [DOI] [PubMed] [Google Scholar]
- 8.Saulnier D, Santos F, Roos S, Mistretta T, Spinler J, Molenaar D. Exploring Metabolic Pathway Reconstruction and Genome-Wide Expression Profiling in Lactobacillus reuteri to Define Functional Probiotic Features. PLoS ONE. 2011;6: 18783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douillard F, Ribbera A, Järvinen H, Kant R, Pietilä T, Randazzo C, et al. Comparative genomic and functional analysis of Lactobacillus casei and Lactobacillus rhamnosus strains marketed as probiotics. Appl. Environ. Microbiol.. 2013;79: 1923–1933. doi: 10.1128/AEM.03467-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Zuo F, Yu R, Zeng Z, Ma H, Chen S. Comparative genome-based identification of a cell wall-anchored protein from Lactobacillus plantarum increases adhesion of Lactococcus lactis to human epithelial cells. Scientific Reports. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altermann E, Russell WM, Azcarate-Peril MA, Barrangou R, Buck BL, McAuliffe O, et al. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc Natl Acad Sci U S A. 2005;102: 3906–3912. doi: 10.1073/pnas.0409188102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek. 2002;82: 279–289. [PubMed] [Google Scholar]
- 13.Cronin M, Ventura M, Fitzgerald GF, van Sinderen D. Progress in genomics, metabolism and biotechnology of bifidobacteria. Int J Food Microbiol. 2011;149: 4–18. doi: 10.1016/j.ijfoodmicro.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 14.FAO, WHO. Guidelines for the Evaluation of Probiotics in Food. Food and Agriculture Organization; 2002; 1–11. [Google Scholar]
- 15.Nishitani Y, Tanoue T, Yamada K, Ishida T, Yoshida M, Azuma T, et al. Lactococcus lactis subsp. cremoris FC alleviates symptoms of colitis induced by dextran sulfate sodium in mice. International Immunopharmacology. 2009;9: 1444–1451. doi: 10.1016/j.intimp.2009.08.018 [DOI] [PubMed] [Google Scholar]
- 16.Radziwill-Bienkowska J, Le D, Szczesny P, Duviau M, Aleksandrzak-Piekarczyk T, Loubière P, et al. Adhesion of the genome-sequenced Lactococcus lactis subsp. cremoris IBB477 strain is mediated by specific molecular determinants. Appl Microbiol Biotechnol. 2016;100: 9605–9617. doi: 10.1007/s00253-016-7813-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Wang Q, Fisher D, Cai M, Chakravartty V, Ye H, et al. Deciphering a unique biotin scavenging pathway with redundant genes in the probiotic bacterium Lactococcus lactis. Scientific Reports. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luerce TD, Gomes-Santos AC, Rocha CS, Moreira TG, Cruz DN, Lemos L, et al. Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis. Gut Pathog. 2014;6: 33 doi: 10.1186/1757-4749-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzoli R, Pessione E, Dufour M, Laroute V, Giuffrida MG, Giunta C, et al. Glutamate-induced metabolic changes in Lactococcus lactis NCDO 2118 during GABA production: combined transcriptomic and proteomic analysis. Amino Acids. 2010;39: 727–737. doi: 10.1007/s00726-010-0507-5 [DOI] [PubMed] [Google Scholar]
- 20.Miyoshi A, Jamet E, Commissaire J, Renault P, Langella P, Azevedo V. A xylose-inducible expression system for Lactococcus lactis. FEMS Microbiol Lett. 2004;239: 205–212. doi: 10.1016/j.femsle.2004.08.018 [DOI] [PubMed] [Google Scholar]
- 21.Servin A. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiology Reviews. 2004;28: 405–440. doi: 10.1016/j.femsre.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 22.Vinderola C, Reinheimer J. Lactic acid starter and probiotic bacteria: a comparative "in vitro" study of probiotic characteristics and biological barrier resistance. Food Research International. 2003;36: 895–904. [Google Scholar]
