Abstract
The purpose of this study was to establish an optimized protocol for the production of alginate-encapsulated canine adipose-derived mesenchymal stem cells (cASCs) and evaluate their suitability for clinical use, including viability, proliferation and in vivo cell retention. Alginate microbeads were formed by vibrational technology and the production of injectable microbeads was performed using various parameters with standard methodology. Microbead toxicity was tested in an animal model. Encapsulated cASCs were evaluated for viability and proliferation in vitro. HEK-293 cells, with or without microencapsulation, were injected into the subcutaneous tissue of mice and were tracked using in vivo bioluminescent imaging to evaluate the retention of transplanted cells. The optimized injectable microbeads were of uniform size and approximately 250 µm in diameter. There was no strong evidence of in vivo toxicity for the alginate beads. The cells remained viable after encapsulation, and there was evidence of in vitro proliferation within the microcapsules. In vivo bioluminescent imaging showed that alginate encapsulation improved the retention of transplanted cells and the encapsulated cells remained viable in vivo for 7 days. Encapsulation enhances the retention of viable cells in vivo and might represent a potential strategy to increase the therapeutic potency and efficacy of stem cells.
Keywords: alginate, canine, injectable, mesenchymal stem cell, microencapsulation
Based on the high potential to treat many degenerative diseases and due to the increasing significance of companion animals, stem cell-based therapies represent a growing field of research in veterinary medicine. Most therapeutic effects of mesenchymal stem cells are thought to occur through a paracrine mechanism by promoting angiogenesis and tissue regeneration, while inhibiting fibrosis, apoptosis and inflammation [14, 21, 22]. However, the low viability and survival of these cells in the disease site are a major barrier to improved success and therapeutic efficacy [14]. Therefore, strategies that focus on increased cell retention and survival have the potential to increase the therapeutic efficacy of cell based therapies.
Microencapsulation of cells using alginate presents a novel and translatable strategy to enhance transplanted cell retention and survival, and ultimately results in enhanced therapeutic potency at the local site of disease [13, 19]. Microencapsulation is also unique compared to other cell transplantation methods, as it results in particles with diameters less than the nominal inner diameter of a 23 gauge hypodermic needle (337 µm) that can be injected directly into the diseased site. This represents a less invasive procedure with localization of therapeutic factors [9].
Alginate was selected as the biomaterial for this process due to several favorable properties; it provides an artificial extracellular matrix that is biocompatible, biodegradable, relatively non-toxic, inexpensive and easy to handle [2, 5, 20]. The porous alginate microcapsules act as a mechanical barrier to prevent the escape and elimination of entrapped cells, while allowing the release of stem cell-produced growth factors and cytokines to the surrounding tissue [20]. The matrix of the alginate microcapsules provides an environment that permits cell colonization, and the membrane acts to immunoisolate encapsulated cells by preventing the penetration of host antibodies and leukocytes [20].
A previous study suggested that microencapsulated stem cells might have greater potential for heart regeneration compared to free stem cells [13]. In vitro and in vivo findings of previous studies also suggested that alginate-based biomaterials were degradable, allowed vascularization and had high biocompatibility [10, 11, 16]. Furthermore, injectable alginate microcapsules enable minimally invasive introduction of stem cells into the body for curing diseases [9, 13]. Therefore, the strategy of cell microencapsulation using alginate could be used for many biomedical applications, such as wound healing, cartilage repair and bone regeneration in veterinary medicine.
In this study, we optimized injectable microbeads by studying the effects of various parameters on the size of the microbeads and hypothesized that alginate microencapsulation of canine adipose tissue-derived mesenchymal stem cells (ASCs) would promote increased cell viability and retention in vivo. We evaluated the cellular influence of alginate encapsulation and investigated the transplantation of microbeads in vivo to evaluate its safety in a rat model.
MATERIALS AND METHODS
Experimental animals
All animal experiments were performed in compliance with guidelines outlined by the Kangwon National University Animal Care Committee. The rats and mice used for the experiments were housed at conventional housing facilities and received standard care. They were maintained in room temperature conditions of 21°C with humidity of 55%, and a 12-hr light-dark cycle with water and food ad libitum. The animals were euthanized using carbon dioxide asphyxiation.
Cell culture
Canine ASCs were isolated from a 2-year-old male beagle using the collagenase perfusion technique according to the methods described in previous articles [8, 18]. Canine ASCs were cultured under standard conditions in high glucose Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 1% antibiotic-antimycotic solution (Thermo Fisher Scientific, Waltham, MA, U.S.A.). The medium was changed at 48-hr intervals until the cells became confluent. After cells reached 90% confluence, they were trypsinized and stored in liquid nitrogen or subcultured. Cell density for the passaging of canine ASCs was approximately 2.5 × 104 cell/cm2. The cryopreserved canine ASCs were used up to passage 5.
HEK-293 cells (Luc-2; Luciferase, HEK293 stable cells, GenTarget Inc., San Diego, CA, U.S.A.), a human embryotic kidney cell line with stable luciferase expression, were purchased from GenTarget Inc. HEK-293 cells were cultured under standard conditions in high glucose DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic solution. The cryopreserved HEK-293 cells were used up to passage 6.
