Abstract
Hand-reared elephant calves that are nursed with milk substitutes sometimes suffer bone fractures, probably due to problems associated with nutrition, exercise, sunshine levels and/or genetic factors. As we were expecting the birth of an Asian elephant (Elephas maximus), we analyzed elephant’s breast milk to improve the milk substitutes for elephant calves. Although there were few nutritional differences between conventional substitutes and elephant’s breast milk, we found a large unknown peak in the breast milk during high-performance liquid chromatography-based amino acid analysis and determined that it was glucosamine (GlcN) using liquid chromatography/mass spectrometry. We detected the following GlcN concentrations [mean ± SD] (mg/100 g) in milk hydrolysates produced by treating samples with 6M HCl for 24 hr at 110°C: four elephant’s breast milk samples: 516 ± 42, three cow’s milk mixtures: 4.0 ± 2.2, three mare’s milk samples: 12 ± 1.2 and two human milk samples: 38. The GlcN content of the elephant’s milk was 128, 43 and 14 times greater than those of the cow’s, mare’s and human milk, respectively. Then, we examined the degradation of GlcN during 0–24 hr hydrolyzation with HCl. We estimated that elephant’s milk contains >880 mg/100 g GlcN, which is similar to the levels of major amino acids in elephant’s milk. We concluded that a novel GlcN-containing milk substitute should be developed for elephant calves. The efficacy of GlcN supplements is disputed, and free GlcN is rare in bodily fluids; thus, the optimal molecular form of GlcN requires a further study.
Keywords: analysis, elephant, glucosamine, hydrolysate, milk
Elephant mothers in zoological gardens sometimes refuse to nurse their calves, because of stress or a lack of social training. However, hand-reared elephant calves that are nursed with milk substitutes sometimes suffer bone fractures. These are supposed to be caused by problems with nutrition, including overnutrition, similar to the bone fractures seen in Great Dane puppies [8]; exercise; the amount of sunshine received; or genetic factors [11]. As far as we know, the nutritional balance of the milk substitutes that are currently supplied to elephant calves does not differ from that of elephant’s breast milk [1]; however, analytical data of the milk were few on minerals, vitamins or other functional components. As we were expecting the birth of an Asian elephant, we carried out a compositional analysis of elephant’s breast milk to develop a novel substitute. During high-performance liquid chromatography (HPLC)-based amino acid (AA) analysis of the acidic hydrolysate of elephant’s breast milk, we found a large unknown peak (UKP) and succeeded in separating it. We subjected the hydrolysate to mass spectrometry and found that the UKP was glucosamine (GlcN).
Elephant’s milk contains more oligosaccharides than other species’ milk, but no previous studies have reported that it contains large amounts of GlcN [17, 24, 27]. To compare the GlcN contents among animal’s and human breast milk, we qualified and partially quantified the amount of GlcN present in breast milk using HPLC-based AA analysis involving post-column ninhydrin derivatization [29]. GlcN levels have also been studied based on the absorbance of GlcN after its ninhydrin derivatization [19], other derivatization methods [21, 31], ultraviolet absorption of GlcN [15] or refractory index detection [14]. A pre-derivatization preparation method was recently described by Association of Analytical Communities (AOAC) on dietary supplements [18, 30]; however, studies examining the most appropriate preparation method are required before this technique can be applied to milk hydrolysates.
MATERIALS AND METHODS
Breast milk and reagents
All experimental procedures were performed according to the ethical rules of the Japanese Association of Zoos and Aquariums (JAZA). We obtained consent from the relevant zoological gardens, mare’s farms and cow’s milk companies to subject the obtained breast milk samples to nutritional analysis. Human milk was collected from two healthy mothers, who provided written informed consent with regard to the use of their milk according to the protocol approved by the ethics committee of Kensyou-kai Medical Institution Inc. (Osaka, Japan; 2014), and the relevant procedures were performed in accordance with the Ethical Principles for Medical Research Involving Human Subjects, WMA Declaration of Helsinki (1964).
A 33-g elephant’s breast milk sample was obtained from Tem (a 19-year-old Asian elephant who was born in Laos) from 207 to 208 days after delivery at Fuji Safari Park. The milk collected on these two days was frozen, mixed and then analyzed. Four samples with weights of 10 g, 40 g, 30 g and 35 g, respectively, were collected from Pooly (a 22–23-year-old Asian elephant who was born in India) at Ichihara Elephant Kingdom at 23, 307, 338 and 381 days after delivery. In addition, a 30-g breast milk sample was obtained at 19 days after delivery from Zuze (a 24-year-old Asian elephant who was born in Latvia, had resided at Kobe Oji Zoo and was moved to Ichihara Elephant Kingdom for delivery). Milk samples from three mares ( >3 g each) were donated by Miyazaki City Phoenix Zoo (Equus caballus, midget pony, at 102–106 days after delivery), Okinawa Zoo and Museum (Equus caballus, Yonaguni horse, at 44 days after delivery) and Kume Stock Farm, Bekkai, Hokkaido (Equus caballus, Ban-ei horse or Banba, at 8 days after delivery). Thirty percent of the triglycerides in mare’s milk are medium-chain triglycerides (MCT; C8, C10 and C12), which is the highest proportion detected in domesticated animal’s milk probably relating to digestibility and absorption [5, 26]. So, we compared mare’s milk with elephant’s breast milk, which has 70% MCT [23]. Three mixtures of raw cow’s (Bos taurus, Holstein) milk for dairy processing (100 g each) were donated by Urahoro Dairy Co., Hosho Milk Co. and Toyo Milk Industry Co., respectively. These samples were filtered through gauze-like fabrics to eliminate grass or feed and then cooled. Human breast milk samples were donated by two volunteers (A and B): (weight: about 20 g) samples A and B were collected on days 167 and 193 after delivery, respectively. All milk samples were frozen as soon as possible and transported at −20°C.