- 23.Mathur S, Singh R. Antibiotic Resistance in Food Lactic Acid Bacteria-A Review. Int J Food Microbiol. 2005;105: 281–295. doi: 10.1016/j.ijfoodmicro.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 24.Oliveira LC, Saraiva TDL, Soares SC, Ramos RTJ, Sá PHCG, Carneiro AR, et al. Genome Sequence of Lactococcus lactis subsp. lactis NCDO 2118, a GABA-Producing Strain. Genome Announc. 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siezen RJ, Bayjanov J, Renckens B, Wels M, van Hijum SAFT, Molenaar D, et al. Complete genome sequence of Lactococcus lactis subsp. lactis KF147, a plant-associated lactic acid bacterium. J Bacteriol. 2010;192: 2649–2650. doi: 10.1128/JB.00276-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Wang Y, Huo G. Complete Genome Sequence of Lactococcus lactis subsp. lactis KLDS4.0325. Genome Announc. 2013;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato H, Shiwa Y, Oshima K, Machii M, Araya-Kojima T, Zendo T, et al. Complete genome sequence of Lactococcus lactis IO-1, a lactic acid bacterium that utilizes xylose and produces high levels of L-lactic acid. J Bacteriol. 2012;194: 2102–2103. doi: 10.1128/JB.00074-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Y, Lu Y, Teng K, Chen M, Zheng H, Zhu Y et al. Complete genome sequence of Lactococcus lactis subsp. lactis CV56, a probiotic strain isolated from the vaginas of healthy women. J Bacteriol. 2011;193: 2886–2887. doi: 10.1128/JB.00358-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCulloch JA, de Oliveira VM, de Almeida Pina AV, Pérez-Chaparro PJ, de Almeida LM, de Vasconcelos JM, et al. Complete Genome Sequence of Lactococcus lactis Strain AI06, an Endophyte of the Amazonian Açaí Palm. Genome Announc. 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolotin A, Quinquis B, Ehrlich SD, Sorokin A. Complete genome sequence of Lactococcus lactis subsp. cremoris A76. J Bacteriol. 2012;194: 1241–1242. doi: 10.1128/JB.06629-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly WJ, Altermann E, Lambie SC, Leahy SC. Interaction between the genomes of Lactococcus lactis and phages of the P335 species. Front Microbiol. 2013;4: 257 doi: 10.3389/fmicb.2013.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wegmann U, O'Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, et al. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol. 2007;189: 3256–3270. doi: 10.1128/JB.01768-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linares DM, Kok J, Poolman B. Genome sequences of Lactococcus lactis MG1363 (revised) and NZ9000 and comparative physiological studies. J Bacteriol. 2010;192: 5806–5812. doi: 10.1128/JB.00533-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siezen RJ, Renckens B, van Swam I, Peters S, van Kranenburg R, Kleerebezem M, et al. Complete sequences of four plasmids of Lactococcus lactis subsp. cremoris SK11 reveal extensive adaptation to the dairy environment. Appl Environ Microbiol. 2005;71: 8371–8382. doi: 10.1128/AEM.71.12.8371-8382.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ainsworth S, Zomer A, de Jager V, Bottacini F, van Hijum SAFT, Mahony J, et al. Complete Genome of Lactococcus lactis subsp. cremoris UC509.9, Host for a Model Lactococcal P335 Bacteriophage. Genome Announc. 2013;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita H, Toh H, Oshima K, Yoshizaki M, Kawanishi M, Nakaya K, et al. Complete genome sequence and comparative analysis of the fish pathogen Lactococcus garvieae. PLoS One. 2011;6: e23184 doi: 10.1371/journal.pone.0023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koornin E, et al. Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci USA. 2006;103: 15611–15616. doi: 10.1073/pnas.0607117103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carvalho AL, Turner DL, Fonseca LL, Solopova A, Catarino T, Kuipers OP, et al. Metabolic and transcriptional analysis of acid stress in Lactococcus lactis, with a focus on the kinetics of lactic acid pools. PLoS One. 2013;8: e68470 doi: 10.1371/journal.pone.0068470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Candela M, Centanni M, Fiori J, Biagi E, Turroni S, Orrico C, et al. DnaK from Bifidobacterium animalis subsp. lactis is a surface-exposed human plasminogen receptor upregulated in response to bile salts. Microbiology. 2010;156: 1609–1618. doi: 10.1099/mic.0.038307-0 [DOI] [PubMed] [Google Scholar]
- 40.Ruiz L, Couté Y, Sánchez B, de los Reyes-Gavilán C, Sanchez J, Margolles A, et al. The cell-envelope proteome of Bifidobacterium longum in an in vitro bile environment. Microbiology. 2009;155: 957–967. doi: 10.1099/mic.0.024273-0 [DOI] [PubMed] [Google Scholar]