Standardization for microencapsulation
Alginate microbeads were produced by vibrational nozzle technology using a Büchi Encapsulator B-395 pro (Büchi Labortechinik AG, Dottikon, Switzerland). The various parameters, such as the nozzle size, vibrational frequency (Hz), flow rate (ml/min) and electrostatic charge (V), were set up and optimized for injectable microbead production with diameters less than the nominal inner diameter of a 23 gauge hypodermic needle (337 µm). The bead morphology was analyzed by light microscopy.
The polymer used was 1.2% sodium alginate solution, and the gelling solution was 100 mM of calcium chloride solution. Sterile filtered isotonic 1.8% sodium alginate solution (Büchi Labortechinik AG) was diluted to 1.2% by normal saline. The gelling solution was produced by combining 11 g of calcium chloride anhydrous powder with 1,000 ml of distilled water. The nozzle size was set at 120 µm for the standardization experiment. Other stable parameters included a syringe pump size of 5 ml and stirrer speed at 80%.
Frequency (Hz), as a factor influencing the bead diameter, was tested at 0, 40, 300, 1,000 and 2,500 Hz. The flow rate was set at 15 ml/min as per recommended for a 120-µm nozzle size, based on the Büchi encapsulator laboratory guide. The electrode was turned off for this experiment. After bead production, 100 microbeads were counted and measured via light microscopy, and the average diameters were determined based on different parameters tested.
The flow rate (ml/min), as a factor influencing the bead diameter, was tested at rates of 15, 18, 23 and 28 ml/min. The frequency was set at 1,000 Hz and determined to be optimal based on the previous experiment. The electrode was also turned off for this experiment. After bead production, a similar process of counting and measuring/averaging the diameters was used to evaluate different parameters tested.
The electrode (V), as a function of bead diameter, was tested at voltage power of 0, 300, 500, 800 and 1,000 V. The flow rate was set at 23 ml/min, and the frequency was set at 1,000 Hz, which were identified as optimal parameters based on previous experiments. A similar evaluation process was used to evaluate the different parameters tested.
In vivo toxicology test of alginate microbead
The toxicity of the microbeads was tested in Sprague Dawley (NaraBiotec, Seoul, Republic of Korea) male rats, 7–8 weeks old with a body weight of 170–200 g. The control group (n=6) received a subcutaneous injection in the dorsal interscapular region containing only PBS, whereas the test group (n=6) received a similar injection with microbeads suspended in PBS. Microbeads were suspended homogenously in PBS before injecting, and each rat received the same volume of microbeads suspension (3 ml per rat). The toxicity was evaluated by assessing blood parameters and through histological evaluation of the injection sites for each group.
Standardized parameters were used for bead production, and blood tests were performed immediately after injection, 1 week after injection and 2 weeks after injection. Whole blood was collected from the jugular vein of each rat and centrifuged for 10 min at 1,500 ×g at 4°C to collect serum. Whole blood was used for hematology, and serum was utilized for serology. The rats were euthanized at week 2 using carbon dioxide asphyxiation, to harvest the tissue at the site of injection. The harvested tissues were rinsed in 1× PBS and were fixed in buffered 10% formalin for 24 hr. The tissues were then embedded in paraffin and sectioned at thickness of 4 µm, and the slides were stained with hematoxylin and eosin (H&E) solution. The histological sections were evaluated for signs of toxicity and inflammation.
Cell viability assay after encapsulation
Canine ASC pellets, at a density of 1 × 105 cell/ml, were combined with 6 ml of 1.2% sodium alginate solution, and standardized process parameters were used for the encapsulation of the cells. After encapsulation, the cell viability and survival rates were evaluated using a LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells (Invitrogen). The reagent solution was mixed according to the manufacturer’s instructions. The encapsulated cell suspension was mixed with reagent solution in a 6-well tissue culture dish and was subsequently incubated for 30 min. Cell survival and viability were quantified using a fluoroscopy microscope.
Proliferation assay for microencapsulated cells
Canine ASC pellets, at a density of 1 × 105 cell/ml, were combined with 6 ml of 1.2% sodium alginate solution, and standardized process parameters were used for the encapsulation of the cells. Cell proliferation, within the microcapsules, was evaluated at day 0, week 1, week 2, week 4 and week 6, by performing an alamarBlue proliferation assay (AbDserotec, Oxford, UK).
After encapsulation, 0.5 ml of the encapsulated cell suspension was placed in a 40-µm nylon cell strainer, which was placed in a well of a 6-well plate filled that contained 5 ml of normal cell culture media. The encapsulated cells were then maintained in standard conditions.
The alamarBlue assay was performed according to manufacturer’s instructions; specifically, the reagent was added at a 10% volume, directly to the medium [3]. To measure proliferation, the strainer containing the encapsulated cells was lifted from the normal culture media and placed in a premixed solution containing 5 ml of PBS and 500 µl of alamarBlue reagent. After 6 hr of incubation, 100 µl of the sample was placed in a 96-well plate, and the absorbance was measured using a microplate reader (Molecular devices, Sunnyvale, CA, U.S.A.) and Softmac pro 5.4.1 software. The samples were analyzed in triplicate, and average values were obtained to calculate the percent reduction of the alamarBlue reagent.