The reagents, standards and mixture solutions were supplied by Wako Pure Chemical (Osaka, Japan), Sigma-Aldrich (St. Louis, MO, U.S.A.), Sigma-Fluka (Rockville, NY, U.S.A.) or Mitsubishi Chemical (Tokyo, Japan).
Compositional analyses, AA and GlcN chromatography
We carried out analyses of nutritional contents; i.e., its moisture, protein, fat, ash, carbohydrate, vitamin and mineral based on the Association of Analytical Communities (AOAC) method for analyzing dairy products [18]. In addition, a milk sample was passed through an ultrafiltration (UF) filter (molecular weight (MW) threshold: 10,000), and then, its lactose and trisaccharide levels were analyzed using refractory index HPLC (RI-HPLC), which was performed with a Shodex KS-801 SUGAR column (8 mm [I.D.]*300 mm [L], Showa Denko Co., Tokyo, Japan), eluted with water at 70°C and a flow rate of 0.8 ml/min, and a refractive index (RI) detector. The peaks were identified using lactose and maltotriose standards, and the area of each peak was quantified. The peak areas of other saccharides were estimated by comparing them with the area of the maltotriose peak using RI detection.
For the AA and GlcN chromatography, we collected 100 mg or 200 mg of milk (3 mg protein), cooled it and hydrolyzed it with 3 ml 6M hydrogen chloride (HCl) for 24 hr at 110°C. Next, we filtered the hydrolysate and then evaporated it at 65°C. After being dried out and dissolved with 5 ml 0.02M HCl, the hydrolysate was filtered through a 0.2-µm filter (Millex-LG, Merk Millipore, Darmstadt, Germany) and then applied to the analyzer. The sample preparation procedures for the Met, Cys and Trp analyses are described in the Supplementary Files. Ten- to 20-µl samples were applied to the AA analysis system. We analyzed the concentrations of AA and GlcN using the L-8900 AA system (Hitachi High-Tech Science Co., Tokyo, Japan) together with post-column ninhydrin derivatization, based on the absorption of the samples at 570 nm. Two different HPLC modes were employed, a 95-min cycle (95-min mode) and a high-resolution 155-min cycle (155-min mode). In the 95-min mode, we used a #2619 packed column set (internal diameter [I.D.]: 4.0 mm, length [L]: 5 mm) as the guard column, a #2622PF packed column (I.D.: 4.6 mm, L: 60 mm) to separate out the AA and a #2650 L packed column (I.D.: 4.6 mm, L: 40 mm) for ammonia filtration. In addition, we used the PF-1, -2 and -4 buffers and the elution gradient programs outlined in the LC050070-ref 02A manual (Hitachi High-Tech Science Co.). In the 155-min mode, we used the same columns as were employed for the 95-min mode. In addition, we used the PF-1, -2, -3 and -4 buffers and the elution gradient programs outlined in the abovementioned manual.
Quantification of AA and GlcN
AA concentrations were calculated by systematically comparing the area of each peak with the relevant standards. AN-2 STD (019–14464) and B STD (010–08644, Wako Pure Chemical) were used as AA standard solutions. Each AA standard solution contained 2.5 µmol/ml of the standard and was diluted 25 times, giving a final concentration of 0.1 µmol/ml. For the calibration of GlcN concentrations, 216.10-mg/20 ml D-glucosamine hydrochloride solution (MW=215.63, Wako Pure Chemical) was diluted 500 times to a final concentration of 0.1 µmol/ml, which was identical to the concentration of the AA standards. The GlcN concentration of each sample was also calculated by comparing the area of each peak with the standard peak area during the HPLC, which was assessed before or after the sample was subjected to the HPLC. The GlcN peak was well separated from AA-peaks in the 155-min mode HPLC (Fig. 1c).
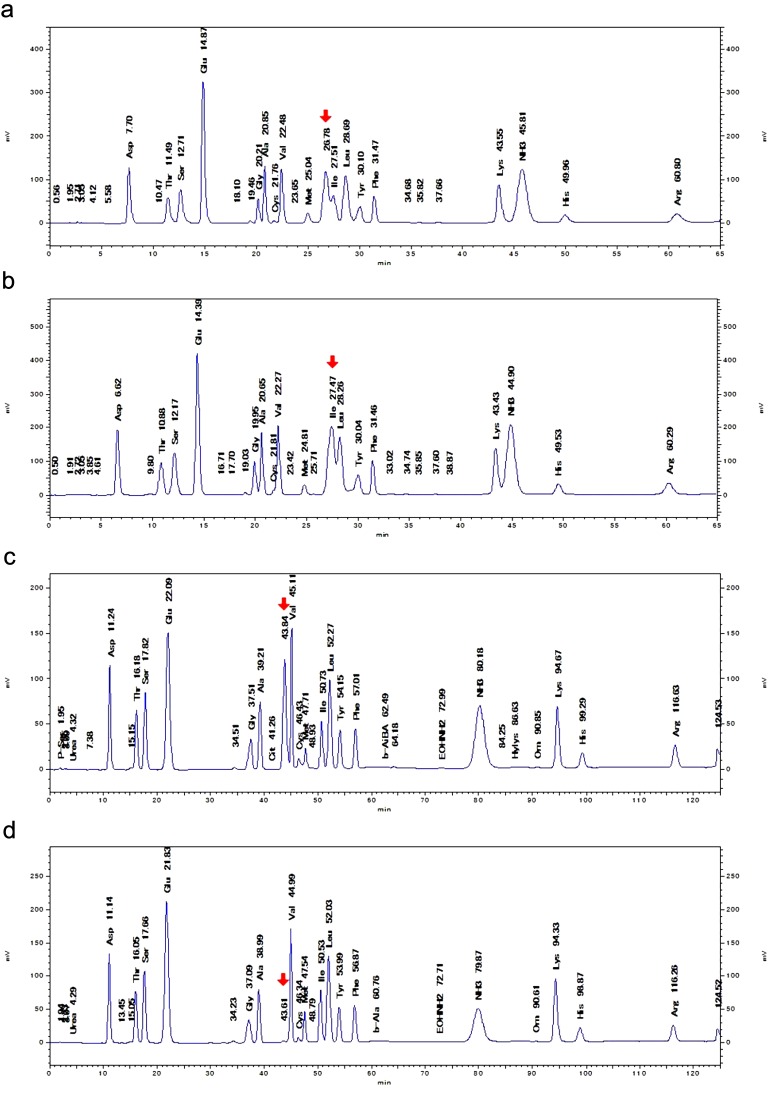
Fig. 1.
