Abstract
A survey of helminths and ectoparasites, including epizoits, was conducted in narrow-ridged finless porpoises (Neophocaena asiaeorientalis) from Japanese five populations using dead stranded or incidentally caught animals. In total, 13 helminth species were found (6 nematodes, 4 trematodes, 2 cestodes and 1 acanthocephalan) in 137 porpoises. A new location record of Stenurus nanjingensis and a new host record of Tetrabothrius sp. were obtained. Eight species of helminth were considered common in the Japanese populations of the finless porpoise: Pharurus sunameri, Pharurus asiaeorientalis, Nasitrema spathulatum, Nasitrema sunameri, Halocercus pingi, Halocercus sunameri, Campula oblonga and Synthesium elongatum. No anisakid nematodes were found. N. spathulatum was found only in the western waters of the Seto Inland Sea. Low prevalence of C. oblonga in the Omura Bay was demonstrated. H. pingi was mostly found in very young porpoises before starting to eat prey, indicating prenatal or transmammary infection. However, a congeneric species, H. sunameri, mainly infected weaned porpoises, indicating that these two species possess different transmission pathways. This study provides information on the geographical distribution and prevalence of helminth parasites in finless porpoises off the Japanese coast.
Keywords: finless porpoise, helminthic fauna, population, prevalence
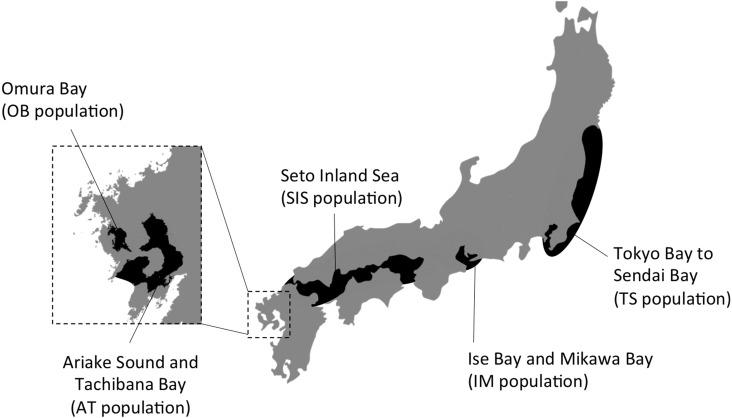
The narrow-ridged finless porpoise (Neophocaena asiaeorientalis) is a small odontocete distributed in eastern Asia, from the Taiwan Strait to the Korean and Japanese coasts [15]. In Japanese waters, five discrete populations have been recognized by morphological and genetic differences (Fig. 1) [40, 41]. These are the Omura Bay (OB), Ariake Sound–Tachibana Bay (AT), Seto Inland Sea (SIS), Ise–Mikawa Bays (IM) and Tokyo Bay–Sendai Bay (TS) populations.
Fig. 1.
Locations of the five known Japanese populations of the finless porpoise.
Parasites of the finless porpoise in Japanese waters have been studied by several authors. In early studies, some new helminth species were described based on the examination of a few hosts [24, 37, 38]. Subsequently, a parasitological study was conducted to understand the faunal differences between different host populations [21]. However, these studies only examined hosts from the SIS and TS populations; no parasitological studies have been conducted on the OB, AT and IM populations. In addition, the number of hosts examined was limited, and no information about the prevalence of parasitic infection was obtained, even in the previously studied populations.
In this study, helminths and ectoparasites, including epizoits, of the narrow-ridged finless porpoise were surveyed in five populations in Japanese waters using animals found dead on the beach (stranding) or incidentally entangled in fishery nets (by-catch). The aims of this study were to reveal the parasitic fauna of the OB, AT and IM populations, as well as to further examine the SIS and TS populations; to investigate the differences between them; and to provide the prevalence of parasitic infection in finless porpoises off the Japanese coast.
MATERIALS AND METHODS
The narrow-ridged finless porpoises examined in this study were collected during the period from 2010 to 2015 from the following areas: the Omura Bay, Ariake Sound, Tachibana Bay, Seto Inland Sea, Ise Bay, Mikawa Bay and the Pacific coast of Chiba and Ibaraki Prefectures. The host animals were autopsied at the stranding site or transported to the laboratory and frozen in −18°C, to be autopsied on another day. In total, 137 porpoises were used in this study; the host information is shown in Table 1.
Table 1. Host animal sampling information: narrow-ridged finless porpoise (Neophocaena asiaeorientalis).
