Abstract
Maropitant, a neurokinin-1 receptor antagonist, may provide analgesic effects by blocking pharmacological action of substance P. Carprofen is a non-steroidal anti-inflammatory drug commonly used for pain control in dogs. The purpose of this study was to evaluate the effect of a combination of maropitant and carprofen on the minimum alveolar concentration for blunting adrenergic response (MAC-BAR) of sevoflurane in dogs. Six healthy adult beagle dogs were anesthetized with sevoflurane four times with a minimum of 7-day washout period. On each occasion, maropitant (1 mg/kg) alone, carprofen (4 mg/kg) alone, a combination of maropitant (1 mg/kg) and carprofen (4 mg/kg), or saline (0.1 ml/kg) was subcutaneously administered at 1 hr prior to the first electrical stimulation for the sevoflurane MAC-BAR determination. The sevoflurane MAC-BAR was significantly reduced by maropitant alone (2.88 ± 0.73%, P=0.010), carprofen alone (2.96 ± 0.38%, P=0.016) and the combination (2.81 ± 0.51%, P=0.0003), compared with saline (3.37 ± 0.56%). There was no significant difference in the percentage of MAC-BAR reductions between maropitant alone, carprofen alone and the combination. The administration of maropitant alone and carprofen alone produced clinically significant sparing effects on the sevoflurane MAC-BAR in dogs. However, the combination of maropitant and carprofen did not produce any additive effect on the sevoflurane MAC-BAR reduction. Anesthetic premedication with a combination of maropitant and carprofen may not provide any further sparing effect on anesthetic requirement in dogs.
Keywords: carprofen, dog, MAC-BAR, maropitant, sevoflurane
In current veterinary practice, administration of multiple analgesics in combination with acting through different mechanisms, “multimodal therapy”, is often advocated to maximize analgesic effect [26]. The multimodal approach for analgesia also provides a great benefit of concomitant reduction of adverse effects with additive or synergistic analgesic effect produced by lower doses of each analgesic [17]. In addition, an administration of analgesics before the patient receives painful stimuli, “preventive analgesia”, is advocated to reduce the requirement of anesthetics during surgery and to minimize post-operative pain in animals [12, 22]. In surgical patients, the multimodal and preventive approach for analgesia is successfully achieved by premedication using multiple analgesics, such as combination of a non-steroidal anti-inflammatory drug (NSAID) and an opioid [12, 22].
Maropitant is a highly selected neurokinin (NK1) receptor antagonist that blocks the pharmacological action of substance P in the central nervous system (CNS) [4, 5]. Maropitant provides antimemetic effects by preventing the substance P from binding with the NK1 receptors located in the vomiting center and the chemo-trigger zone [5, 23]. On the other hand, the substance P binds to the NK1 receptors located in the dorsal horn of spinal cord with high affinity and produces increases in pain signal in cats [11]. Therefore, maropitant is expected to provide analgesic effects by preventing the substance P from binding with the NK1 receptors located in the dorsal horn. It is reported that maropitant decreased the anesthetic requirements in dogs [1, 7].
NSAIDs produce analgesic, anti-inflammatory and anti-pyretic effects by inhibiting arachidonate cyclooxygenase (COX), thereby inhibiting the production of prostaglandins [35]. There are three isozymes of COX: COX-1 which is normally present in a variety of organs and is constitutive under physiological conditions, COX-2 which is induced by inflammatory stimuli and pathological conditions, and COX-3 which is encoded by the same gene as COX-1 and present in the brain [6]. Carprofen is a COX-2 selective NSAID and commonly used for treatment for post-operative pain and inflammation in dogs. It is also reported that carprofen reduced the anesthetic requirements in normal healthy dogs without inflammation [38]. Prostaglandins are known to facilitate the release of substance P from the central nerve terminals of primary sensory nerves [19]. Malmberg et al. [20] showed that NSAIDs exerted a direct spinal antinociceptive action by blocking the hyperalgesia induced by the activation of spinal NK1 receptors. These findings indicate that NSAIDs may produce their analgesic effect by the direct spinal action besides peripheral anti-inflammatory action. Therefore, NSAIDs including carprofen may reduce the anesthetic requirement by the spinal antinociceptive action through the prevention of substance P release.
The potency of inhalation anesthetics traditionally has been evaluated by use of the concept of minimum alveolar concentration (MAC) to prevent movements, which is the alveolar concentration of inhalation anesthetic agent at 1 atmosphere that prevents movement in 50% of population exposed to a noxious stimulation [27, 36]. Several studies provide evidence that volatile inhalant anesthetic agents act primarily within the spinal cord to decrease movement in response to a noxious stimulation [3, 28] and produce immobility mainly by acting on the spinal ventral horn [2, 15]. Therefore, the MAC could reflect the suppression of motor neurons at the ventral horn in the spinal cord [15]. On the other hand, minimum alveolar concentration for blunting adrenergic response (MAC-BAR) is defined as the minimum anesthetic concentration that prevents an autonomic response to a noxious stimulation [16, 18, 21, 29]. The MAC-BAR is a useful measure of anesthetic effect on autonomic pathways in the subcortical centers (spinal cord and brainstem) and may provide important information to diminish the intraoperative neuroendocrine stress response [29]. Two previous studies suggested that a preventive administration of analgesic reduced the MAC-BAR of sevoflurane in dogs [18, 32].