In vivo bioluminescent imaging of injected microencapsulated cells
A HEK-293 (Luc-2) cell pellet, at a density of 1 × 107 cell/ml, was combined with 6 ml of 1.2% sodium alginate solution, and standardized process parameters were used for the encapsulation of the cells.
ICR male mice (NaraBiotec), 8–10 weeks old with a body weight of 16–20 g, were used for this experiment. The control group and test groups were injected with 1 ml of 1 × 107 non-encapsulated or encapsulated HEK-293T (Luc-2) cells, respectively. The survival rates of the encapsulated HEK-293 (Luc-2) cells were then compared to those of the non-encapsulated HEK-293T (Luc-2) cells. In vivo bioluminescent imaging was performed immediately after injection, and 1 d, 3 d and 7 d post-injection, using a Xenogen IVIS 200 (Xenogen IVIS 200, PerkinElmer, Waltham, MA, U.S.A.) in Chuncheon Center of Korea Basic Science Institute. The animals were injected with 100 µl of D-lucferin (D-lucferin, PerkinElmer) intraperitoneally 15 min before imaging.
Statistical analysis
Data are expressed as the mean ± SD. Statistical analysis was performed with appropriate software (SPSS version 20, SPSS, Chicago, IL, U.S.A.). Student’s t test was performed to assess differences between two groups. A Kruskal-Wallis test was used to assess differences between two or more groups. A post hoc test was also performed with a Mann-Whitney U test. A P value <0.05 was considered significant.
RESULTS
Dimensional and morphological characterization of microbeads
The effects of altered frequency (Hz), flow rate (ml/min) and electrode (V) on bead diameter and shape were assessed using a standardized process. The objective of the process was to determine optimal parameters to produce microbeads ideal for injecting with a 23-gauge hypodermic needle.
Microcapsules formed using optimal conditions, specifically a frequency of 1,000 Hz, a flow rate of 23 ml/min and electrode of 1,000 V, were approximately 250 µm in diameter (Fig. 1). The diameter of the microcapsules tended to decrease with higher frequency, higher flow rate and higher voltage. However, results suggested that an excessive frequency of 2,500 Hz produced microbeads with increased diameter. Furthermore, an excessive flow rate of 28 ml/min produced microbeads with smaller diameter; however, severe flaring out of the sodium alginate solution resulted in significant loss of the solution.
Fig. 1.
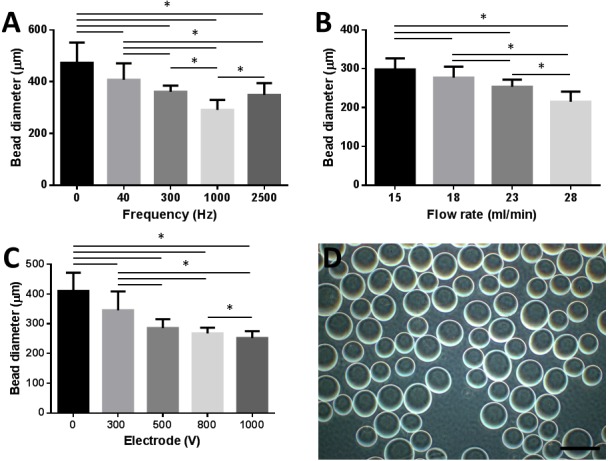
Standardization of optimal bead size for injection. (A) Microbead diameter (µm) as a function of increasing frequency (Hz). (B) Microbead diameter (µm) as a function of increasing flow rate (ml/min). (C) Microbead diameter (µm) as a function of increasing electrode (V). (D) Light microscope image of microbeads; bar=500 µm.
In vivo biocompatibility and toxicity of alginate microbeads
A toxicology test was performed to determine the biocompatibility and toxicity of alginate microbeads in vivo. Toxicity was assessed in rats through subcutaneous injection of the beads and subsequent blood testing and histological evaluation of the subcutaneous tissue
No significant toxicity was indicated by blood chemistry evaluation of the control group and the alginate microcapsule-injected group (Fig. 2 and Supplementary Tables 1 and 2). Histological evaluation of the microcapsule injection site showed that the beads could be detected 2 weeks after implantation and that degradation and phagocytosis of the bead material were occurring without significant inflammation (Fig. 3).
Fig. 2.
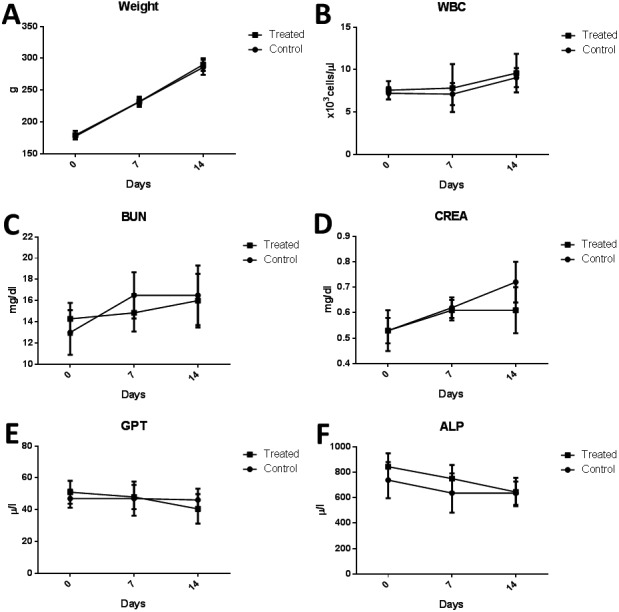
In vivo toxicology evaluation of alginate microbeads. Weight and blood chemistry evaluation of the PBS injected group and the alginate microbead injected group, which showed no significant difference between the groups at 0 days, 1 week and 2 weeks after injection. (A) Weight. (B) White blood cell count. (C) Blood urea nitrogen. (D) Creatinine. (E) Glutamic-pyruvic transaminase. (F) Alanine transaminase.