| Location (Population) | Sampling period | Number of animals | Category based on body length |
|||
|---|---|---|---|---|---|---|
| <90 cm | 90−110 cm | >110 cm | Unclear | |||
| Omura Bay (OB) | 2010−2015 | 50 | 17 | 7 | 26 | - |
| Ariake Sound, Tachibana Bay (AT) | 2010−2015 | 42 | 6 | 10 | 25 | 1 |
| Seto Inland Sea (SIS) | 2012−2015 | 24 | 5 | 2 | 17 | - |
| Ise Bay, Mikawa Bay (IM) | 2014 | 19 | 14 | 0 | 5 | - |
| Pacific coast of Chiba and Ibaraki prefecture (TS) | 2013−2014 | 2 | 1 | 0 | 1 | - |
| Total | 137 | 43 | 19 | 74 | 1 | |
The parasitological examination followed the methodology described previously [21]. The surface of the host animal was investigated before the autopsy to search for ectoparasites and epizoits. The lungs and liver were cross-sectioned and observed macroscopically, then sliced into 1 to 2 cm thickness and washed in fresh water with slight pressure by hands. The stomach and intestine were opened entirely and washed in fresh water. The cranial sinus was opened and rinsed in running water. All of the washes obtained were left to stand, and then, the precipitation was observed under a stereomicroscope to collect any helminths. In some cases, this method could not be applied to all organs and tissues owing to post-mortem change, including organ loss or degradation. Only cross-sectioning and macroscopic observations were applied to highly degraded organs, and extremely degraded organs were not examined. Totally cranial sinus of 118, lungs of 114, liver of 69, stomach of 49 and intestine of 88 porpoises were examined. When parasites were found in other tissues or organs during the autopsy, they were also collected.
Nematodes were fixed in 70% or 99% ethanol, or 10% neutral buffered formalin, and cleared for observation using Gater’s solution. The worms were identified morphologically and counted. The nematodes in the cranial sinus were often very numerous. In such cases, the total number of worms was estimated based on the proportion of the weight of the worms [21]. One hundred entire worms were randomly selected from the collected worms and identified; then, the wet weights of the 100 worms and the total worms were measured. The number of worms of each identified species was estimated from its proportion in the sample, using the following equation: (number of identified worms)×(total worms weight)/(100 worms weight). The identification of the female lungworm was very difficult owing to morphological similarity between species; therefore, only male lungworms were identified.
Trematodes, cestodes and acanthocephalans were flattened between a pair of slide glasses and fixed in 70% ethanol. After fixation, the worms were stained with alum-carmine, Semichon’s carmine or Heidenhain’s iron hematoxylin, and mounted in Canada balsam. They were then identified morphologically and counted.
The identification was based on species descriptions or identification keys. All specimens are deposited in the Marine Mammal Research Laboratory, Nagasaki University (NU MMRL Parasite Collection Nematoda 2–21, 24, 28–31, 33–44, 46, 47, 50–91, 96–100, 104–114, 118–125, 128, 129, 132, 133, 136–193, 195–207, 213; Digenea 4–13, 18–20, 22–24, 28–40, 42–53, 56–58, 72–83, 88, 89, 91, 92, 95–124, 127–132, 135–149, 151, 152; Cestoda 1, 9, 69, 71; Acanthocephala 3).
The prevalence of infection was calculated based only on the organs/tissues that had been washed and microscopically observed. Many helminths with indirect life cycles are transmitted to their definitive host through dietary ingestion, including ingestion of infective larvae. Therefore, whether the host has started to eat prey or not must be considered for the prevalence calculation. Based on logistic models, Shirakihara et al. [31] estimated that narrow-ridged finless porpoises start to eat solid food and wean at 98 cm and 101 cm in body length, respectively. In their models, a porpoise is very unlikely (5%) to be consuming solid food when it is 92 cm long, and to be consuming milk when it is 109 cm long. Therefore, in this study, <90 cm long porpoises were regarded as nursing (do not eat prey) and >110 cm long porpoises were considered weaned (only eat prey). Porpoises of 90–110 cm body length were regarded as weaning (in transition between nursing and weaned). The prevalence of infection was calculated separately for these three growth categories.
The differences in prevalence among host populations or between growth categories were tested using Fisher’s exact test. Pairwise Fisher’s exact test with the Benjamini–Hochberg procedure was used for multiple comparisons between host populations. All statistical analysies were performed using R, version 3.3.1 [29], and the statistical package fmsb [23] was used in multiple comparisons.
RESULTS
The identified species and sites of infection are shown in Table 2. In total, 13 species of helminths, 6 of nematodes, 4 of trematodes, 2 of cestodes and 1 of acanthocephalans were found. No ectoparasites or epizoits were found. Most of the helminths were consistently found in a specific infection site; however, some worms were in other sites. Pharurus sunameri and Pharurusasiaeorientalis were mainly found in the cranial sinus, with a few worms occurring in the respiratory organs. Halocercus pingi was very long; in cases of severe infection, the worms reached from the bronchiole to the trachea. H. pingi was also found in the uterus of one young porpoise. Campula oblonga and Synthesium elongatum were found in the liver/bile ducts and the intestine, respectively. These trematodes were also found in the stomach, but no other helminths occurred in the stomach. The detailed investigation and identification of Tetrabothrius sp. were suspended, because only a single specimen was obtained. The general appearance of Corynosoma sp. was consistent with the description of unidentified specimens by Kuramochi et al. [21].