- 41.Nader-Macías M, Otero M, Espeche M, Maldonado N. Advances in the design of probiotic products for the prevention of major diseases in dairy cattle. Journal of Industrial Microbiology & Biotechnology. 2008;35: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 42.de Jong A, van Heel AJ, Kok J, Kuipers OP. BAGEL2: mining for bacteriocins in genomic data. Nucleic Acids Res. 2010;38: W647–51. doi: 10.1093/nar/gkq365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cotter P, Hill C, Ross P. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3 (10): 777–788. doi: 10.1038/nrmicro1273 [DOI] [PubMed] [Google Scholar]
- 44.Siegers K, Entian K. Genes involved in immunity to the lantibiotic nisin produced by Lactococcus lactis 6F3. Applied and environmental microbiology. 1995;61: 1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvarez-Sieiro P, Montalbán-López M, Mu D, Kuipers O. Bacteriocins of lactic acid bacteria: extending the family. Applied Microbiology and Biotechnology. 2016;100: 2939–2951. doi: 10.1007/s00253-016-7343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barinov A, Loux V, Hammani A, Nicolas P, Langella P, Ehrlich D, et al. Prediction of surface exposed proteins in Streptococcus pyogenes, with a potential application to other gram-positive bacteria. Proteomics. 2009;9: 61–73. doi: 10.1002/pmic.200800195 [DOI] [PubMed] [Google Scholar]
- 47.Santos AR, Carneiro A, Gala-García A, Pinto A, Barh D, Barbosa E, et al. The Corynebacterium pseudotuberculosis in silico predicted pan-exoproteome. BMC Genomics. 2012;13 Suppl 5: S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Stoeckert CJJ, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13: 2178–2189. doi: 10.1101/gr.1224503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moran N. Microbial Minimalism: Genome Reduction in Bacterial Pathogens. Cell. 2002;108: 583–586. [DOI] [PubMed] [Google Scholar]
- 50.Fernández E, Alegría Á, Delgado S, Martín M, Mayo B. Comparative phenotypic and molecular menetic profiling of wild Lactococcus lactis subsp. lactis strains of the lactis and cremoris genotypes isolated from starter-free cheeses made of raw milk. Appl. Environ. Microbiol.. 2011;77: 5324–5335. doi: 10.1128/AEM.02991-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godon J, Delorme C, Ehrlich S, Renault P. Divergence of Genomic Sequences between Lactococcus lactis subsp. lactis and Lactococcus lactis subsp. cremoris. Appl Environ Microbiol. 1992;58: 4045–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nomura M, Kimoto H, Someya Y, Susuki I. Novel characteristic for distinguishing Lactococcus lactis subsp. lactis from subsp. cremoris. International Journal of Systematic Bacteriology. 1999;49: 163–166. doi: 10.1099/00207713-49-1-163 [DOI] [PubMed] [Google Scholar]
- 53.van Hylckama Vlieg J, Rademaker J, Bachmann H, Molenaar D, Kelly W, Siezen R. Natural diversity and adaptive responses of Lactococcus lactis. Curr Opin Biotechnol. 2006;17: 183–190. doi: 10.1016/j.copbio.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 54.Salminen S, von Wright A, Morelli L, Marteau P, Brassart D, de Vos W, et al. Demonstration of safety of probiotics—a review. International Journal of Food Microbiology. 1998;44: 93–106. [DOI] [PubMed] [Google Scholar]
- 55.Casjens S. Prophages and bacterial genomics: what have we learned so far? Mol Microbiol. 2003;49: 277–300. [DOI] [PubMed] [Google Scholar]
- 56.Sharples GJ, Bolt EL, Lloyd RG. RusA proteins from the extreme thermophile Aquifex aeolicus and lactococcal phage r1t resolve Holliday junctions. Mol Microbiol. 2002;44: 549–559. [DOI] [PubMed] [Google Scholar]
- 57.Mateos LM, Ordóñez E, Letek M, Gil JA. Corynebacterium glutamicum as a model bacterium for the bioremediation of arsenic. Int Microbiol. 2006;9: 207–215. [PubMed] [Google Scholar]
- 58.Achour-Rokbani A, Cordi A, Poupin P, Bauda P, Billard P. Characterization of the ars gene cluster from extremely arsenic-resistant Microbacterium sp. strain A33. Appl Environ Microbiol. 2010;76: 948–955. doi: 10.1128/AEM.01738-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blauwkamp TA, Ninfa AJ. Antagonism of PII signalling by the AmtB protein of Escherichia coli. Mol Microbiol. 2003;48: 1017–1028. [DOI] [PubMed] [Google Scholar]