As mentioned above, the multimodal and preventive approach for analgesia is successfully achieved by premedication using multiple analgesics [12, 22]. However, it is suspected that further sparing effect on anesthetic requirement during surgery may not be achieved by a combination of maropitant and carprofen because the prevention of substance P release provided by carprofen through the inhibition of prostaglandine synthesis may be incompetent to increase the antinociceptive effect under the presence of maropitant that can prevent the substance P from binding with the NK1 receptors in the spinal cord. We hypothesized that premedication with a combination of maropitant and carprofen might not provide any further sparing effect on anesthetic requirement in dogs. As far as the authors know, the interaction between maropitant and carprofen has not been described in any animal species. The purpose of this study was to evaluate the interaction between maropitant and carprofen on sparing of the MAC-BAR of sevoflurane in dogs.
MATERIALS AND METHODS
Experimental animals
Six intact adult beagle dogs (3 males and 3 females), 2.3 ± 1.0 (mean ± standard deviation [S.D.]; range 1.0 to 3.0) years old and weighed 10.3 ± 1.5 (8.0 to 13.5) kg, were anesthetized with sevoflurane four times with a minimum of 7-day washout period. We adopted the same number of females and males in order to offset the consequences of gender difference. The dogs received a subcutaneous injection of saline on the first occasion (Control group), maropitant alone on the second occasion (MARP group), carprofen alone on the third occasion (CARP group), and a combination of maropitant and carprofen on the last occasion (MARP-CARP group) at 1 hr prior to the first electrical stimulation for the determination of sevoflurane MAC-BAR. The dogs were judged to be in good to excellent health based upon a physical examination, blood cells count and serum biochemical determination. Food was withheld from the dogs for 12 hr before anesthesia, but allowed free access to water. The dogs were cared for according to the principles of the “Guide for the Care and Use of Laboratory animals” prepared by Rakuno Gakuen University. The Animal Care and Use Committee of Rakuno Gakuen University approved this study (Approval No. VH23B13).
Anesthesia and instrumentation
In all the dogs, anesthesia was induced by mask induction using sevoflurane (Sevoflo, DS Pharma Animal Health Co., Ltd., Osaka, Japan) in oxygen. The dogs were orotracheally intubated after the induction of anesthesia and anesthetized with oxygen and sevoflurane in left lateral recumbency. The cephalic vein and the dorsal pedal artery were catheterized with 22-guage catheters (Supercath, Medikit Co., Ltd., Tokyo, Japan). Arterial blood pressure was directly measured by connecting the catheter that was placed in the dorsal pedal artery to a pressure transducer (BD DTXTM Plus DT-4812, Japan Becton, Dickinson and Co., Fukushima, Japan) that was placed and zeroed at the level of the mid-sternum. During anesthesia, the partial pressure of end-tidal CO2 (PETCO2) was maintained between 35 and 40 mmHg by intermittent positive pressure ventilation (IPPV) using a time-cycled ventilator (Nuffield Anesthesia Ventilator Series 200, Penlon, Abingdon Oxon, U.K.). The dogs were administered lactated Ringer’s solution at a rate of 10 ml/kg/hr intravenously through the catheter that was placed in the cephalic vein. Esophageal temperature was maintained between 37.5 and 38.5 ○C using a heating pad and a warm air blanket in the dogs.
Esophageal temperature (○C), heart rate (beats/min), lead II of the electrocardiogram, respiratory rate (breaths/min), mean arterial blood pressure (MABP; mmHg), saturation of hemoglobin with oxygen measured by pulse oxymetry (SpO2;%), PETCO2 (mmHg) and end-tidal concentration of sevoflurane (ETSEV;%) were monitored using a veterinary patient monitoring system (BP-608V, Omron Colin Co., Ltd., Tokyo, Japan). The esophageal temperature was measured using an electric thermometer probe placed orally into the thoracic esophagus. According to the previous reports [28, 31,32,33], a commercially available adaptor (Air way adaptor L-shape, Omron Colin Co., Ltd.) modified with an 8-Fr feeding tube (Atom Indwelling Feeding Tube, Atom medical Co., Ltd., Tokyo, Japan) was placed at the Y-piece of the breathing circuit. The feeding tube passed through the endotracheal tube so that its tip rested in the thoracic portion of the trachea. Gas samples were drawn from the proximal end of the endotracheal tube using the feeding tube at a rate of 200 ml/min. A side-stream capnograph and anesthetic agent monitor were used to determine respiratory rate, PETCO2 and ETSEV. The anesthetic agent monitor was calibrated at the start of each experiment using a commercial calibration kit (AG calibration gas and adaptor set, Omlon Colin Co., Ltd.).