Fig. 3.
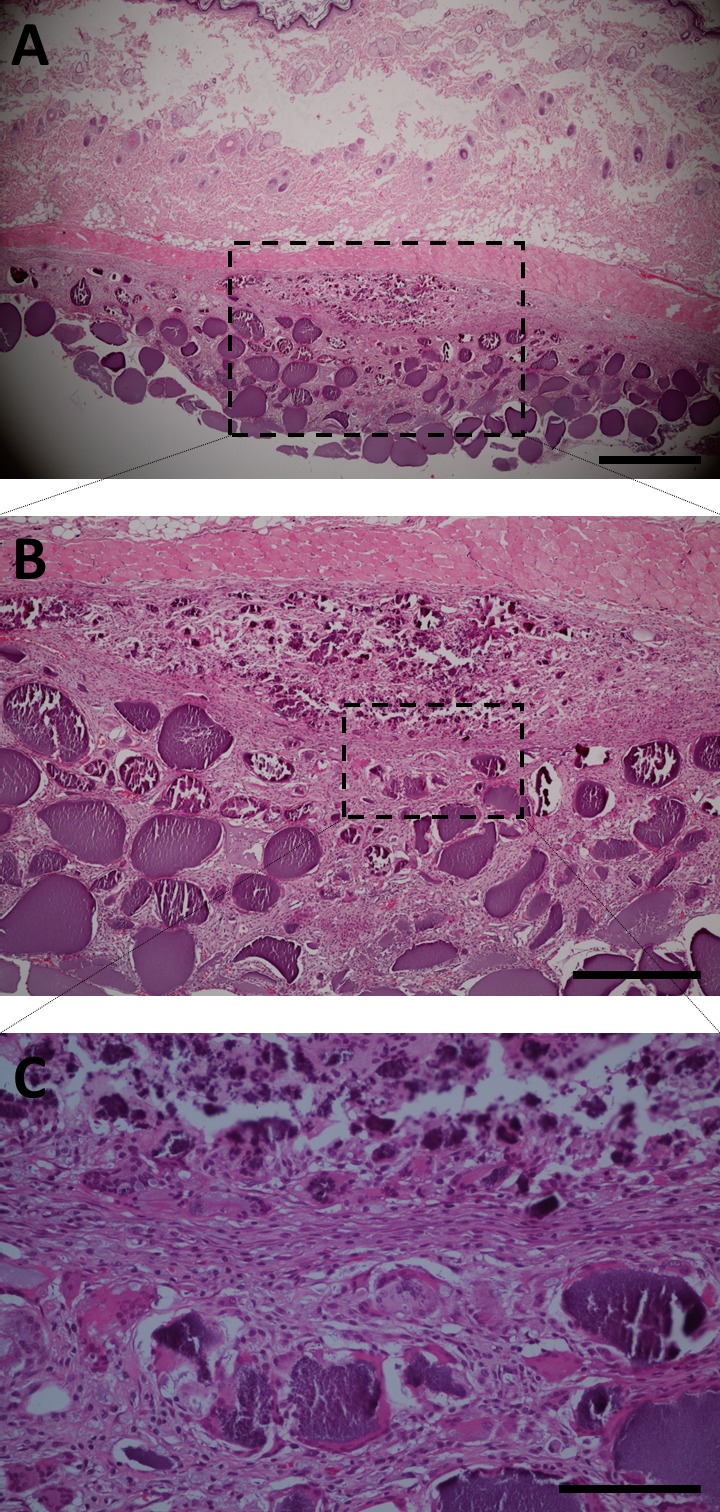
Photomicrograph of the H&E stained sections of the subcutaneous tissue injected with alginate microcapsules at week 2. Degradation and phagocytosis of the bead material are shown. (A) The alginate microcapsules were detectable 2 weeks after implantation; bar=500 µm. (B) Inset −higher magnification image of the boxed area; bar=250 µm. (C) Inset −higher magnification image of the boxed area; bar=125 µm.
Cell viability after encapsulation
The effect of encapsulation on cell viability and survival was assessed using a LIVE/DEAD Viability/Cytotoxicity Kit immediately after the encapsulation process.