- 60.Martinussen J, Schallert J, Andersen B, Hammer K. The pyrimidine operon pyrRPB-carA from Lactococcus lactis. J Bacteriol. 2001;183: 2785–2794. doi: 10.1128/JB.183.9.2785-2794.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moya A, Peretó J, Gil R, Latorre A. Learning how to live together: genomic insights into prokaryote–animal symbioses. Nature Reviews Genetics. 2008;9: 218–229. doi: 10.1038/nrg2319 [DOI] [PubMed] [Google Scholar]
- 62.Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M, et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother. 2001;45: 105–116. doi: 10.1128/AAC.45.1.105-116.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Bambeke F, Balzi E, Tulkens PM. Antibiotic efflux pumps. Biochem Pharmacol. 2000;60: 457–470. [DOI] [PubMed] [Google Scholar]
- 64.Zapun A, Contreras-Martel C, Vernet T. Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol Rev. 2008;32: 361–385. doi: 10.1111/j.1574-6976.2007.00095.x [DOI] [PubMed] [Google Scholar]
- 65.Tynkkynen S, Singh KV, Varmanen P. Vancomycin resistance factor of Lactobacillus rhamnosus GG in relation to enterococcal vancomycin resistance (van) genes. Int J Food Microbiol. 1998;41: 195–204. [DOI] [PubMed] [Google Scholar]
- 66.Charteris WP, Kelly PM, Morelli L, Collins JK. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot. 1998;61: 1636–1643. [DOI] [PubMed] [Google Scholar]
- 67.Lebeer S, Vanderleyden J, De Keersmaecker SCJ. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev. 2008a;72: 728–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Even S, Lindley ND, Cocaign-Bousquet M. Transcriptional, translational and metabolic regulation of glycolysis in Lactococcus lactis subsp. cremoris MG1363 grown in continuous acidic cultures. Microbiology. 2003;149: 1935–1944. doi: 10.1099/mic.0.26146-0 [DOI] [PubMed] [Google Scholar]
- 69.O'Sullivan E, Condon S. Relationship between acid tolerance, cytoplasmic pH, and ATP and H+-ATPase levels in chemostat cultures of Lactococcus lactis. Appl Environ Microbiol. 1999;65: 2287–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christensen JE, Dudley EG, Pederson JA, Steele JL. Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek. 1999;76: 217–246. [PubMed] [Google Scholar]
- 71.Sanders J, Venema G, Kok J. Environmental stress responses in Lactococcus lactis. FEMS Microbiology Reviews. 1999;23: 483–501. [Google Scholar]
- 72.Xanthopoulos V, Litopoulou-Tzanetaki E, Tzanetakis N. In vitro study of Lactobacillus species strains on bile tolerance and cholesterol removal In: Lactic acid bacteria–lactic 97 1997;Presses Universitaires de Caen. [Google Scholar]
- 73.Morelli L. In Vitro Selection of Probiotic Lactobacilli: A Critical Appraisal. Curr. Issues Intest. Microbiol. 2000;1: 59–67. [PubMed] [Google Scholar]
- 74.Frees D, Vogensen F, Ingmer H. Identification of proteins induced at low pH in Lactococcus lactis. International Journal of Food Microbiology. 2003;87: 293–300. [DOI] [PubMed] [Google Scholar]
- 75.Hibbing M, Fuqua C, Parsek M, Peterson S. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8: 15–25. doi: 10.1038/nrmicro2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soto G, Hultgren S. Bacterial Adhesins: Common Themes and Variations in Architecture and Assembly. Journal of Bacteriology. 1999;181: 1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kawada M, Chen C, Arihiro A, Nagatani K, Watanabe T, Mizoguchi E. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Lab Invest. 2008;88: 883–895. doi: 10.1038/labinvest.2008.47 [DOI] [PubMed] [Google Scholar]
- 78.Kamba A, Shibata Y, Mizoguchi E. Potential roles of chitin in mucosal inflammation. Microbial pathogens and strategies for combating them: science, technology and education. 2013; 1853–1863. [Google Scholar]
- 79.Vaaje-Kolstad G, Bunaes AC, Mathiesen G, Eijsink VGH. The chitinolytic system of Lactococcus lactis ssp. lactis comprises a nonprocessive chitinase and a chitin-binding protein that promotes the degradation of alpha- and beta-chitin. FEBS J. 2009;276: 2402–2415. [DOI] [PubMed] [Google Scholar]