MAC-BAR determination
Following the instrumentation, the dogs received a subcutaneous injection of maropitant (1 mg/kg, 10 mg/ml; Cerenia Injectable, Zoetis Japan, Tokyo, Japan) in MARP group, a subcutaneous injection of carprofen (4 mg/kg, 50 mg/ml; Rimadyl Injectable, Zoetis Japan) in CARP group, subcutaneous injections of maropitant (1 mg/kg) and carprofen (4 mg/kg) in MARP-CARP group or a subcutaneous injection of saline (0.1 ml/kg) in Control group. The MAC-BAR determination began after the dogs were allowed to equilibrate for 60 min at ETSEV 3.0%. The MAC-BAR of sevoflurane was determined according to the previous studies [32, 37]. Briefly, the MAC-BAR of sevoflurane was determined by judging the dogs’ response to a noxious electrical stimulus (50 V, 50 Hz, 10 msec) applied to their right upper gingival for 10 sec using an electrical stimulator (SEN3301, Nihon Koden Co., Tokyo, Japan). Positive response for the MAC-BAR determination was fixed and increased in either heart rate or MABP over 15% above the pre-stimulation value recorded at 1 min before applying the electrical stimulus during a 30 sec observation period following the 10 sec stimulation period. When the dog exhibited the positive response, the ETSEV was increased by 10 to 20%, and the dog was retested after 20 min of re-equilibration. When the dog did not exhibit the positive response, the ETSEV was decreased by 10 to 20%, and the dog was retested after 20 min of re-equilibration. The MAC-BAR was determined as the mean of the ETSEV at which the dog did not demonstrate positive responses and next lower concentration tested (i.e., the highest concentration at which the dog demonstrated positive responses to the electrical stimulus). The MAC-BAR for each dog was determined in triplicate by the same person (S. F.).
The percentage of MAC-BAR reduction was calculated as: Percentage MAC-BAR reduction=(MAC-BAR of Control group –MAC-BAR of each treatment group)/ MAC-BAR of Control group ×100.
Statistical analysis
The statistical analyses were performed using State Mate III for Windows (ATMS, Tokyo, Japan). The data were reported as means ± S.D. from 6 dogs in each group and were confirmed for normality and homogenesity of variance of the data by using Kolmogov-Smirnov and Bartlett tests. The sevoflurane MAC-BAR, percentage of MAC-BAR reduction and time taken to obtain the triplicate data for the sevoflurane MAC-BAR were analyzed by using one-way factorial ANOVA and Tukey test between overall groups, because their normality and homogenesity of variance were confirmed. The heart rate and MABP were analyzed by using Friedman test between the overall groups, because of their non-normality and/or heteroscedasticity. The MAC-BAR was also compared between the Control group and each treatment group by using paired t-test.
A drug interaction between maropitant and carprofen was evaluated according to a previous report in dogs [38]. Briefly, the drug interaction was evaluated by comparing the changes in the sevoflurane MAC-BAR, heart rate and MABP produced by the administration of maropitant and carprofen with those produced by the administration of carprofen alone. A paired data of the sevoflurane MAC-BAR, heart rate and MABP from the Control and CARP groups were used for analyzing the changes produced by the administration of carprofen alone. Another paired data of those from the MARP and MARP-CARP groups were used for analyzing the changes produced by the administration of maropitant and carprofen. The changes in these paired data were analyzed by using two-way repeated measures ANOVA. If a significant difference was detected with a statistical interaction, the drug interaction between maropitant and carprofen was judged to be synergic or antagonistic. If a significant difference was obtained without any statistical interaction, the drug interaction was judged to be additive. For all analyses, values of P<0.05 were considered significant.
RESULTS
The sevoflurane MAC-BAR in each dog is summarized in Table 1. It took 217 ± 35 min, 234 ± 48 min, 226 ± 42 min and 248 ± 76 min to obtain the triplicate data for the sevoflurane MAC-BAR in the MARP, CARP, MARP-CARP and Control groups, respectively. At the completion of sevoflurane MAC-BAR determinations, all the dogs did not show any clinically relevant inflammatory symptoms, such as swelling and redness, at the gingival area that was applied the noxious electrical stimuli. The sevoflurane MAC-BAR was 2.88 ± 0.77% for the MARP, 2.96 ± 0.38% for the CARP, 2.81 ± 0.51% for the MARP-CARP and 3.37 ± 0.56% for the Control group. The sevoflurane MAC-BAR for each treatment group was significantly lower than that for the Control group (P=0.010 for the MARP, P=0.016 for the CARP and P=0.0003 for the MARP-CARP groups), although there was no significant difference between the overall groups. The percentage of MAC-BAR reduction was 15.0 ± 16.0% for the MARP, 10.2 ± 17.6% for the CARP and 16.2 ± 8.8% for the MARP-CARP group. There was no significant difference in the percentage of MAC reductions between the treatment groups.