AA chromatograms of hydrolysates of elephant’s milk and cow’s milk. a, The hydrolysate of Tem’s (an elephant) milk showed twin peaks composed of an UKP (arrow) and the Ile peak when HPLC was performed in 95-min mode. b, The hydrolysate of Zuze’s (an elephant) milk showed a fused peak composed of the UKP and the Ile peak (arrow) when HPLC was performed in 95-min mode. c, The UKP peak (arrow) detected in the hydrolysate of Zuze’s milk moved and separated when HPLC was performed in 155-min mode. d, The hydrolysate of a mixture of cow’s milk (Urahoro) showed a tiny UKP peak (arrow) when HPLC was performed in 155-min mode.
Methods for separating and quantifying GlcN levels based on AA HPLC combined with ninhydrin post-derivatization were established, and the linearity of the GlcN measurements was calibrated by Zacharius in J Chromatog. [29]. This approach has been adopted as a standard method for use with the HPLC system (Hitachi-High Tech Science Co.) [12]. Calibration with standard GlcN should be sufficient for determining the concentration of GlcN in elephant’s milk, because the Hitachi HPLC system makes it possible to measure the levels of 40 kinds of amino acids and other monoamine compounds, including GlcN, via HLPC combined with ninhydrin post-derivatization.
Retention times (RT) of GlcN, galactosamine (GalN) and mannosamine (ManN)
After we had characterized the UKP, we analyzed the hydrolysate of Zuze’s breast milk using GlcN, GalN and ManN standards; the relevant AA standard mixtures; and the AA analysis system in both the 155-min and 95-min modes. Twenty-µl samples of the GlcN, GalN and ManN standards were used at a concentration of 0.067 µmol/ml, and their RT and peak areas were measured. The concentration of the UKP (GlcN) was initially calculated in Ala equivalents, but after it had been identified, it was quantified with standard glucosamine hydrochloride.
LC/mass spectrometry (MS) procedure
We used an LC-20A chromatograph (Shimadzu, Kyoto, Japan) and an Intrada AA (3 µm resin) column (ID: 2 mm, L: 150 mm, Imtakt Corp., Kyoto, Japan) together with solution A (0.2% folic acid/acetonitrile) and solution B (0.2 ml 100 mM ammonium acetate at 40°C and a flow rate of 0.2 ml/min according to the following gradient: A/B: 50/50 (10 min) to 0/100 (5 min) to 0/100 (1 min) and finally to 50/50 (5 min). The MS was performed with an LTQ system (a linear ion trap mass spectrometer; Thermo Fisher Scientific, Yokohama, Japan) that included a triple scan system. A 2-µl sample was injected and analyzed according to the manufacturer’s instructions. Full, selected ion monitoring and automatic MS/MS scans were periodically repeated.
RESULTS
Detection of fused and separate twin peaks
We analyzed the composition of the elephant’s milk samples (Table 1 ), which did not differ from previously reported data [1], and then analyzed the AA levels of the four elephant’s breast milk samples by HPLC. In the 95-min mode, which is often used for food analysis, the chart for Tem’s milk exhibited a twin peak composed of an Ile peak and an UKP and contained similar levels of all AA to those reported previously [9], whereas these two peaks had fused together in the chart for Zuze’s milk (Fig. 1a, b). Thereafter, we subjected the samples to HPLC using the 155-min mode [20] (Fig. 1c, d), and the RT of the UKP shifted markedly after the change in the analytical method. As we found UKP in the hydrolysates, but few such peaks were detected in the unhydrolyzed elephant’s milk (Table 4, Supplementary Files) [6], we compared the UKP detected in the hydrolysates of human, cow’s and mare’s milk with those found in elephant’s milk (Table 2). There were large differences in the UKP contents of these samples.