The encapsulation of the cell could be seen in microscopic images. The cells were almost embedded in the alginate and cell-alginate mixture solidified in contact with gelling solution (100 mM of calcium chloride). Microcapsules containing canine ASCs were mostly spherical in shape with diameters that ranged from approximately 250–300 µm (Fig. 4). Fluorescence imaging of the encapsulated canine ASCs after performing the LIVE/DEAD Viability/Cytotoxicity assay showed that almost all cells were viable (Fig. 4). Canine ASCs viability was 97.36% after encapsulation, and washing. Live cells are represented by green fluorescence, while dead cells are represented by red fluorescence.
Fig. 4.
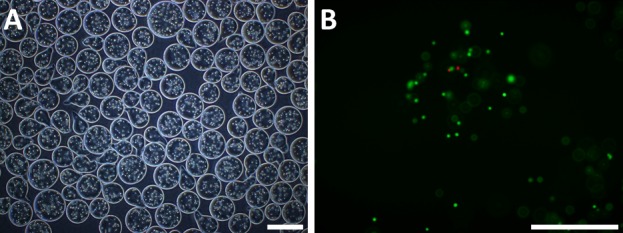
Encapsulated canine ASCs. (A) Microscopic appearance of microencapsulated canine ASCs. Light microscopic appearance of encapsulated canine ASCs showing a bead diameter of approximately 250 µm; bar=500 µm. (B) Cell viability immediately after encapsulation. Live cell cytoplasm appeared green, while dead cells had a red-stained nucleus; bar=250 µm.
In vitro proliferation of the microencapsulated cells
Cell proliferation and viability within the microcapsules, for up to 6 weeks in standard cultivation, were assessed by an alamarBlue assay.
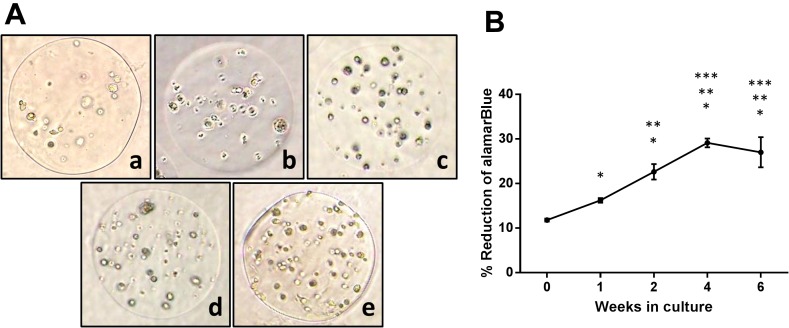
Light microscopic images of the microencapsulated canine ASCs showed increasing cell proliferation within the beads at day 0, week 1, week 2 and week 4 (Fig. 5). The percent reduction of alamarBlue increased with time; however, there was no significant increase in reduction when comparing weeks 4 and 6.
Fig. 5.
AlamarBlue percent reduction and associated photomicrographs, of the encapsulated canine ASCs at 0 hr (a), 1 week (b), 2 weeks (c), 4 weeks (d) and 6 weeks (e). (A) Light microscopic images of the canine ASCs showed an increase in cell proliferation within the beads with time. (B) The percent reduction of alamarBlue increased with time; however, there was no significant increase in reduction when comparing weeks 4 to 6.
In vivo retention of injected microencapsulated cells
After injection, the in vivo survival rate of the encapsulated luciferase-expressing stable cell line in mice was evaluated and compared to that of non-encapsulated cells using bioluminescent imaging up to 7 d post-injection.
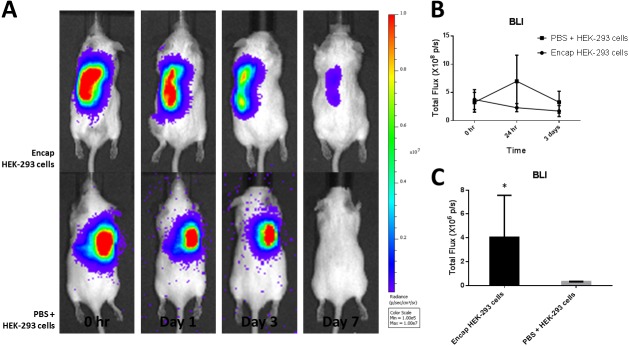
In vivo bioluminescence imaging at 0 hr, 1 day, 3 days and 7 days post-injection showed greater cell retention in mice administered encapsulated HEK-293T (Luc-2) cells compared to those receiving direct cell injection (Fig. 6). The encapsulated HEK-293T (Luc-2) cells were still viable at 7 days post-injection; however, the non-encapsulated HEK-293T (Luc-2) cells could not be detected at 7 days post-injection. The total flux (108 p/s) was not significantly different between the test group and the control group at 0 hr, 1 day and 3 days post-injection; however, at 7 days post-injection, the test group had a significantly increased total flux (106 p/s) compared to that in the control group. The results showed that encapsulated HEK-293T (Luc-2) cells had increased retention in vivo compared to non-encapsulated HEK-293T (Luc-2) cells.
Fig. 6.