Table 1. Minimum alveolar concentration for blunting adrenergic response (MAC-BAR) of sevoflurane in each dog treated with subcutaneous administration of saline (Control), maropitant alone (MARP), carprofen alone (CARP) or a combination of maropitant and carprofen (MARP-CARP).
| Dogs | Age (years) |
Sex | Sevoflurane MAC-BAR (%) |
|||
|---|---|---|---|---|---|---|
| Control | MARP | CARP | MARP-CARP | |||
| No.1 | 1 | female | 3.45 | 3.75 | 3.35 | 2.67 |
| No.2 | 3 | female | 4.20 | 3.72 | 2.95 | 3.68 |
| No.3 | 3 | female | 3.25 | 3.05 | 2.28 | 2.27 |
| No.4 | 1 | male | 3.75 | 2.57 | 3.23 | 3.15 |
| No.5 | 1 | male | 2.85 | 2.40 | 2.85 | 2.62 |
| No.6 | 2 | male | 2.72 | 1.80 | 3.12 | 2.50 |
| Mean ± S.D. of MAC-BAR | 3.37 ± 0.56 | 2.88 ± 0.77 b) | 2.96 ± 0.38 a) | 2.81 ± 0.51 b) | ||
| Percentage MAC-BAR reduction | - | 15.0 ± 16.0 % | 10.2 ± 17.6% | 16.2 ± 8.8% | ||
S.D.: standard deviation. The percentage of MAC-BAR reduction was calculated as: (MAC-BAR of Control group –MAC-BAR of each treatment group)/ MAC-BAR of Control group ×100. Significant difference compared to the Control group: a) P<0.05, b) P<0.01.
Heart rate and MABP at the MAC-BAR determination are summarized in Table 2. In each dog, normothermia was achieved by using a heating pad and a warm air blanket. Good oxygenation and eucapnia were also achieved by both oxygen inhalation and IPPV. There was no significant difference in the heart rate. Clinically relevant hypotension (MABP <60 mmHg) was observed at the MAC-BAR determination in 4 dogs (67%) of the Control group (the lowest MABP: 45, 47, 49 and 52 mmHg in each dog), 3 dogs (50%) of the MARP group (the lowest MABP: 43, 47 and 52 mmHg in each dog) and 2 dogs (33%) of the MARP-CARP group (the lowest MABP: 50 and 58 mmHg in each dog). No dog showed hypotension in the CARP group.
Table 2. Heart rate and mean arterial blood pressure at the determination of minimum alveolar concentration for blunting adrenergic response (MAC-BAR) of sevoflurane in dogs treated with subcutaneous administration of saline (Control), maropitant alone (MARP), carprofen alone (CARP) or a combination of maropitant and carprofen (MARP-CARP).
| Control | MARP | CARP | MARP-CARP | |
|---|---|---|---|---|
| Heart rete (beats/min) | 108 ± 16 | 106 ± 11 | 105 ± 7 | 97 ± 16 |
| Mean arterial blood pressure (mmHg) a) | 66 ± 23 | 63 ± 19 | 77 ± 7 | 73 ± 18 |
Data from 3 observations recorded immediately prior to electrical stimulation that produced changes in response to stimulation were obtained from each dog. Significant difference between groups: a) P<0.01.
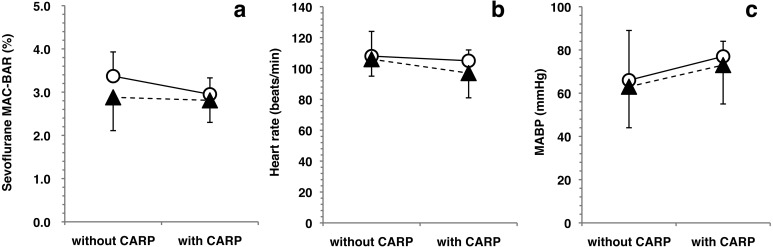
Changes in the sevoflurane MAC-BAR, heart rate and MABP produced by the administration of carprofen alone and the combination of maropitant and carprofen are shown in Fig. 1. There was no significant difference between the changes produced by the carprofen alone and the combination of maropitant and carprofen.
Fig. 1.