Table 1. Nutritional composition of elephant’s breast milk.
| Name | Pooly | Zuze | Pooly | Tem | Mean | SD |
|---|---|---|---|---|---|---|
| Sample weight | 40 g | 30 g | 10 g | 33 g | ||
| Sample collection time | 307 d | 19 d | 23 d | 207−208 d | ||
| Moisture (%) | 82.6 | 84.5 | 82.1 | 78.4 | 81.9 | 2.2 |
| Protein (%) | 3.5 | 3.6 | 3.1 | 3.0 | 3.3 | 0.3 |
| Fat (%) | 7.6 | 3.7 | 7.4 | 12.0 | 7.7 | 2.9 |
| Carbohydrates (%) | 5.8 | 7.8 | 7.0 | 6.2 | 6.7 | 0.8 |
| Ash (%) | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.0 |
| Energy (kcal/100 g) | 106 | 79 | 107 | 145 | 109 | |
| Lactose (%) | 3.34 | 2.68 | NT | 2.91 | 3.0 | 0.3 |
| Trisaccharides (%) | 0.00 | 1.25 | NT | 0.55 | 0.6 | 0.6 |
| Other saccharides (%) | 4.42 | 3.48 | NT | 2.67 | 3.5 | 0.9 |
General nutritional assay data and refractory index HPLC saccharide data (%) are shown. d: days after delivery, SD: standard deviation; energy values were calculated using the Atwater method, NT: not tested.
Table 4. The changes in the levels of GlcN during the HCl hydrolysis of milk and standard GlcNAc.
| Hydrolysis time (hr) |
3 hr/24 hr |
||||||
|---|---|---|---|---|---|---|---|
| GlcN concentration mg/100 g | 0 | 1 | 3 | 9 | 10 | 24 | ratio |
| Elephant’s milk (Zuze) | 4 | 795 | 931 | 797 | 527 | 1.7 | |
| Elephant’s milk (Pooly) | 966 | 572 | |||||
| Cow’s milk mixture (Hosho) | 7 | 3 | 2.6 | ||||
| Cow’s milk mixture (Toyo) | 7 | 3 | |||||
| Mare’s milk (Yonaguni) | 20 | 31 | 29 | 19 | 1.6 | ||
| Human milk (A) | 0 | 55 | 51 | 33 | 30 | 1.6 | |
| Human milk (B) | 0 | 20 | 34 | 26 | 25 | ||
| 1,200 mg GlcNAc added to 100 g cow’s milk mixture (Urahoro) |
223 | 572 | 949 | 789 | 558 | 1.7 | |
We monitored the time course of the GlcN levels from the start of hydrolysis (time 0) to 1, 3, 9, 10 and 24 hr. The maximum GlcN concentration was observed after approximately 3 hr hydrolysis, and the 3 hr: 24 hr GlcN level ratios ranged from 1.6 to 2.6. The 1,200 mg GlcNAc is equivalent to 1,000 mg GlcN.
Table 2. AA and GlcN levels of milk hydrolysates obtained via 24 hr treatment with 6M HCl at 110°C.
Characterization of the UKP and the detection of GlcN by LC/MS
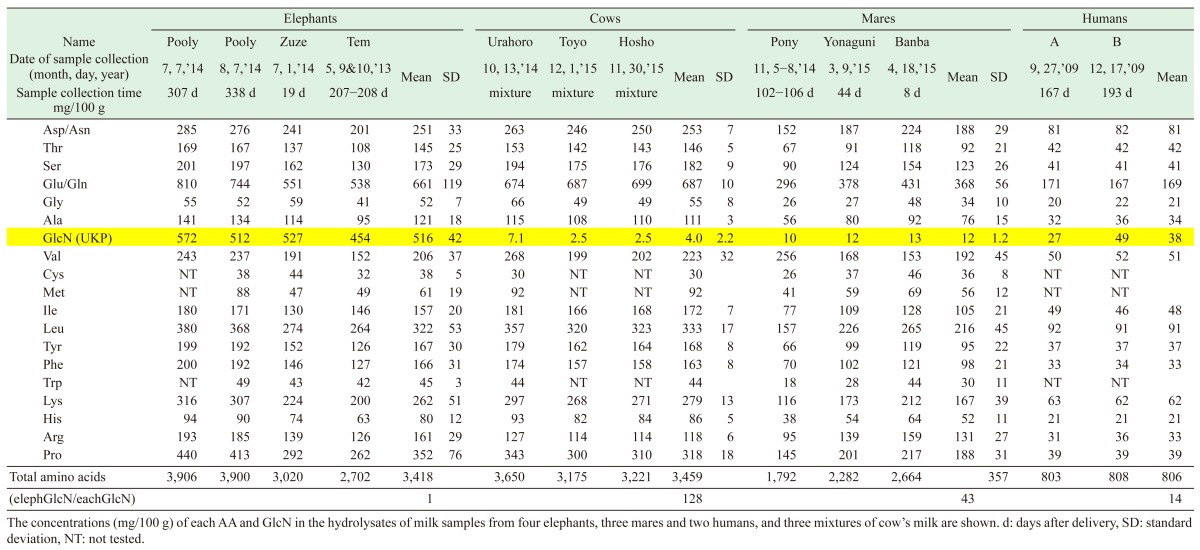
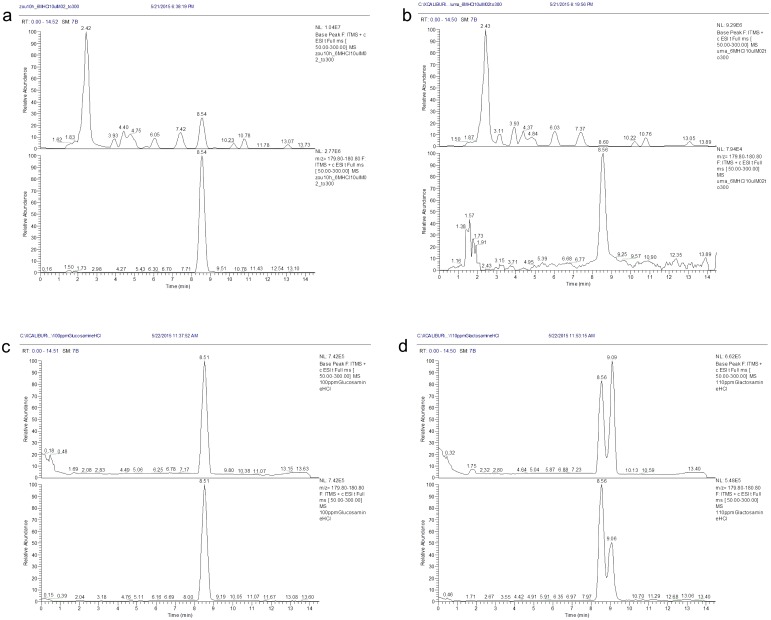
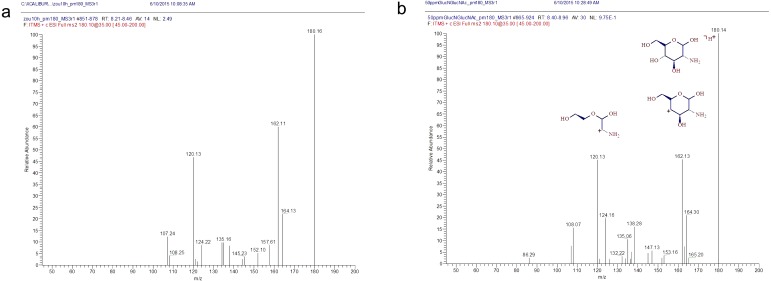
In terms of its AA profile, the elephant’s milk was more similar to the cow’s milk than the mare’s milk (Table 2). However, Banba’s (a mare) milk exhibited smaller relative standard deviation (SD) values for each AA than Hosho’s (a cow) milk (Table 3); therefore, we adopted the mare’s milk as a reference during the LC/MS analysis. We prepared hydrolysates of the milk from Zuze and Banba using the same