In vivo optical bioluminescence imaging (BLI) showed greater retention and survival for encapsulated HEK-293 (Luc-2) cells compared to those of direct injection. (A) BLI image of representative animal from each group (n=5), showing increased cell retention in the animal administered encapsulated cells at day 7. (B) Quantification of BLI signal in regions of interest in the subcutaneous tissue at the total flux level of 108 p/s at 0 hr, 1 day and 3 days showed no significant difference between the groups. (C) Quantification of BLI signal in regions of interest in the subcutaneous tissue at the total flux level of 106 p/s showed significantly greater signal in the animals treated with encapsulated cells at day 7.
DISCUSSION
Stem cell-based therapies have great potential for the treatment of several diseases in veterinary medicine; however, the low cell viability at the disease site prevents therapeutic efficacy [14, 21, 22]. Numerous studies have focused on increasing stem cell retention and survival rates; however, the application of stem cell encapsulation in veterinary science is lacking. Previous successful studies incorporating alginate microencapsulation with human mesenchymal stem cells in various organs imply its promising future for applications in the veterinary sciences, such as tissue repair and regeneration, and the development of artificial organs [1, 4, 6, 7, 17, 23]. Alginate-based encapsulation provides a new strategy for veterinary stem cell therapy. In this study, we have successfully evaluated the retention potential of viable HEK-293 cells in alginate microencapsulation using an in vivo mouse model, and our findings suggest that it could be a novel method for enhancing the therapeutic potential of stem cells for several diseases in the veterinary medical field.
To improve cell-based therapies, the cell delivery materials are one of the major factors contributing to cell survival. In general, encapsulated cells can be protected from both mechanical stress and the host immune system [15]. Furthermore, cell encapsulation within biocompatible materials that are capable of bidirectional diffusion of nutrients, oxygen and waste would allow for enhanced cell function and cell survival [9]. In the present study, canine ASCs were cultured for up to 6 weeks using standard cultivation and exhibited cell proliferation over time. The increase in the reduction of alamarBlue until week 4 indicates that alginate microcapsules provide an environment that supports cell colonization and proliferation, and confirms the free exchange of nutrients, oxygen and metabolites to maintain metabolic functions. The absence of significant alamarBlue reduction at week 6 suggests that there might be a maximum proliferation potential within the microcapsules or that alginate might lack the bioactive ligands necessary for cell anchoring [15, 23]. An injectable scaffold can provide a microenvironment for cells and can be employed for local therapeutics including local injection for treatment of osteoarthritis or nonunion. We demonstrated that encapsulated cells were retained longer in the injected site as assessed by bioluminescence imaging. Our results clearly address the alginate microenvironment supports and retention in vivo. Increased cell proliferation in a time dependent manner increased cell retention in vivo, suggesting that the alginate microbeads formed in this study were able to overcome low cell survival at the injection site. Therefore, the results of this study indicate that encapsulation is a successful technique to increase viable cell retention for a prolonged period in vivo and that this technique can be applied for enhanced efficacy of stem cell therapy in the veterinary medical field.
In this study, HEK-293 (luc-2) cells were used for the BLI, though canine ASCs transfected with luciferase genes should have been used for a more correct representation of the enhanced in vivo cell retention. We thought that using luciferase stable cell lines was able to show a representation of the enhanced in vivo cell retention of alginate microencapsulation technique. Because transfected stem cells with luciferase gene would require many cell passages and stem cells are not immortal, viability of stem cells could be decreased significantly with increasing passages. However, a previous study successfully performed the transduction of human mesenchymal stem cells with a lentivirus expressing firefly luciferase and concluded that in vivo BLI showed greater retention of cells in animals treated with encapsulated stem cells compared to delivery by direct injection [13]. Further in vivo studies will be needed to determine, if alginate based microencapsulation can be used to sustain survival of canine ASCs over a desirable period of time in vivo.
Alginate based microencapsulation has received interest for drug delivery and cell encapsulation. One common problem faced in alginate microencapsulation is the production of small beads that are replicable and similar in size and morphology. The objective of this was to determine optimal process parameters for the production of microbeads that are ideal for injection with a 23-gauge hypodermic needle, as the injection system is an important consideration for cell-based therapies. The diameters of the microcapsules formed with the optimized parameters (frequency of 1,000 Hz, flow rate of 23 ml/min and electrode of 1,000 V) were sufficiently small enough to pass through the nominal inner diameter of the 23-gauge hypodermic needle, making the microcapsules ideal for injection. Furthermore, in the present study, canine ASC and HEK-293T cells were well encapsulated in alginate microbeads, and the cell encapsulation process did not affect the diameter and shape of microbeads. In this study, the effect of the encapsulation process on cell viability and survival rate was also tested. The results of the LIVE/DEAD Viability/Cytotoxicity assay suggested that the encapsulation process had little or no significant adverse effects on cell viability and survival rate. The high survival rate of the cells indicates that the technique permits optimal use of extracted cells.