Drug interactions between maropitant and carprofen in the sevoflurane MAC-BAR, heart rate and mean arterial blood pressure (MABP). Plots and error bars represent mean values and standard deviations, respectively. Open circles (○) showed the paired data from the Control (without CARP) and CARP (with CARP) groups. Closed triangles (▲) showed the paired data from the MARP (without CARP) and MARP-CARP (with CARP) groups. There was no significant difference in the sevoflurane MAC-BAR (a), heart rate (b) and MABP (c) between the changes produced by carprofen alone and the combination of maropitant and carprofen. Therefore, it indicated that there was no drug interaction between maropitant and carprofen on the reduction of sevoflurane MAC-BAR, heart rate and MABP in dogs.
DISCUSSION
In the present study, the administration of maropitant and carprofen alone decreased the sevoflurane MAC-BAR by 15.0% and 10.2%, respectively. However, the combination of maropitant and carprofen did not produce any further reduction in the sevoflurane MAC-BAR. In addition, there was no drug interaction between maropitant and carprofen on the reduction of sevoflurane MAC-BAR, heart rate and MABP in dogs. Anesthetic premedication with the combination of maropitant and carprofen may not provide additive effect on sevoflurane requirement.
As we hypothesized, the combination of maropitant and carprofen did not show any additive effects on the reduction of sevoflurane MAC-BAR in dogs. Maropitant can provide analgesic effect by blocking the pharmacological action of substance P at the spinal cord and brain [1, 7, 30]. NSAIDs produce analgesic and anti-inflammatory effects by inhibiting the production of prostaglandins [35]. Prostaglandins can facilitate substance-P release from the central nerve terminals of C-fibers [25]. Therefore, it was thought that the inhibition of substance P release from the C-fibers provided by carprofen became incompetent to reduce the sevoflurane MAC-BAR because maropitant blocked the substance P from binding with the NK1 receptors in the spinal cord at the same time. Consequently, further sparing effect on anesthetic requirement was not achieved by the combination of maropitant and carprofen in our dogs.
The terminal ends of primary sensory nerve C- and Aδ-fibers recognize and transform nociceptive stimuli into electrical pain signals that are carried to the dorsal horn of the spinal cord where the pain signals are immediately relayed to the brain through the brainstem [25]. In the spinal dorsal horn and brain, various neurotransmitters (ex, glutamate, substance P, calcitonin gene-related peptide, etc.) and their corresponding receptors are involved in relaying pain signals [25]. Substance P is an undecapeptide member of the tachykinin neuropeptide family and acts as a neurotransmitter and as a neuromodulator associated with inflammatory process and pain in the spinal cord and brain [10, 14]. Since the endogenous receptor for substance P is NK1 receptor [13], it is anticipated that NK1 receptor antagonists, such as maropitant, can provide analgesic effect by blocking the pharmacological action of substance P in the spinal cord and brain [1, 7, 30]. Actually, Boscan et al. [6] showed that maropitant reduced the anesthetic requirement during noxious visceral stimulation of the ovary in dogs. In addition, Alvillar et al. [1] showed that maropitant had a sparing effect on the sevoflurane MAC in dogs. Similar to these previous studies, the clinical antiemetic dose of maropitant (1 mg/kg) produced a significant reduction in the sevoflurane MAC-BAR by 15.0% in our dogs. The present study reconfirmed that maropitant provides a sparing effect on anesthetic requirement in dogs [1, 7, 28].
The tissue damage and inflammation produce the local release of prostaglandins and bradykinin at the site of injury [25]. The locally released prostaglandins and bradykinin activate C-fibers and cause the release of glutamate and substance P at the central nerve terminals of C-fibers [25]. These neuropeptides induce increase in intracellular calcium concentration and releasing nitric oxides and prostaglandins from the central nerve terminals of C-fibers and produce hyperalgesia [25]. NSAIDs including carprofen produce peripheral analgesic and anti-inflammatory effects by inhibiting arachidonate COX, thereby inhibiting the production of prostaglandins [25, 34]. In addition, there are mounting evidences that NSAIDs have antinociceptive effects at the level of the central nervous system [20, 34, 35]. Recent studies have shown the production of antinociceptive effect with direct NSAIDs administration to both the supraspinal and spinal structures [34]. The COX-2 isoform affects acute hyperalgesia at the level of the central nervous system [35]. Malmberg et al. [20] showed that NSAIDs exerted a direct spinal antinociceptive action by blocking the hyperalgesia induced by the activation of spinal NK1 receptors. These findings indicate that NSAIDs may produce their analgesic effect by the direct spinal action as well as peripheral anti-inflammatory action. The MAC or MAC-BAR is defined as the minimum anesthetic concentration that prevents a movement or an autonomic response in 50% of population exposed to a noxious stimulation, respectively [18, 21, 27, 29, 36]. In the present study, the sevoflurane MAC-BAR was determined by judging the dogs’ response to a noxious electrical stimulus, and it took 3–5 hr after the first electrical stimulation. It cannot be denied that the electrical stimulation might cause local inflammatory response at the site applied the electrical stimulation during the sevoflurane MAC-BAR determination, although the dogs did not show any clinically relevant inflammatory symptoms. It was thought that carprofen provided the sparing effect on sevoflurane requirement through the direct spinal action and peripheral anti-inflammatory effect in the dogs of CARP group.