samples as were employed for the AA analysis and subjected them to LC/MS to obtain m/z values (Fig. 2). The peaks for all of the AA differed by < 2-fold between the two samples. However, the hydrolysate of Zuze’s milk demonstrated a peak at a RT of 8–9 min in its base-peak chromatogram (m/z of 2–300) and the MS chromatogram of MH+ (m/z of 180), which was >30 times larger than that detected in Banba’s hydrolysate (Fig. 2a, b, c). We estimated that the UKP was derived from a protonated particle with an m/z of 179. We also detected a dehydrated fragment peak (m/z: 162) using MS/MS, which we considered might have been derived from an amino sugar. By comparing the abovementioned LC/MS m/z data with those for GlcN-HCl and GalN-HCl standards, we found that GalN produced two peaks with RT of 8.56 and 9.09 min, which differed from the values obtained for Zuze’s milk (Fig. 2d). A parent ion with an m/z of 180 and daughter ions with m/z of 162 and 120, respectively, were detected in Zuze’s hydrolysate using MS/MS, which matched well with the parent and fragment ions produced by the GlcN standard (Fig. 3).
Table 3. Comparison of the AA profiles of elephant’s, mare’s and cow’s milk.
| Elephant | Mare | Elephant/mare | Cow | Elephant/cow | |
|---|---|---|---|---|---|
| Name, sample collection time | Zuze, 19 d | Banba, 8 d | Hosho | ||
| Hydrolysis time (hr) | 10 | 24 | 24 | ||
| Concentration of AA or GlcN | mg/100 g | mg/100 g | mg/100 g | ||
| Asp/Asn | 245 | 224 | 1.09 | 250 | 0.98 |
| Thr | 145 | 118 | 1.23 | 143 | 1.01 |
| Ser | 177 | 154 | 1.15 | 176 | 1.01 |
| Glu/Gln | 505 | 431 | 1.17 | 699 | 0.72 |
| Gly | 53 | 48 | 1.10 | 49 | 1.08 |
| Ala | 120 | 92 | 1.30 | 110 | 1.09 |
| Val | 188 | 153 | 1.23 | 202 | 0.93 |
| Cys | NT | 46 | NT | ||
| Met | NT | 69 | NT | ||
| Ile | 120 | 128 | 0.94 | 168 | 0.71 |
| Leu | 284 | 265 | 1.07 | 323 | 0.88 |
| Tyr | 156 | 119 | 1.31 | 164 | 0.95 |
| Phe | 153 | 121 | 1.26 | 158 | 0.97 |
| Trp | NT | 44 | NT | ||
| Lys | 234 | 212 | 1.10 | 271 | 0.86 |
| His | 76 | 64 | 1.19 | 84 | 0.90 |
| Arg | 145 | 159 | 0.91 | 114 | 1.27 |
| Pro | 304 | 217 | 1.40 | 310 | 0.98 |
| Total amino acids | 2,905 | 2,664 | 1.09 | 3,221 | 0.90 |
| SD of the ratios | 0.13 | 0.13 | |||
| Relative standard deviation (SD/total AA) | 0.12 | 0.15 | |||
The AA composition of the elephant’s milk was more similar to that of the cow’s milk than the mare’s milk, but the relative SD values for the AA levels of the mare’s milk were lower than those of the AA levels of the cow’s milk. So, we used mare’s milk as a reference during the LC/MS assay of elephant’s milk. d: days after delivery, SD: standard deviation, NT: not tested.
Fig. 2.