Table 2. List of the helminth species obtained in the Japanese finless porpoises in this study.
| Class | Predominant site of infection |
Other sites | References for identification |
|---|---|---|---|
| Species | |||
| Nematoda | |||
| Pharurus sunameri | cranial sinus | trachea, lung | [38] |
| P. asiaeorientalis | cranial sinus | trachea | [27] |
| Stenurus nanjingensis | cranial sinus | [34] | |
| Halocercus pingia) | lung | trachea, uterus | [26, 36] |
| H. sunameria) | lung | [38] | |
| H. tauricaa) | lung | [4] | |
| Trematoda | |||
| Nasitrema sunameri | cranial sinus | [37] | |
| N. spathulatum | cranial sinus | [24] | |
| Campula oblonga | liver/bile duct | stomach, intestine | [37] |
| Synthesium elongatum | intestine | stomach, duodenum | [24] |
| Cestoda | |||
| Diphyllobothrium fuhrmanni | intestine | [18] | |
| Tetrabothrius sp. | intestine | [13] | |
| Acanthocephala | |||
| Corynosoma sp. | intestine | [21] | |
a) Only male worms were identified.
Table 3 shows each helminth species with the number of infected hosts, and the estimated mean, minimum and maximum intensity (number of worms of positive hosts) for each population and the total of the populations. In total, cranial sinus nematodes were found in 80 porpoises, including 4 hosts in which the worms were highly degraded and unidentifiable. Both P. sunameri and P. asiaeorientalis occurred in 68 hosts. Stenurus nanjingensis was found together with P. sunameri and P. asiaeorientalis in 2 hosts. The species of the genus Nasitrema were found in 62 hosts, including 3 hosts in which the worms were highly degraded and unidentifiable. Both species of this genus were found together in 40 hosts, and coinfection of any trematode and any nematode species occurred in 61 hosts. Lungworms were found in 64 hosts, but were unidentifiable in 29 of these owing to degradation or fragmentation. H. pingi and H. sunameri were not found in the same host, but H. taurica was found with H. sunameri in 2 hosts.
Table 3. Number of infected hosts and intensity of helminths in five Japanese populations of the finless porpoise (OB: Omura Bay, AT: Ariake Sound-Tachibana Bay, SIS: Seto Inland Sea, IM: Ise-Mikawa Bays, TS: Tokyo Bay-Sendai Bay).
| Total |
OB |
AT |
SIS |
IM |
TS |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Infected host |
Intensity |
Infected host |
Intensity |
Infected host |
Intensity |
Infected host |
Intensity |
Infected host |
Intensity |
Infected host |
Intensity |
|||||||||||||||||||
| Species | mean | min. | max. | mean | min. | max. | mean | min. | max. | mean | min. | max. | mean | min. | max. | mean | min. | max. | |||||||||||||
| Cranial sinus / Examinedhost | 118 | 47 | 39 | 19 | 11 | 2 | |||||||||||||||||||||||||
| P. sunameri | 75 | 809 | 1 | 5,824a) | 28 | 903 | 2 | 5,824a) | 26 | 805 | 2 | 3,324a) | 15 | 750 | 9 | 1,906a) | 5 | 421 | 1 | 1,147a) | 1 | - | 1,069a) | - | |||||||
| P. asiaeorientalis | 69 | 370 | 1 | 2,496a) | 27 | 417 | 5 | 2,496a) | 23 | 239 | 1 | 1,380a) | 15 | 538 | 5 | 1,483a) | 3 | 138 | 6 | 235a) | 1 | - | 267a) | - | |||||||
| S. nanjingensis | 2 | - | 1 | 22 | 2 | - | 1 | 22 | 0 | - | - | - | 0 | - | - | - | 0 | - | - | - | 0 | - | - | - | |||||||
| N. sunameri | 48 | 19 | 1 | 252 | 17 | 7 | 1 | 36 | 16 | 33 | 1 | 252 | 9 | 11 | 2 | 39 | 5 | 30 | 4 | 55 | 1 | - | 17 | - | |||||||
| N. spathulatum | 51 | 15 | 1 | 82 | 19 | 8 | 2 | 31 | 19 | 21 | 1 | 82 | 13 | 18 | 1 | 78 | 0 | - | - | - | 0 | - | - | - | |||||||
| Lung / Examined host | 114 | 45 | 27 | 21 | 19 | 2 | |||||||||||||||||||||||||
| H. pingi | 22 | 63 | 1 | 302 | 13 | 80 | 2 | 302 | 3 | 2 | 1 | 3 | 1 | - | 10 | - | 5 | 66 | 1 | 130 | 0 | - | - | - | |||||||
| H. sunameri | 13 | 12 | 1 | 58 | 6 | 10 | 1 | 32 | 3 | 13 | 6 | 21 | 3 | 20 | 1 | 58 | 1 | - | 1 | - | 0 | - | - | - | |||||||
| H. taurica | 2 | - | 1 | 1 | 1 | - | 1 | - | 1 | - | 1 | - | 0 | - | - | - | 0 | - | - | - | 0 | - | - | - | |||||||
| Liver / Examined host | 69 | 24 | 15 | 12 | 16 | 2 | |||||||||||||||||||||||||
| C. oblonga | 18 | 30 | 1 | 123 | 1 | - | 5 | - | 7 | 8 | 1 | 30 | 4 | 31 | 9 | 53 | 5 | 45 | 5 | 123 | 1 | - | 122 | - | |||||||
| Intestine / Examined host | 88 | 31 | 23 | 17 | 15 | 2 | |||||||||||||||||||||||||
| S. elongatum | 16 | 13 | 1 | 45 | 3 | 10 | 6 | 12 | 2 | - | 12 | 45 | 8 | 12 | 1 | 40 | 3 | 11 | 1 | 26 | 0 | - | - | - | |||||||
| D. fuhrmanni | 3 | - b) | - b) | 123 | 1 | - | - b) | - | 0 | - | - | - | 1 | - | - b) | - | 0 | - | - | - | 1 | - | 123 | - | |||||||
| Tetrabothrius sp. | 1 | - | 1 | - | 0 | - | - | - | 1 | - | 1 | - | 0 | - | - | - | 0 | - | - | - | 0 | - | - | - | |||||||
| Corynosoma sp. | 1 | - | 426 | - | 0 | - | - | - | 0 | - | - | - | 0 | - | - | - | 0 | - | - | - | 1 | - | 426 | - | |||||||
a) Intensity was calculated using the number of identified worms and the ratio of the wet weight of the identified worms to the wet weight of total worms. b) Exact intensity was unclear, because only fragments of strobila were obtained.