There were some limitations that might affect our interpretation. Firstly, we did not show actual changes at the level of substance P in the spinal cord, although our speculation could be appropriate to discuss the results of the present study. Secondly, the dogs did not receive the drug administration at random. It cannot be denied that the order of drug administrations might affect our results, although the sevoflurane MAC-BAR determination was highly objective because its determination is based on the increase in heart rate and/or MABP following the electrical stimulus. Thirdly, the electrical noxious stimulation using the MAC-BAR determination is different from surgical tissue damage that produces various degrees of local and systemic inflammatory response as well as noxious stimulus in clinical cases.
In the present study, clinically relevant hypotension was observed at the MAC-BAR determination in some dogs of the Control, MARP and MARP-CARP groups, while no dog showed hypotension in the CARP group. Sevoflurane has a dose-dependent depressant effect on cardiorespiratory function in dogs [24]. A subcutaneous injection of carprofen (4 mg/kg) alone decreased the sevoflurane MAC by 12.1% and provided an improvement of arterial blood pressure in dogs [38]. The sevoflurane MAC-BAR for the control group was significantly higher than those for MARP, CARP and MARP-CARP groups. However, there was no statistically significant difference in the sevoflurane MAC-BAR between MARP, CARP and MARP-CARP groups. It was reported that the administration of maropitant decreased arterial blood pressure in dogs and rats [7,8,9]. Therefore, practitioners should exercise caution when administering maropitant to dogs as a pre-medication, because of the hypotension produced during anesthesia.
In conclusion, the subcutaneous administration of maropitant or carprofen alone produced a clinically relevant sparing effect of the MAC-BAR of sevoflurane in dogs. However, the combination of maropitant and carprofen did not produce any additive effect on the sevoflurane MAC-BAR reduction in dogs. It is thought that premedication with a combination of maropitant and carprofen may not provide any further sparing effect on anesthetic requirement during surgery in dogs.
REFERENCES
- 1.Alvillar B. M., Boscan P., Mama K. R., Ferreira T. H., Congdon J., Twedt D. C.2012. Effect of epidural and intravenous use of the neurokinin-1 (NK-1) receptor antagonist maropitant on the sevoflurane minimum alveolar concentration (MAC) in dogs. Vet. Anaesth. Analg. 39: 201–205. doi: 10.1111/j.1467-2995.2011.00670.x [DOI] [PubMed] [Google Scholar]
- 2.Antognini J. F., Carstens E.1999. Increasing isoflurane from 0.9 to 1.1 minimum alveolar concentration minimally affects dorsal horn cell responses to noxious stimulation. Anesthesiology 90: 208–214. doi: 10.1097/00000542-199901000-00027 [DOI] [PubMed] [Google Scholar]
- 3.Antognini J. F., Schwartz K.1993. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology 79: 1244–1249. doi: 10.1097/00000542-199312000-00015 [DOI] [PubMed] [Google Scholar]
- 4.Benchaoui H. A., Cox S. R., Schneider R. P., Boucher J. F., Clemence R. G.2007. The pharmacokinetics of maropitant, a novel neurokinin type-1 receptor antagonist, in dogs. J. Vet. Pharmacol. Ther. 30: 336–344. doi: 10.1111/j.1365-2885.2007.00877.x [DOI] [PubMed] [Google Scholar]
- 5.Benchaoui H. A., Siedek E. M., De La Puente-Redondo V. A., Tilt N., Rowan T. G., Clemence R. G.2007. Efficacy of maropitant for preventing vomiting associated with motion sickness in dogs. Vet. Rec. 161: 444–447. doi: 10.1136/vr.161.13.444 [DOI] [PubMed] [Google Scholar]
- 6.Borer-Weir K.2013. Analgesia. pp. 101–134. In: Veterinary Anaesthesia (Clarke, K. W., Trim, C. M. and Hall, L. W. eds.) Saunders, St. Louis. [Google Scholar]
- 7.Boscan P., Monnet E., Mama K., Twedt D. C., Congdon J., Steffey E. P.2011. Effect of maropitant, a neurokinin 1 receptor antagonist, on anesthetic requirements during noxious visceral stimulation of the ovary in dogs. Am. J. Vet. Res. 72: 1576–1579. doi: 10.2460/ajvr.72.12.1576 [DOI] [PubMed] [Google Scholar]