LC/MS chromatograms of elephant’s milk, mare’s milk, a GlcN standard and a GalN standard. a, The chromatographic peak produced by the hydrolysate of Zuze’s (an elephant) milk. b, The peak produced by the hydrolysate of Banba’s (a mare) milk was only 1/35th of the size of the peak produced by the hydrolysate of Zuze’s milk at an m/z value of 180. c, The GlcN standard exhibited the same RT and peak m/z value (m/z 180) as the hydrolysates of Zuze’s and Banba’s milk. d, The GalN standard displayed a different type of m/z 180 peak, which might have been caused by the presence of anomers.
Fig. 3.
MS/MS spectra of the elephant’s milk and standard GlcN. a, The hydrolysate of Zuze’s (an elephant) milk produced a parent ion with an m/z value of 180 and daughter ions with m/z values of 162 and 120. b, The GlcN standard produced a parent ion with an m/z value of 180 and daughter ions with m/z values of 162 and 120. The estimated structures of the ion molecules are shown schematically.
Further HPLC and peak identification with standard reagents
As we suspected that the UKP represented GlcN, we subjected the hydrolysate of Zuze’s breast milk to a further HPLC-based AA analysis with GlcN, GalN and ManN standards. The RT of these standards shifts when the chromatographic conditions are changed [12]. The RT of the GlcN standard was located between those of Met and Ile in the 95-min mode, whereas it was located between those of Ala and Val in the 155-min mode (the column and buffer were not changed), and these shifts coincided with the shift in the UKP. The RT of the GalN and ManN standards also shifted, but these standards had different RT from the UKP. The RT of the AA shifted, but their order did not (Fig. 1). The HPLC was performed in the 155-min mode without a guard column (because the guard column was stained) during this analysis, and the standards exhibited the following RT: GlcN, 42.1 min; ManN, 43.7 min; and GalN, 45.1 min, whereas the RT of the hydrolysate’s UKP (after 3 hr hydrolysis) was 42.0 min. In the 95-min mode, the RT of the GlcN standard was 27.5 min, and that of the UKP peak of the hydrolysate (3 hr), overlapped with that of the Ile (27.6 min). In each mode, the RT of GlcN was similar to that of the UKP peak of the elephant’s milk hydrolysate.
We concluded that the UKP represented GlcN because its RT and RT shift coincided with those exhibited by the GlcN standard in both HPLC conditions, and the parent and daughter ions detected using LC/MS/MS also supported this suggestion.
In the HPLC-based AA analysis, the UKP peak area calibration with the Ala and GlcN standards produced very stable results. The peak area ratio of these standards, Ala/GlcN, in five different HPLC runs performed over three months were 1.12, 1.11, 1.11, 1.10 and 1.12, respectively; the mean Ala/GlcN ratio was 1.11; and the relative SD value was 0.64%. Other AA standards showed comparable relative SD values to Ala during repeated chromatography sessions performed over several years. So, we initially calibrated the UKP with the Ala standard and then calibrated it with the glucosamine hydrochloride standard after we had determined it to be GlcN.
The GlcN concentrations of the milk hydrolysates [mean ± SD] (mg/100 g) produced by 24 hr treatment with 6M HCl at 110°C were as follows: four elephant’s breast milk samples: 516 ± 42, three cow’s milk mixtures: 4.0 ± 2.2, three mare’s milk samples: 12 ± 1.2 and two human milk samples: 38. The GlcN content of the elephant’s milk was 128, 43 and 14 times greater than those observed in the cow’s, mare’s and human milk, respectively (Table 2). The mare’s milk exhibited a similarly high MCT content to the elephant’s milk; however, it did not contain much GlcN.
DISCUSSION
Estimation of GlcN concentrations by optimal hydrolysis
We used an AA analysis method to determine the levels of GlcN; however, the hydrolysis method was a little stronger than optimal [21, 29], and the most appropriate conditions might differ according to the species or concentration of the examined milk. So, we investigated the time course of the GlcN concentration during the hydrolysis of Zuze’s milk with 6M HCl at 110°C for 0, 1, 3, 10 or 24 hr. Milk from other animals and humans were also examined for 0–24 hr. As we only had a few milk samples, two elephant’s milk, two cow’s milk, one mare’s milk and two human milk samples were tested. During the 24-hr hydrolyzation process, the GlcN concentration peaked at 3 hr, when it was 1.6 to 1.7 times greater than that seen at 24 hr, except in the case of cow’s milk. In addition, we examined the time course of N-acetyl glucosamine (GlcNAc) degradation to GlcN in milk. The 1,200 mg/100 g GlcNAc standard, which was equivalent to 1,000 mg/100 g GlcN, exhibited the same changes. The GlcN concentration peaked at 3 hr, when it was 1.7 times greater than that seen at 24 hr (Table 4). Almost all GlcNAc (949 mg/100 g GlcN) was recovered within the 3-hr hydrolysate. However, the elephant’s milk and the other milk samples might have included heparan sulfate or other saccharides containing GlcN (derived from cell debris). Therefore, we considered that it was not possible to estimate the original concentration of GlcNAc in breast milk based on a standard GlcNAc calibration curve in this study. Since the cow’s milk contained little GlcN and its chromatograph contained noise, the associated data were less reliable. The maximum GlcN concentrations (mean, in mg/100 g) after 3 hr hydrolyzation were as follows: elephant: 949, cow: 7, mare: 31 and human: 42 (Table 4). The 3-hr hydrolysate of the elephant’s milk contained 135, 31 and 23 times more GlcN than the cow’s, mare’s and human milk, respectively, and these variations were comparable with the fold differences for the 24-hr hydrolysates shown in Table 2 (128, 43 and 14 times). The peak GlcN content of the elephant’s milk was estimated to be as follows based on the data from Table 2 and Table 4: 516*1.7=880 mg/100 g.