Prevalence is shown as percentages in Table 4. The prevalence of all cranial sinus helminths was higher in >110 cm hosts than in <90 cm hosts in all populations. The prevalence of P. sunameri, P. asiaeorientalis and N. sunameri in >110 cm hosts was not significantly different among populations (Fisher’s exact test, P>0.05). The prevalence of N. spathulatum was significantly different between IM and OB, AT and SIS populations (Pairwise Fisher’s exact test with Benjamini–Hochberg procedure, adjusted P<0.05). H. pingi was frequently found in <90 cm hosts in four of the populations, and its prevalence was not significantly different among populations (Fisher’s exact test, P>0.05). In the total of the populations, the prevalence of H. pingi in <90 cm hosts was significantly higher than in >110 cm hosts (Fisher’s exact test, P<0.01). H. sunameri was only found in 90–110 cm and >110 cm hosts in four populations. Its prevalence in >110 cm hosts was not significantly different among populations (Fisher’s exact test, P>0.05), but it was significantly higher than in <90 cm hosts in the total of the populations (Fisher’s exact test, P<0.01). C. oblonga was found only in >110 cm hosts, and its prevalence in OB was significantly lower than in AT, SIS and IM (Pairwise Fisher’s exact test with Benjamini–Hochberg procedure, adjusted P<0.05). S. elongatum was frequently found in >110 cm hosts, and its prevalence was not significantly different among populations (Fisher’s exact test, P>0.05).
Table 4. Prevalence of helminths in five Japanese populations of the finless porpoise (OB: Omura Bay, AT: Ariake Sound-Tachibana Bay, SIS: Seto Inland Sea, IM: Ise-Mikawa Bays, TS: Tokyo Bay-Sendai Bay).
| Prevalence (%) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Total |
OB |
AT |
SIS |
IM |
TS |
|||||||||||||
| Species | <90 | 90−110 | >110 | <90 | 90−110 | >110 | <90 | 90−110 | >110 | <90 | 90−110 | >110 | <90 | 90−110 | >110 | <90 | 90−110 | >110 | |
| cm | cm | cm | cm | cm | cm | cm | cm | cm | cm | cm | cm | cm | cm | cm | cm | cm | cm | ||
| Cranial sinus / Examined hosta) | 21 | 10 | 50 | 8 | 4 | 15 | 4 | 5 | 15 | 2 | 1 | 14 | 6 | 0 | 5 | 1 | 0 | 1 | |
| P. sunameri | 10 | 40 | 98 | 25 | 75 | 100 | 0 | 0 | 93 | 0 | 100 | 100 | 0 | - | 100 | 0 | - | 100 | |
| P. asiaeorientalis | 14 | 40 | 88 | 25 | 75 | 100 | 0 | 0 | 80 | 50 | 100 | 93 | 0 | - | 60 | 0 | - | 100 | |
| S. nanjingensis | 0 | 0 | 4.0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | - | 0 | |
| N. sunameri | 0 | 10 | 70 | 0 | 25 | 67 | 0 | 0 | 67 | 0 | 0 | 64 | 0 | - | 100 | 0 | - | 100 | |
| N. spathulatum | 0 | 10 | 72 | 0 | 0 | 80 | 0 | 0 | 80 | 0 | 100 | 86 | 0 | - | 0 | 0 | - | 0 | |
| Lung / Examined hosta) | 34 | 10 | 50 | 11 | 4 | 17 | 4 | 4 | 14 | 4 | 2 | 13 | 14 | 0 | 5 | 1 | 0 | 1 | |
| H. pingi | 35 | 20 | 2.0 | 45 | 25 | 0 | 25 | 25 | 7.1 | 25 | 0 | 0 | 36 | - | 0 | 0 | - | 0 | |
| H. sunameri | 0 | 10 | 20 | 0 | 25 | 24 | 0 | 0 | 14 | 0 | 0 | 23 | 0 | - | 20 | 0 | - | 0 | |
| H. taurica | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | - | 0 | |
| Liver / Examined hosta) | 18 | 8 | 32 | 6 | 3 | 14 | 2 | 4 | 8 | 0 | 1 | 5 | 10 | 0 | 5 | 0 | 0 | 0 | |
| C. oblonga | 0 | 13 | 47 | 0 | 0 | 7.1 | 0 | 25 | 63 | - | 0 | 80 | 0 | - | 100 | - | - | - | |
| Intestine / Examined hosta) | 24 | 13 | 48 | 8 | 6 | 15 | 4 | 5 | 14 | 1 | 2 | 13 | 10 | 0 | 5 | 1 | 0 | 1 | |
| S. elongatum | 4.2 | 7.7 | 29 | 0 | 17 | 13 | 0 | 0 | 14 | 100 | 0 | 54 | 0 | - | 60 | 0 | - | 0 | |
| D. fuhrmanni | 0 | 0 | 4.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7.7 | 0 | - | 0 | 0 | - | 100 | |
| Tetrabothrius sp. | 0 | 0 | 2.1 | 0 | 0 | 0 | 0 | 0 | 7.1 | 0 | 0 | 0 | 0 | - | 0 | 0 | - | 0 | |
| Corynosoma sp. | 0 | 0 | 2.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | - | 100 | |
a) Only hosts which were washed and microscopically observed are included.