- 8.Cloutier F., Ongali B., Deschamps K., Brouillette J., Neugebauer W., Couture R.2006. Upregulation of tachykinin NK-1 and NK-3 receptor binding sites in the spinal cord of spontaneously hypertensive rat: impact on the autonomic control of blood pressure. Br. J. Pharmacol. 148: 25–38. doi: 10.1038/sj.bjp.0706694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culman J., Wiegand B., Spitznagel H., Klee S., Unger T.1995. Effects of the tachykinin NK1 receptor antagonist, RP 67580, on central cardiovascular and behavioural effects of substance P, neurokinin A and neurokinin B. Br. J. Pharmacol. 114: 1310–1316. doi: 10.1111/j.1476-5381.1995.tb13348.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datar P., Srivastava S., Coutinho E., Govil G.2004. Substance P: structure, function, and therapeutics. Curr. Top. Med. Chem. 4: 75–103. doi: 10.2174/1568026043451636 [DOI] [PubMed] [Google Scholar]
- 11.Duggan A. W., Hendry I. A., Morton C. R., Hutchison W. D., Zhao Z. Q.1988. Cutaneous stimuli releasing immunoreactive substance P in the dorsal horn of the cat. Brain Res. 451: 261–273. doi: 10.1016/0006-8993(88)90771-8 [DOI] [PubMed] [Google Scholar]
- 12.Epstein M. E., Rodanm I., Griffenhagen G., Kadrlik J., Petty M. C., Robertson S. A., Simpson W., AHAAAAFP2015. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J. Feline Med. Surg. 17: 251–272. doi: 10.1177/1098612X15572062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grady E. F., Garland A. M., Gamp P. D., Lovett M., Payan D. G., Bunnett N. W.1995. Delineation of the endocytic pathway of substance P and its seven-transmembrane domain NK1 receptor. Mol. Biol. Cell 6: 509–524. doi: 10.1091/mbc.6.5.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison S., Geppetti P.2001. Substance p. Int. J. Biochem. Cell Biol. 33: 555–576. doi: 10.1016/S1357-2725(01)00031-0 [DOI] [PubMed] [Google Scholar]
- 15.Jinks S. L., Bravo M., Hayes S. G.2008. Volatile anesthetic effects on midbrain-elicited locomotion suggest that the locomotor network in the ventral spinal cord is the primary site for immobility. Anesthesiology 108: 1016–1024. doi: 10.1097/ALN.0b013e3181730297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katoh T., Kobayashi S., Suzuki A., Iwamoto T., Bito H., Ikeda K.1999. The effect of fentanyl on sevoflurane requirements for somatic and sympathetic responses to surgical incision. Anesthesiology 90: 398–405. doi: 10.1097/00000542-199902000-00012 [DOI] [PubMed] [Google Scholar]
- 17.Kehlet H.2009. Multimodal approach to postoperative recovery. Curr. Opin. Crit. Care 15: 355–358. doi: 10.1097/MCC.0b013e32832fbbe7 [DOI] [PubMed] [Google Scholar]
- 18.Love L., Egger C., Rohrbach B., Cox S., Hobbs M., Doherty T.2011. The effect of ketamine on the MACBAR of sevoflurane in dogs. Vet. Anaesth. Analg. 38: 292–300. doi: 10.1111/j.1467-2995.2011.00616.x [DOI] [PubMed] [Google Scholar]
- 19.Ma W., Eisenach J. C.2003. Intraplantar injection of a cyclooxygenase inhibitor ketorolac reduces immunoreactivities of substance P, calcitonin gene-related peptide, and dynorphin in the dorsal horn of rats with nerve injury or inflammation. Neuroscience 121: 681–690. doi: 10.1016/S0306-4522(03)00497-4 [DOI] [PubMed] [Google Scholar]
- 20.Malmberg A. B., Yaksh T. L.1992. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science 257: 1276–1279. doi: 10.1126/science.1381521 [DOI] [PubMed] [Google Scholar]
- 21.March P. A., Muir W. W., 3rd2003. Minimum alveolar concentration measures of central nervous system activation in cats anesthetized with isoflurane. Am. J. Vet. Res. 64: 1528–1533. doi: 10.2460/ajvr.2003.64.1528 [DOI] [PubMed] [Google Scholar]
- 22.Mathews K., Kronen P. W., Lascelles D., Nolan A., Robertson S., Steagall P. V., Wright B., Yamashita K.2014. Guidelines for recognition, assessment and treatment of pain: WSAVA Global Pain Council members and co-authors of this document. J. Small Anim. Pract. 55: E10–E68. doi: 10.1111/jsap.12200 [DOI] [PubMed] [Google Scholar]