Genetic factors and dietary effects on GlcN content
Zuze came from Latvia, Tem was born in Laos, and Pooly came from India [13]; therefore, the high levels of GlcN detected in their milk were unlikely to have been due to genetic factors. None of the elephants were given GlcN supplements while they were nursing their calves. In addition, the GlcN content of the milk samples did not vary much among the three elephants (Table 2); therefore, GlcN is assumed to be species specific. Improvements in cattle breeding and the bacteria and protozoa in the calf rumen might also influence the GlcN content of cow’s milk.
Milk components, oligosaccharides and GlcN
The concentrations of milk components generally do not differ markedly among species. In previous studies, interspecies differences of <10-fold were detected in the levels of milk solids, protein, fat, carbohydrate, vitamins and minerals [16], and interspecies differences of <2-fold were found for AA concentrations [9]. Kunz et al. [17] and Uemura et al. [27] investigated elephant’s milk using fine chromatography. They quantified the saccharide levels of the milk using characterized standards, and their data indicated that elephant’s milk contains lower GlcN concentrations than were detected in the present study. We detected large amounts of GlcN, because most saccharide chains would have been hydrolyzed. Oligosaccharides have been investigated in terms of their effects on microflora or calcium absorption, but little is known about oligosaccharides containing GlcN and their role in bone development [3, 4, 10].
Bone development and GlcN
The large interspecies differences in GlcN concentrations might be related to the evolution of milk [28] and could have important implications for human and animal health. Elephants’ bones account for 25% of their body weight and could be a good model of vertebrate bones. In addition, the GlcN content of elephant’s milk did not vary much among mother elephants (Table 2). GlcN is assumed to be important for bone growth, because amino sugars play significant roles in chondrocyte production. Hyaluronan is composed of GlcNAc and glucuronic acid, and fills spaces in chondrocytes, where cartilage forms and is subsequently replaced by bone. So, GlcN is one of the most important factors for bone development as well as bone remodeling in adults [2, 7, 25].
Milk substitutes
The currently available elephant’s milk substitutes contain little GlcN, as they are mainly composed of cow’s milk products, so we concluded that GlcN should be added to such milk substitutes, although the efficacy of GlcN supplements in humans is still disputed [22]. Besides, we cannot supply large amounts (1,000 mg/100 g) of GlcN during the early stages of life at first, because of safety concerns regarding the use of GlcN in elephant calves. We could not collect a sufficient volume of samples because of ethical reasons and did not elucidate the molecular form of GlcN in each milk sample (Supplementary Files), and studies are necessary to precisely quantify GlcN levels; however, we hastened to publish this initial study so that other researchers could start further investigations, and zoological gardens could improve their hand-rearing techniques, which would aid elephant proliferation.
Supplementary
Acknowledgments
We would like to thank Fuji Safari Park, Ichihara Elephant Kingdom, Miyazaki Safari Park, Okinawa Zoo and Museum and Kumegawa Ban-Eiba Farming for donating the elephant’s and equine breast milk samples. We are also profoundly grateful to the two volunteers who provided us with samples of their breast milk. Last but not least, we thank all of the staff at Kobe Oji Zoo, especially Kumiko Hanaki, Akiko Yamada and Eiko Shimokawa (all vets) who taught us much about elephants and collected the milk samples.
REFERENCES
- 1.Abbondanza F. N., Power M. L., Dickson M. A., Brown J., Oftedal O. T.2013. Variation in the composition of milk of Asian elephants (Elephas maximus) throughout lactation. Zoo Biol. 32: 291–298. doi: 10.1002/zoo.21022 [DOI] [PubMed] [Google Scholar]
- 2.Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., Walter P.2015. Molecular Biology of the Cell. 6th ed., Garland Science, New York and London. [Google Scholar]
- 3.Albrecht S., Lane J. A., Mariño K., Al Busadah K. A., Carrington S. D., Hickey R. M., Rudd P. M.2014. A comparative study of free oligosaccharides in the milk of domestic animals. Br. J. Nutr. 111: 1313–1328. doi: 10.1017/S0007114513003772 [DOI] [PubMed] [Google Scholar]
- 4.Bode L.2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22: 1147–1162. doi: 10.1093/glycob/cws074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breckenridge W. C., Kuksis A.1967. Molecular weight distributions of milk fat triglycerides from seven species. J. Lipid Res. 8: 473–478. [PubMed] [Google Scholar]
- 6.Cavalli C., Teng C., Battaglia F. C., Bevilacqua G.2006. Free sugar and sugar alcohol concentrations in human breast milk. J. Pediatr. Gastroenterol. Nutr. 42: 215–221. doi: 10.1097/01.mpg.0000189341.38634.77 [DOI] [PubMed] [Google Scholar]
- 7.Cooper K. L., Oh S., Sung Y., Dasari R. R., Kirschner M. W., Tabin C. J.2013. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 495: 375–378. doi: 10.1038/nature11940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dämmrich K.1991. Relationship between nutrition and bone growth in large and giant dogs. J. Nutr. 121Suppl: S114–S121. [DOI] [PubMed] [Google Scholar]
- 9.Davis T. A., Nguyen H. V., Garcia-Bravo R., Fiorotto M. L., Jackson E. M., Lewis D. S., Lee D. R., Reeds P. J.1994. Amino acid composition of human milk is not unique. J. Nutr. 124: 1126–1132. [DOI] [PubMed] [Google Scholar]
- 10.Difilippo E., Willems H. A., Vendrig J. C., Fink-Gremmels J., Gruppen H., Schols H. A.2015. Comparison of milk oligosaccharides pattern in colostrum of different horse breeds. J. Agric. Food Chem. 63: 4805–4814. doi: 10.1021/acs.jafc.5b01127 [DOI] [PubMed] [Google Scholar]
- 11.Emanuelson K.2006. Neonatal care and hand rearing. pp. 233–241. In: Biology, Medicine, and Surgery of Elephants (Fowler, M.E. and Mikota, S.K. eds.), Blackwell Publishing, Ames. [Google Scholar]
- 12.Hitachi Technical Data. 1990. Separation of amino sugar and amino acids. Technical data LC103. Hitachi High-Tech Science Co. Tokyo. [Google Scholar]
- 13.Japanese Association of Zoos and Aquariums. 2014. Elephant Studbook 2014 in Japan. JAZA, Tokyo. [Google Scholar]
- 14.Japan Food Research Laboratories. 2016. Announcement for glucosamine and N-acetylglucosamine analysis. Tokyo. http://www.jfrl.or.jp/.