DISCUSSION
Three species obtained in this study include several synonyms. Pharurus sunameri and Pharurus asiaeorientalis were originally described as Pseudostenurus sunameri Yamaguti, 1951 [38] and Pharurus asiaeorientalis Petter, 1982 [27], respectively. The former was included in the genus Pharurus [27]; however, the name Pseudostenurus sunameri has been used in several later studies [14, 21, 26]. The latter was renamed and transferred to genus Otophocaenurus [21]. Both genera (Pseudostenurus and Otophocaenurus) were considered synonyms of the genus Pharurus [3]. The present study follows this classification. S. elongatum was originally described as Orthosplanchnus elongatus Ozaki, 1935 [24]; subsequently, it was transferred to the genus Odhneriella [39] and then to Hadwenius [1]. Recently, Hadwenius was considered as a synonym of the genus Synthesium [11]. Therefore, in this study, a new combination Synthesium elongatum n. comb. is used.
A new location record and a new host record were obtained in this study. S. nanjingensis was originally described based on specimens obtained from a finless porpoise in Nanjing, China [34]. The detection of this species in the present study is the first record in Japanese waters, although the worms were very rare and were only found in two hosts of the OB population. Tetrabothrius sp. was detected for the first time in the genus Neophocaena.
This study is the first report about the helminths found in finless porpoises in the OB, AT and IM populations. It also adds further information on the SIS and TS populations, which have been studied previously [21]. Eight species of helminths: P. sunameri, P. asiaeorientalis, N. spathulatum, N. sunameri, H. pingi, H. sunameri, C. oblonga and S. elongatum were commonly found and are considered common parasitic helminths of finless porpoises in Japan. In marine mammal helminths, anisakid nematodes in the stomach have been reported in several cetaceans [12, 16, 17]. However, no anisakids were found in this study. This is in line with previous findings: another study on the finless porpoises in the SIS and TS populations also found no anisakids [21]. This might be due to the highly coastal habitat of the finless porpoise [14, 19]. On the other hand, Pilleri [28] reported Anisakis typica in two hosts of the finless porpoise in Pakistan. Jefferson and Wang [15] proposed that the genus Neophocaena includes at least two species: N. asiaeorientalis in east Asia and N. phocaenoides in the Indian Ocean; both species show extensive geographical variation. Although the prevalence of anisakid infection in N. phocaenoides is not reported, Pilleri’s report might indicate an inter-species or regional difference in anisakid infection in the genus Neophocaena, which probably reflects a difference in local habitat in the genus.
Comparing the helminthic fauna in the different host populations, the species composition or prevalence of helminths was more or less different between each adjacent population. N. spathulatum was not found in the IM and TS populations. Although the number of examined hosts from TS was limited, a previous study also found no N. spathulatum in the four hosts examined [21]. N. spathulatum is considered absent or less prevalent in IM and TS populations than in the other populations. Corynosoma sp. was found only in the TS population. Kuramochi et al. [21] examined the intestines of three adult finless porpoises from TS and obtained some specimens of the genus Corynosoma from all of them. Based on general morphology, these worms were regarded as the same species. This acanthocephalan species is considered specific to TS. The prevalence of C. oblonga in the OB population was lower than in the other populations, with the exception of TS. The only available information on C. oblonga infection in the TS population is from Kuramochi et al. [21]. In their study, one of three examined adult porpoises was infected. If the data from the present study and Kuramochi et al. [21] are combined, the best estimate of the prevalence of C. oblonga in the TS population increases to 50% (2/4), higher than the OB population.