Several important factors that determine the mechanical properties of the alginate microbeads include the source of the alginate, the concentration of the gelling solution and the polymerization time. In several studies of alginate encapsulation, increasing the alginate concentration, up to 1.5–2.25%, led to an increase in comet- or lentil-shaped bead morphology [16]. The increase in sodium alginate concentration also led to an increase in interruptions in the spraying process due to the higher viscosity of the solution [6]. Furthermore, an increase in calcium chloride concentration from 20 mM to 150 mM resulted in beads with decreased diameter; however, they had suboptimal mechanical properties that made the microbeads sensitive to shear stress and an uncontrollable degradation rate [6]. Jitrauch et al. concluded that an increase in polymerization time from 10 to 15 min resulted in physically stable microbeads; however, a further increase from 15 to 20 min resulted in brittle microbeads [7]. The study suggested that a polymerization time of 10 min might have been too short for effective cross-linking and equilibration of external calcium between the core of the alginate and the external medium [7]. In our study, the effect of polymerization time was not investigated; this might have influenced the degradation rate of the microbeads in vivo. Histological evaluation of the alginate beads injected in rats showed that cells associated with subcutaneously injected alginate beads were still viable two weeks after injection. Other studies on the bead degradation rate in vivo, such as that by Paul et al., also concluded that microcapsules tend to degrade after 3–4 weeks and confirmed that polymeric microcapsules completely disappeared after 10 weeks [16]. Paul et al. used 1.5% sodium alginate solution and 100 mM of calcium chloride solution with 20 min of polymerization time to make calcium-alginate beads containing human ASCs [16]. In the present study, 1.2% sodium alginate solution was used, and other parameters were similar to those of the previous study. Although the exact effect of the concentration of alginate on the degradation time of microbeads in vivo was not clear, it could be inferred that the degradation time would not be longer than 10 weeks in this study. To improve microcapsule cell-based therapies, the effect of microbead materials and polymerization times on the in vivo degradation rate must be further investigated.
Alginate is a biomaterial with numerous applications in biomedical science and engineering due to its favorable properties including biocompatibility and ease of gelation. Although the biocompatibility of alginate has been extensively evaluated in vitro and in vivo, debate exists regarding the impact of alginate composition. The immunogenic response at the injection or implantation sites might be attributed to impurities in the alginate. A previous study reported that no significant inflammatory response was observed when gels formed from commercially available, highly purified alginate were subcutaneously injected into mice [12]. Similarly, commercially purchased sodium alginate was used in the present study, and results from blood work and histological evaluation indicated that the alginate beads were biocompatible and biodegradable, and had no toxicity in vivo. Therefore, it is concluded that the alginate beads are safe and ideal for in vivo application.
Specific problems remain to be solved, as alginate-based biomaterials are currently unable to meet certain design parameters, such as a controllable degradation rate and mechanical rigidity. Several possible cross-linking strategies, using different chemical molecules, might yield more applicable and suitable biomaterials for use in various tissues and organs. Furthermore, to improve viability and proliferation of the encapsulated cells, alginate modification with cell recognition factors is needed for improved cell attachment that allows long-term growth within the alginate structures. In a review of alginate-based biomaterials for regenerative medicine applications, it was concluded that although alginate exhibits good biocompatibility, it is composed of inert monomers that inherently lack bioactive ligands necessary for cell anchoring [20]. Therefore, by cross-linking the biomaterials to arginine-glycine-aspartic acid molecules, to introduce ligands for cell attachment, improved cell attachment and proliferation within the beads could be achieved [8, 15, 23].
In conclusion, an optimized protocol for the production of canine ASC-microbeads has been established in this study. Data presented in this study indicate that calcium cross-linked alginate microcapsules were able to act as substrate and support the in vitro proliferation of canine ASCs. Based on the use of sodium alginates as cell encapsulation materials, this work demonstrates that it has potential to act as an injectable, cell immobilizing, 3D scaffold in tissue engineering. Thus, injectable microencapsulated cells could represent a novel strategy for stem cell therapy in the veterinary medical field.
Supplementary
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A01051759).
REFERENCES
- 1.Abbah S. A., Lu W. W., Chan D., Cheung K. M., Liu W. G., Zhao F., Li Z. Y., Leong J. C., Luk K. D.2006. In vitro evaluation of alginate encapsulated adipose-tissue stromal cells for use as injectable bone graft substitute. Biochem. Biophys. Res. Commun. 347: 185–191. doi: 10.1016/j.bbrc.2006.06.072 [DOI] [PubMed] [Google Scholar]
- 2.Bhat A., Hoch A. I., Decaris M. L., Leach J. K.2013. Alginate hydrogels containing cell-interactive beads for bone formation. FASEB J. 27: 4844–4852. doi: 10.1096/fj.12-213611 [DOI] [PubMed] [Google Scholar]