- 15.Japan Health and Nutrition Food Association. 2015. Official Notice #68. Public Announcement of Standard for Qualification and Specification of N-acetylglucosamine. Tokyo. [Google Scholar]
- 16.Jenness R., Sloan R. E.1970. The composition of milks of various species. Dairy Sci. Abstr. 32: 599–612. [Google Scholar]
- 17.Kunz C., Rudloff S., Schad W., Braun D.1999. Lactose-derived oligosaccharides in the milk of elephants: comparison with human milk. Br. J. Nutr. 82: 391–399. doi: 10.1017/S0007114599001798 [DOI] [PubMed] [Google Scholar]
- 18.Latimer G. W. J.2016. Official Methods of Analysis of AOAC INTERNATIONAL, 20th ed., Rockville. [Google Scholar]
- 19.Leane M. M., Nankervis R., Smith A., Illum L.2004. Use of the ninhydrin assay to measure the release of chitosan from oral solid dosage forms. Int. J. Pharm. 271: 241–249. doi: 10.1016/j.ijpharm.2003.11.023 [DOI] [PubMed] [Google Scholar]
- 20.Le Boucher J., Charret C., Coudray-Lucas C., Giboudeau J., Cynober L.1997. Amino acid determination in biological fluids by automated ion-exchange chromatography: performance of Hitachi L-8500A. Clin. Chem. 43: 1421–1428. [PubMed] [Google Scholar]
- 21.Li B., Zhang J., Bu F., Xia W.2013. Determination of chitosan with a modified acid hydrolysis and HPLC method. Carbohydr. Res. 366: 50–54. doi: 10.1016/j.carres.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 22.McAlindon T. E., Bannuru R. R., Sullivan M. C., Arden N. K., Berenbaum F., Bierma-Zeinstra S. M., Hawker G. A., Henrotin Y., Hunter D. J., Kawaguchi H., Kwoh K., Lohmander S., Rannou F., Roos E. M., Underwood M.2014. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 22: 363–388. doi: 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 23.McCullagh K. G., Widdowson E. M.1970. The milk of the African elephant. Br. J. Nutr. 24: 109–117. doi: 10.1079/BJN19700014 [DOI] [PubMed] [Google Scholar]
- 24.Osthoff G., Dickens L., Urashima T., Bonnet S. L., Uemura Y., van der Westhuizen J. H.2008. Structural characterization of oligosaccharides in the milk of an African elephant (Loxodonta africana africana). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 150: 74–84. doi: 10.1016/j.cbpb.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 25.Stoppoloni D., Politi L., Leopizzi M., Gaetani S., Guazzo R., Basciani S., Moreschini O., De Santi M., Scandurra R., Scotto d’Abusco A.2015. Effect of glucosamine and its peptidyl-derivative on the production of extracellular matrix components by human primary chondrocytes. Osteoarthritis Cartilage 23: 103–113. doi: 10.1016/j.joca.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 26.Tantibhedhyangkul P., Hashim S. A.1978. Medium-chain triglyceride feeding in premature infants: effects on calcium and magnesium absorption. Pediatrics 61: 537–545. [PubMed] [Google Scholar]
- 27.Uemura Y., Asakuma S., Yon L., Saito T., Fukuda K., Arai I., Urashima T.2006. Structural determination of the oligosaccharides in the milk of an Asian elephant (Elephas maximus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 145: 468–478. doi: 10.1016/j.cbpa.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 28.Urashima T., Fukuda K., Messer M.2012. Evolution of milk oligosaccharides and lactose: a hypothesis. Animal 6: 369–374. doi: 10.1017/S1751731111001248 [DOI] [PubMed] [Google Scholar]
- 29.Zacharius R. M.1976. Quantitative determination of hexosamines in glycoprotein by ion-exchange chromatography. J. Chromatogr. A 125: 421–427. doi: 10.1016/S0021-9673(00)83373-7 [DOI] [PubMed] [Google Scholar]
- 30.Zhou J. Z., Waszkuc T., Mohammed F.2005. Determination of glucosamine in raw materials and dietary supplements containing glucosamine sulfate and/or glucosamine hydrochloride by high-performance liquid chromatography with FMOC-Su derivatization: collaborative study. J. AOAC Int. 88: 1048–1058. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X., Cai J., Yang J., Su Q.2005. Determination of glucosamine in impure chitin samples by high-performance liquid chromatography. Carbohydr. Res. 340: 1732–1738. doi: 10.1016/j.carres.2005.01.045 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.