Not only for the finless porpoise, information on the lifecycles of marine mammal helminths is generally very scarce. C. oblonga has been found in the harbor porpoise (Phocoena phocoena) [7, 12, 32, 33], Dall’s porpoise (Phocaenoides dalli) [6] and the common dolphin (Delphinus delphis) [12] from a wide range of habitats in the northern hemisphere. An atypical-host infection has also been reported in the common thresher shark (Alopias vulpinus) [2]. Based on the feeding habits of these hosts, the transmission of C. oblonga is suspected to involve the consumption of fish. On the other hand, N. spathulatum has only been found in finless porpoises in Japan, and there is no information available about its lifecycle. In this study, N. spathulatum was found exclusively in porpoises that were longer than 90 cm, which were designated weaning or weaned (see Materials and Methods). Therefore, one or multiple prey species might serve as intermediate/paratenic hosts. From stomach content analyses, the finless porpoise is known to be an opportunistic feeder, which feeds on several fishes, cephalopods, crustaceans and other marine organisms [20, 30, 31]. The differences in the presence and prevalence of helminths found in this study might reflect differences in the available prey species in each population.
The prevalence of H. pingi was higher in <90 cm porpoises, which were considered nursing (see Materials and Methods), than in >110 cm porpoises, considered weaned. The preferential infection by H. pingi of neonate finless porpoises (the exact species are unknown) has been reported in Hong Kong, and prenatal or transmammary infection has also been suspected [25, 26]. The present study also supports this possibility. Such vertical transmission has also been suspected during H. lagenorhynchi infection of the bottlenose dolphin (Tursiops truncatus) [8, 10] and the common dolphin (D. delphis) [35]. If the vertical transmission hypothesis is true, it is strange that H. pingi is almost not found in >110 cm porpoises. If the worms are transmitted from the mother to the fetus or the neonate, the prevalence of infected adults should be equal to or higher than that of calves. The pathogenicity of H. pingi could increase the fatality of severely infected neonates, which would result in high numbers of young animals infected by H. pingi being stranded. Thus, the prevalence of H. pingi in <90 cm porpoises might appear higher than in >110 cm porpoises. On the other hand, a congeneric species H. sunameri was found frequently in >110 cm porpoises, but not found in <90 cm porpoises, indicating that H. sunameri infects porpoises via infected prey. In lungworms found in the harbor porpoise, a correlation between the host age and the number of species of lungworms has been observed [5], and a heteroxenous lifecycle, i.e., the existence of intermediate host species, has been suggested [22]. Although the species reported in the harbor porpoise are different from those in the finless porpoise [4, 9, 12], a heteroxenous lifecycle of finless porpoise lungworms is plausible. The presence of completely different infection routes between the two congenerics (the vertical transmission of H. pingi and the trans-prey infection of H. sunameri) is very interesting. Such shifts in lungworm species with the host’s growth have not been reported in other cetaceans. To verify the infection routes, detection of H. pingi in fetuses or milk, and the identification of infective H. sunameri larvae from prey species are needed.
Acknowledgments
This study was financially supported by the Sasakawa Scientific Research Grant from The Japan Science Society. We also thank the following institutes and aquaria for the collection of stranded and by-caught porpoises: the Kujukushima Pearl Sea Resort (Nagasaki); Saga Pref. Space and Science Museum (Saga); Graduate School of Science and Technology, Kumamoto University (Kumamoto); Oita Marine Palace Aquarium “Umitamago” (Oita); Center for Marine Environmental Studies (CMES) and Leading Academia in Marine and Environment Pollution Research (LaMer), Ehime University (Ehime); Osaka Museum of Natural History (Osaka); Laboratory of Fish Stock Enhancement, Graduate School of Bioresources, Mie University (Mie); Sea Turtle and Finless Porpoise Conservation and Research Club of Mie University “Kameppuri” (Mie); Toba Aquarium (Mie); Minami-chita Beach Land Aquarium (Aichi); Toyohashi Museum of Natural History (Aichi); Choshi Ocean Institute (Chiba); Ibaraki Prefectural Oarai Aquarium (Ibaraki); and Department of Zoology, National Museum of Nature and Science (Ibaraki).