- 23.Mathis A., Lee K., Alibhai H. I.2011. The use of maropitant to prevent vomiting induced by epidural administration of preservative free morphine through an epidural catheter in a dog. Vet. Anaesth. Analg. 38: 516–517. doi: 10.1111/j.1467-2995.2011.00645.x [DOI] [PubMed] [Google Scholar]
- 24.Mutoh T., Nishimura R., Kim H. Y., Matsunaga S., Sasaki N.1997. Cardiopulmonary effects of sevoflurane, compared with halothane, enflurane, and isoflurane, in dogs. Am. J. Vet. Res. 58: 885–890. [PubMed] [Google Scholar]
- 25.Muir W. W., 3rd2002. Physiology and pathophysiology of pain. pp. 13–45. In: Handbook of Veterinary Pain Management, 3rd ed. (Gaynor, J. S. and Muir, W. W. eds.) Mosby, St. Louis. [Google Scholar]
- 26.Muir W. W., 3rd2002. Choosing and administering the right analgesic therapy. pp. 329–345. In: Handbook of Veterinary Pain Management, 3rd ed. (Gaynor, J. S. and Muir, W. W. eds.) Mosby, St. Louis. [Google Scholar]
- 27.Muir W. W., 3rd2007. Considerations for general anesthesia. pp. 7–30. In: Lumb and Jones’ VeterinaryAnesthesia and Analgesia, 4th ed. (Tranquilli, W. J., Thurmon, J. C. and Grimm, K. A. eds.), Blackwell Publishing, Ames. [Google Scholar]
- 28.Rampil I. J., Mason P., Singh H.1993. Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology 78: 707–712. doi: 10.1097/00000542-199304000-00014 [DOI] [PubMed] [Google Scholar]
- 29.Roizen M. F., Horrigan R. W., Frazer B. M.1981. Anesthetic doses blocking adrenergic (stress) and cardiovascular responses to incision--MAC BAR. Anesthesiology 54: 390–398. doi: 10.1097/00000542-198105000-00008 [DOI] [PubMed] [Google Scholar]
- 30.Sakurada T., Katsumata K., Yogo H., Tan-No K., Sakurada S., Ohba M., Kisara K.1995. The neurokinin-1 receptor antagonist, sendide, exhibits antinociceptive activity in the formalin test. Pain 60: 175–180. doi: 10.1016/0304-3959(94)00107-P [DOI] [PubMed] [Google Scholar]
- 31.Seddighi R., Egger C. M., Rohrbach B. W., Hobbs M., Doherty T. J.2012. Effect of nitrous oxide on the minimum alveolar concentration for sevoflurane and the minimum alveolar concentration derivatives that prevent motor movement and autonomic responses in dogs. Am. J. Vet. Res. 73: 341–345. doi: 10.2460/ajvr.73.3.341 [DOI] [PubMed] [Google Scholar]
- 32.Tamura J., Itami T., Ishizuka T., Fukui S., Ooyama N., Miyoshi K., Sano T., Yamashita K.2014. Sparing effect of robenacoxib on the minimum alveolar concentration for blunting adrenergic response (MAC-BAR) of sevoflurane in dogs. J. Vet. Med. Sci. 76: 113–117. doi: 10.1292/jvms.13-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ura T., Higuchi H., Taoda M., Sato T.1999. Minimum alveolar concentration of sevoflurane that blocks the adrenergic response to surgical incision in women: MACBAR. Eur. J. Anaesthesiol. 16: 176–181. doi: 10.1097/00003643-199903000-00007 [DOI] [PubMed] [Google Scholar]
- 34.Vangeas H., Schaible H. G.2001. Prostaglandins and cyclooxygenases in the spinal cord. Prog. Neurobiol. 64: 327–363. doi: 10.1016/S0301-0082(00)00063-0 [DOI] [PubMed] [Google Scholar]
- 35.Yaksh T. L., Dirig D. M., Malmberg A. B.1998. Mechanism of action of nonsteroidal anti-inflammatory drugs. Cancer Invest. 16: 509–527. doi: 10.3109/07357909809011705 [DOI] [PubMed] [Google Scholar]
- 36.Yamashita K., Iwasaki Y., Umar M. A., Itami T.2009. Effect of age on minimum alveolar concentration (MAC) of sevoflurane in dogs. J. Vet. Med. Sci. 71: 1509–1512. doi: 10.1292/jvms.001509 [DOI] [PubMed] [Google Scholar]
- 37.Yamashita K., Furukawa E., Itami T., Ishizuka T., Tamura J., Miyoshi K.2012. Minimum alveolar concentration for blunting adrenergic responses (MAC-BAR) of sevoflurane in dogs. J. Vet. Med. Sci. 74: 507–511. doi: 10.1292/jvms.11-0274 [DOI] [PubMed] [Google Scholar]
- 38.Yamashita K., Okano Y., Yamashita M., Umar M. A., Kushiro T., Muir W. W.2008. Effects of carprofen and meloxicam with or without butorphanol on the minimum alveolar concentration of sevoflurane in dogs. J. Vet. Med. Sci. 70: 29–35. doi: 10.1292/jvms.70.29 [DOI] [PubMed] [Google Scholar]