- 80.Verma A, Brissette CA, Bowman A, Stevenson B. Borrelia burgdorferi BmpA is a laminin-binding protein. Infect Immun. 2009;77: 4940–4946. doi: 10.1128/IAI.01420-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pan W, Li P, Liu Z. The correlation between surface hydrophobicity and adherence of Bifidobacterium strains from centenarians' faeces. Anaerobe. 2006;12: 148–152. doi: 10.1016/j.anaerobe.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 82.Steinberg R, Lima M, Oliveira N, Miyoshi A, Nicoli J, Neumann E, et al. Effect of intestinal colonization by two Lactobacillus strains on the immune response of gnotobiotic mice. Beneficial Microbes. 2014;5: 409–419. doi: 10.3920/BM2013.0075 [DOI] [PubMed] [Google Scholar]
- 83.Nardi R, Santoro M, Oliveira J, Pimenta A, Ferras V, Benchetrit L, et al. Purification and molecular characterization of antibacterial compounds produced by Lactobacillus murinus strain L1. Journal of Applied Microbiology. 2005;99: 649–656. doi: 10.1111/j.1365-2672.2005.02632.x [DOI] [PubMed] [Google Scholar]
- 84.Flemming HC, Wingender J. Relevance of microbial extracellular polymeric substances (EPSs)—Part II: Technical aspects. Water Sci Technol. 2001;43: 9–16. [PubMed] [Google Scholar]
- 85.Denou E, Pridmore RD, Berger B, Panoff J, Arigoni F, Brüssow H. Identification of genes associated with the long-gut-persistence phenotype of the probiotic Lactobacillus johnsonii strain NCC533 using a combination of genomics and transcriptome analysis. J Bacteriol. 2008;190: 3161–3168. doi: 10.1128/JB.01637-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agren J, Sundström A, Håfström T, Segerman B. Gegenees: fragmented alignment of multiple genomes for determining phylogenomic distances and genetic signatures unique for specified target groups. PLoS One. 2012;7: e39107 doi: 10.1371/journal.pone.0039107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lagesen K, Hallin P, Rødland EA, Staerfeldt H, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35: 3100–3108. doi: 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edgar R. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004;32: 1792–1797. doi: 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One. 2010;5: e11147 doi: 10.1371/journal.pone.0011147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soares SC, Geyik H, Ramos RTJ, de Sá PHCG, Barbosa EGV, Baumbach J, et al. GIPSy: Genomic island prediction software. J Biotechnol. 2015;232: 2–11. doi: 10.1016/j.jbiotec.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 91.Alikhan N, Petty N, Zakour N, Beatson S. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12: 402 doi: 10.1186/1471-2164-12-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. PHAST: a fast phage search tool. Nucleic Acids Res. 2011;39: W347–52. doi: 10.1093/nar/gkr485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saier MHJ, Reddy VS, Tamang DG, Västermark A. The transporter classification database. Nucleic Acids Res. 2014;42: D251–8. doi: 10.1093/nar/gkt1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karp PD, Paley S, Romero P. The Pathway Tools software. Bioinformatics. 2002;18 Suppl 1: S225–32. [DOI] [PubMed] [Google Scholar]
- 95.Caspi R, Altman T, Dale JM, Dreher K, Fulcher CA, Gilham F, et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2010;38: D473–9. doi: 10.1093/nar/gkp875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soares SC, Trost E, Ramos RTJ, Carneiro AR, Santos AR, Pinto AC, et al. Genome sequence of Corynebacterium pseudotuberculosis biovar equi strain 258 and prediction of antigenic targets to improve biotechnological vaccine production. J Biotechnol. 2013;167: 135–141. doi: 10.1016/j.jbiotec.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 97.de Melo AL, Machado CR, Pereira LH. Host cell adhesion to Schistosoma mansoni larvae in the peritoneal cavity of naive mice. Histological and scanning electron microscopic studies. Rev Inst Med Trop Sao Paulo. 1993;35: 17–22. [DOI] [PubMed] [Google Scholar]
- 98.Silva BC, Jung LRC, Sandes SHC, Alvim LB, Bomfim MRQ, Nicoli JR, et al. In vitro assessment of functional properties of lactic acid bacteria isolated from faecal microbiota of healthy dogs for potential use as probiotics. Benef Microbes. 2013;4: 267–275. doi: 10.3920/BM2012.0048 [DOI] [PubMed] [Google Scholar]
- 99.Pelletier C, Bouley C, Cayuela C, Bouttier S, Bourlioux P, Bellon-Fontaine M. Cell surface characteristics of Lactobacillus casei subsp. casei, Lactobacillus paracasei subsp. paracasei, and Lactobacillus rhamnosus strains. Appl Environ Microbiol. 1997;63: 1725–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Branco KMGR Nardi RMD, Moreira JLS Nunes AC, Farias LM Nicoli JR, et al. Identification and in vitro production of Lactobacillus antagonists from women with or without bacterial vaginosis. Braz J Med Biol Res. 2010;43: 338–344. [DOI] [PubMed] [Google Scholar]