REFERENCES
- 1.Adams A. M., Rausch R. L.1989. A revision of the genus Orthosplanchnus Odhner, 1905 with consideration of the genera Odhneriella Skriabin, 1915 and Hadwenius Price, 1932 (Digenea: Campulidae). Can. J. Zool. 67: 1268–1278. doi: 10.1139/z89-181 [DOI] [Google Scholar]
- 2.Adams A. M., Hoberg E. P., McAlpine D. F., Clayden S. L.1998. Occurrence and morphological comparisons of Campula oblonga (Digenea: Campulidae), including a report from an atypical host, the thresher shark, Alopias vulpinus. J. Parasitol. 84: 435–438. doi: 10.2307/3284507 [DOI] [PubMed] [Google Scholar]
- 3.Anderson R. C.2009. Strongylida Metastrongyloidea. pp. 178–217. In: Keys to the Nematode Parasites of Vertebrates: Archival Volume. (Anderson, R. C., Chabaud, A. G. and Willmott, S. eds.), CABI, Wallingford. [Google Scholar]
- 4.Arnold P. W., Gaskin D. E.1975. Lungworms (Metastrongyloidea: Pseudaliidae) of harbor porpoise Phocoena phocoena (L. 1758). Can. J. Zool. 53: 713–735. doi: 10.1139/z75-087 [DOI] [PubMed] [Google Scholar]
- 5.Balbuena J. A., Aspholm P. E., Andersen K. I., Bjørge A.1994. Lung-worms (Nematoda: Pseudaliidae) of harbour porpoises (Phocoena phocoena) in Norwegian waters: patterns of colonization. Parasitology 108: 343–349. doi: 10.1017/S0031182000076186 [DOI] [PubMed] [Google Scholar]
- 6.Conlogue G. J., Ogden J. A., Foreyt W. J.1985. Parasites of the Dall’s porpoise (Phocoenoides dalli True). J. Wildl. Dis. 21: 160–166. doi: 10.7589/0090-3558-21.2.160 [DOI] [PubMed] [Google Scholar]
- 7.Dailey M., Stroud R.1978. Parasites and associated pathology observed in cetaceans stranded along the Oregon coast. J. Wildl. Dis. 14: 503–511. doi: 10.7589/0090-3558-14.4.503 [DOI] [PubMed] [Google Scholar]
- 8.Dailey M., Walsh M., Odell D., Campbell T.1991. Evidence of prenatal infection in the bottlenose dolphin (Tursiops truncatus) with the lungworm Halocercus lagenorhynchi (Nematoda: Pseudaliidae). J. Wildl. Dis. 27: 164–165. doi: 10.7589/0090-3558-27.1.164 [DOI] [PubMed] [Google Scholar]
- 9.Delyamure S. L. 1955. Helminthofauna of marine mammals (ecology and phylogeny) Gel’mintologicheskaya Laboratoria, Akademiya Nauk SSSR, Moskova. English translation by Israel Program for Scientific Translations, Jerusalem, 1968. [Google Scholar]
- 10.Fauquier D. A., Kinsel M. J., Dailey M. D., Sutton G. E., Stolen M. K., Wells R. S., Gulland F. M. D.2009. Prevalence and pathology of lungworm infection in bottlenose dolphins Tursiops truncatus from southwest Florida. Dis. Aquat. Organ. 88: 85–90. doi: 10.3354/dao02095 [DOI] [PubMed] [Google Scholar]
- 11.Gibson D. I.2005. Family Brachycladiidae Odhner, 1905. pp. 641–652. In: Keys to the Trematoda, Vol. 2. (Jones, A., Bray, R. A. and Gibson, D. I. eds.), CABI and the Natural History Museum, London. [Google Scholar]
- 12.Gibson D. I., Harris E. A., Bray R. A., Jepson P. D., Kuiken T., Baker J. R., Simpson V. R.1998. A survey of the helminth parasites of cetaceans stranded on the coast of England and Wales during the period 1990–1994. J. Zool. (Lond.) 244: 563–574. doi: 10.1111/j.1469-7998.1998.tb00061.x [DOI] [Google Scholar]
- 13.Hoberg E. P.1994. Order Tetrabothriidea Baer, 1954. pp. 295–304. In: Keys to the Cestode Parasites of Vertebrates. (Khalil, L. F., Jones, A. and Bray, R. A. eds.), CABI, Wallingford. [Google Scholar]
- 14.Jefferson T. A., Hung S. K.2004. Neophocaena phocaenoides. Mamm. Species 746: 1–12. doi: 10.1644/746 [DOI] [Google Scholar]
- 15.Jefferson T. A., Wang J. Y.2011. Revision of the taxonomy of finless porpoises (genus Neophocaena): the existence of two species. J. Mar. Anim. Ecol. 4: 3–16. [Google Scholar]
- 16.Kagei N., Oshima T., Takemura A.1967. Survey of Anisakis spp. (Anisakidae: Nematoda) in marine mammals of the coast of Japan. Kisechugaku Zasshi 16: 427–435(in Japanese). [Google Scholar]
- 17.Kikuchi S., Hayashi S., Nakajima M.1967. Studies on anisakiasis in dolphins. Kisechugaku Zasshi 16: 156–166(in Japanese). [Google Scholar]
- 18.Kamo H., Maejima J., Yazaki S., Fukumoto S.1982. Notes on morphology and taxonomy of the genus Diphyllobothrium found from some marine mammals in Japanese environs. Yonago Acta Med. 33: 261–270(in Japanese). [Google Scholar]
- 19.Kasuya T.1999. Finless porpoise Neophocaena phocaenoides (G. Cuvier, 1829). pp. 411–442. In: Handbook of Marine Mammals, Vol. 6. (Ridgway, S. H. and Harrison, R. eds.), Academic Press, San Diego. [Google Scholar]
- 20.Kataoka T., Kitamura S., Sekido M., Yamamoto K.1976. On the feeding habit of finless porpoise (Neophocaena phocaenoides). Mie Seibutsu 24: 29–36(In Japanese). [Google Scholar]
- 21.Kuramochi T., Kikuchi T., Okamura H., Tatsukawa T., Doi H., Nakamura K., Sakakibara S.2000. Parasitic helminth and epizoit fauna of finless porpoise in the Inland Sea of Japan and the western North Pacific with a preliminary note on faunal difference by host’s local population. Mem. Natn. Sci. Mus. Tokyo 33: 83–95. [Google Scholar]
- 22.Lehnert K., von Samson-Himmelstjerna G., Schaudien D., Bleidorn C., Wohlsein P., Siebert U.2010. Transmission of lungworms of harbour porpoises and harbour seals: molecular tools determine potential vertebrate intermediate hosts. Int. J. Parasitol. 40: 845–853. doi: 10.1016/j.ijpara.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 23.Nakazawa M.2015. fmsb: Functions for Medical Statistics Book with some Demographic Data. R package version 0.5.2. https://CRAN.R-project.org/package=fmsb.