- 3.Bonnier F., Keating M. E., Wróbel T. P., Majzner K., Baranska M., Garcia-Munoz A., Blanco A., Byrne H. J.2015. Cell viability assessment using the Alamar blue assay: a comparison of 2D and 3D cell culture models. Toxicol. In Vitro 29: 124–131. doi: 10.1016/j.tiv.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 4.Capone S. H., Dufresne M., Rechel M., Fleury M. J., Salsac A. V., Paullier P., Daujat-Chavanieu M., Legallais C.2013. Impact of alginate composition: from bead mechanical properties to encapsulated HepG2/C3A cell activities for in vivo implantation. PLoS ONE 8: e62032. doi: 10.1371/journal.pone.0062032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasperini L., Mano J. F., Reis R. L.2014. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 11: 20140817. doi: 10.1098/rsif.2014.0817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gryshkov O., Pogozhykh D., Zernetsch H., Hofmann N., Mueller T., Glasmacher B.2014. Process engineering of high voltage alginate encapsulation of mesenchymal stem cells. Mater. Sci. Eng. C 36: 77–83. doi: 10.1016/j.msec.2013.11.048 [DOI] [PubMed] [Google Scholar]
- 7.Jitraruch S., Dhawan A., Hughes R. D., Filippi C., Soong D., Philippeos C., Lehec S. C., Heaton N. D., Longhi M. S., Mitry R. R.2014. Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure. PLOS ONE 9: e113609. doi: 10.1371/journal.pone.0113609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang B. J., Ryu H. H., Park S. S., Koyama Y., Kikuchi M., Woo H. M., Kim W. H., Kweon O. K.2012. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton’s jelly for treating bone defects. J. Vet. Sci. 13: 299–310. doi: 10.4142/jvs.2012.13.3.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim P. H., Yim H. G., Choi Y. J., Kang B. J., Kim J., Kwon S. M., Kim B. S., Hwang N. S., Cho J. Y.2014. Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J. Control. Release 187: 1–13. doi: 10.1016/j.jconrel.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 10.Landázuri N., Levit R. D., Joseph G., Ortega-Legaspi J. M., Flores C. A., Weiss D., Sambanis A., Weber C. J., Safley S. A., Taylor W. R.2016. Alginate microencapsulation of human mesenchymal stem cells as a strategy to enhance paracrine-mediated vascular recovery after hindlimb ischaemia. J. Tissue Eng. Regen. Med. 10: 222–232. doi: 10.1002/term.1680 [DOI] [PubMed] [Google Scholar]
- 11.Lee C. S., Nicolini A. M., Watkins E. A., Burnsed O. A., Boyan B. D., Schwartz Z.2014. Adipose stem cell microbeads as production sources for chondrogenic growth factors. J. Stem Cells Regen. Med. 10: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J., Lee K. Y.2009. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm. Res. 26: 1739–1744. doi: 10.1007/s11095-009-9884-4 [DOI] [PubMed] [Google Scholar]
- 13.Levit R. D., Landázuri N., Phelps E. A., Brown M. E., García A. J., Davis M. E., Joseph G., Long R., Safley S. A., Suever J. D., Lyle A. N., Weber C. J., Taylor W. R.2013. Cellular encapsulation enhances cardiac repair. J. Am. Heart Assoc. 2: e000367. doi: 10.1161/JAHA.113.000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linero I., Chaparro O.2014. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLOS ONE 9: e107001. doi: 10.1371/journal.pone.0107001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murua A., Portero A., Orive G., Hernández R. M., de Castro M., Pedraz J. L.2008. Cell microencapsulation technology: towards clinical application. J. Control. Release 132: 76–83. doi: 10.1016/j.jconrel.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 16.Paul A., Chen G., Khan A., Rao V. T., Shum-Tim D., Prakash S.2012. Genipin-cross-linked microencapsulated human adipose stem cells augment transplant retention resulting in attenuation of chronically infarcted rat heart fibrosis and cardiac dysfunction. Cell Transplant. 21: 2735–2751. doi: 10.3727/096368912X637497 [DOI] [PubMed] [Google Scholar]
- 17.Penolazzi L., Tavanti E., Vecchiatini R., Lambertini E., Vesce F., Gambari R., Mazzitelli S., Mancuso F., Luca G., Nastruzzi C., Piva R.2010. Encapsulation of mesenchymal stem cells from Wharton’s jelly in alginate microbeads. Tissue Eng. Part C Methods 16: 141–155. doi: 10.1089/ten.tec.2008.0582 [DOI] [PubMed] [Google Scholar]
- 18.Ryu H. H., Kang B. J., Park S. S., Kim Y., Sung G. J., Woo H. M., Kim W. H., Kweon O. K.2012. Comparison of mesenchymal stem cells derived from fat, bone marrow, Wharton’s jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J. Vet. Med. Sci. 74: 1617–1630. doi: 10.1292/jvms.12-0065 [DOI] [PubMed] [Google Scholar]
- 19.Serra M., Correia C., Malpique R., Brito C., Jensen J., Bjorquist P., Carrondo M. J., Alves P. M.2011. Microencapsulation technology: a powerful tool for integrating expansion and cryopreservation of human embryonic stem cells. PLoS ONE 6: e23212. doi: 10.1371/journal.pone.0023212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J., Tan H.2013. Alginate-based biomaterials for regenerative medicine applications. Materials (Basel) 6: 1285–1309. doi: 10.3390/ma6041285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volk S. W., Theoret C.2013. Translating stem cell therapies: the role of companion animals in regenerative medicine. Wound Repair Regen. 21: 382–394. doi: 10.1111/wrr.12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitworth D. J., Banks T. A.2014. Stem cell therapies for treating osteoarthritis: prescient or premature? Vet. J. 202: 416–424. doi: 10.1016/j.tvjl.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 23.Yu J., Du K. T., Fang Q., Gu Y., Mihardja S. S., Sievers R. E., Wu J. C., Lee R. J.2010. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 31: 7012–7020. doi: 10.1016/j.biomaterials.2010.05.078 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.