- 101.Charteris WP, Kelly PM, Morelli L, Collins JK. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol. 1998;84: 759–768. [DOI] [PubMed] [Google Scholar]
- 102.Paule BJA, Meyer R, Moura-Costa LF, Bahia RC, Carminati R, Regis LF, et al. Three-phase partitioning as an efficient method for extraction/concentration of immunoreactive excreted-secreted proteins of Corynebacterium pseudotuberculosis. Protein Expr Purif. 2004;34: 311–316. doi: 10.1016/j.pep.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 103.Gilar M, Olivova P, Daly AE, Gebler JC. Two-dimensional separation of peptides using RP-RP-HPLC system with different pH in first and second separation dimensions. J Sep Sci. 2005;28: 1694–1703. [DOI] [PubMed] [Google Scholar]
- 104.Geromanos SJ, Vissers JPC, Silva JC, Dorschel CA, Li G, Gorenstein MV, et al. The detection, correlation, and comparison of peptide precursor and product ions from data independent LC-MS with data dependant LC-MS/MS. Proteomics. 2009;9: 1683–1695. doi: 10.1002/pmic.200800562 [DOI] [PubMed] [Google Scholar]
- 105.Silva JC, Denny R, Dorschel CA, Gorenstein M, Kass IJ, Li G, et al. Quantitative proteomic analysis by accurate mass retention time pairs. Anal Chem. 2005;77: 2187–2200. doi: 10.1021/ac048455k [DOI] [PubMed] [Google Scholar]
- 106.Li G, Vissers JPC, Silva JC, Golick D, Gorenstein MV, Geromanos SJ. Database searching and accounting of multiplexed precursor and product ion spectra from the data independent analysis of simple and complex peptide mixtures. Proteomics. 2009;9: 1696–1719. doi: 10.1002/pmic.200800564 [DOI] [PubMed] [Google Scholar]
- 107.Curty N, Kubitschek-Barreira PH, Neves GW, Gomes D, Pizzatti L, Abdelhay E, et al. Discovering the infectome of human endothelial cells challenged with Aspergillus fumigatus applying a mass spectrometry label-free approach. J Proteomics. 2014;97: 126–140. doi: 10.1016/j.jprot.2013.07.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
L. lactis subsp. lactis NCDO 2118 (top) was used as a reference for the comparison analyses. The genomes are represented according to the nucleotide conservation and synteny. Low similarity regions are represented as white regions inside the blocks, highlighted by a red (*). Regions of deletions are represented as blank spaces between the blocks, letter (A). Insertion regions are highlighted with the letter (B), and inversion regions are represented by the letter (C). To perform the genome synteny analysis, we used the software Mauve, which compares the genomes by identifying and clustering homologous genes between the genomes into large collinear blocks of genes [89]. The most conserved genome compared to L. lactis NCDO 2118 was L. lactis KF147. Between these two strains, it is possible to see some regions of: deletion; insertion; inversion and specific areas with low or no similarity with the reference genome. The comparison of those features with other strains shows: a deletion on the genome position 1,200,000 of Lactococcus lactis subsp. lactis IO-1; a big inversion region in Lactococcus lactis subsp. lactis AI06 in the range from 800,000 to 1,600,000; a small insertion near the genome position 200,000 of L. lactis KLDS 40325 (in green); and a block on Lactococcus lactis subsp. lactis S0 (2,000,000 position) with low similarity to the reference genome.
(TIF)
(A) L. lactis subsp. lactis NCDO 2118 growth under acid stress conditions. Blue: (LL) L. lactis without acid contact. Red: (LLAT) L. lactis under acid treatment. (B) L. lactis growth under intestinal conditions. Blue: (LL) L. lactis without salt contact salt. Red: (LLOG) L. lactis growth with 0.3% ox gall.
(TIF)
The metabolic pathways were predicted using the software Pathway Tools.
(XLS)
(XLSX)
Bacteriocin regions were predicted using BAGEL.
(XLS)
The exclusive, secreted and expressed proteins were predicted using the software OrthoMCL, SurfG+ and proteomics analyses, respectively.
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.