- 24.Ozaki Y.1935. Trematode parasites of Indian porpoise Neophocaena phocaenoides Gray. J. Sci. Hiroshima Univ. 3: 115–138. [Google Scholar]
- 25.Parsons E. C. M., Jefferson T. A.2000. Post-mortem investigations on stranded dolphins and porpoises from Hong Kong waters. J. Wildl. Dis. 36: 342–356. doi: 10.7589/0090-3558-36.2.342 [DOI] [PubMed] [Google Scholar]
- 26.Parsons E. C. M., Overstreet R. M., Jefferson T. A.2001. Parasites from Indo-Pacific hump-backed dolphins (Sousa chinensis) and finless porpoises (Neophocaena phocaenoides) stranded in Hong Kong. Vet. Rec. 148: 776–780. doi: 10.1136/vr.148.25.776 [DOI] [PubMed] [Google Scholar]
- 27.Petter A. J., Pilleri G.1982. Pharurus asiaeorientalis new species, metastrongylid nematode, parasite of Neophocaena asiaeorientalis (Phocoenidae, Cetacea). Invest. Cetacea 13: 141–148. [Google Scholar]
- 28.Pilleri G.1974. First record of Anisakis typica (Nematoda, Ascaridata) in Delphinus tropicalis and Neophocaena phocaenoides off the coast of Pakistan. Invest. Cetacea 5: 339–340. [Google Scholar]
- 29.R Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- 30.Shirakihara M., Shirakihara K., Takemura A.1992. Records of the finless porpoise (Neophocaena phocaenoides) in the waters adjacent to Kanmon Pass, Japan. Mar. Mamm. Sci. 8: 82–85. doi: 10.1111/j.1748-7692.1992.tb00128.x [DOI] [Google Scholar]
- 31.Shirakihara M., Seki K., Takemura A., Shirakihara K., Yoshida H., Yamazaki T.2008. Food habits of finless porpoises Neophocaena phocaenoides in Western Kyushu, Japan. J. Mammal. 89: 1248–1256. doi: 10.1644/07-MAMM-A-264.1 [DOI] [Google Scholar]
- 32.Siebert U., Tolley K., Víkingsson G. A., Ólafsdottir D., Lehnert K., Weiss R., Baumgärtner W.2006. Pathological findings in harbour porpoises (Phocoena phocoena) from Norwegian and Icelandic waters. J. Comp. Pathol. 134: 134–142. doi: 10.1016/j.jcpa.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 33.Smith F. R., Threlfall W.1973. Helminths of some mammals from Newfoundland. Am. Midl. Nat. 90: 215–218. doi: 10.2307/2424284 [DOI] [Google Scholar]
- 34.Tao J.1983. A new species and a new Chinese record of nematodes from porpoise Neophocaena phocaenoides. Acta Zootaxonomica. Sin. 8: 350–353. [Google Scholar]
- 35.Tomo I., Kemper C. M., Lavery T. J.2010. Eighteen-year study of South Australian dolphins shows variation in lung nematodes by season, year, age class, and location. J. Wildl. Dis. 46: 488–498. doi: 10.7589/0090-3558-46.2.488 [DOI] [PubMed] [Google Scholar]
- 36.Wu H. W.1929. On Halocercus pingi n. sp. a lung-worm from the porpoise, Neomeris phocoenoides. J. Parasitol. 15: 276–279. doi: 10.2307/3271983 [DOI] [Google Scholar]
- 37.Yamaguti S.1951. Studies on the Helminth Fauna of Japan. Part 45. Trematodes of marine mammals. Arb. Med. Fak. Okayama 7: 283–294 with 2 Plates. [Google Scholar]
- 38.Yamaguti S.1951. Studies on the Helminth Fauna of Japan. Part 46. Nematodes of marine mammals. Arb. Med. Fak. Okayama, 7: 295–306 with 3 Plates. [Google Scholar]
- 39.Yamaguti S.1971. CAMPULIDAE Odhner, 1926. pp. 724–729. In: Synopsis of Digenetic Trematodes of Vertebrates, Vol. 1. (Yamaguti, S. ed.), Keigaku Publ. Co., Tokyo. [Google Scholar]
- 40.Yoshida H., Shirakihara K., Shirakihara M., Takemura A.1995. Geographic variation in the skull morphology of the finless porpoise Neophocaena phocaenoides in Japanese waters. Fish. Sci. 61: 555–558. [Google Scholar]
- 41.Yoshida H., Yoshioka M., Shirakihara M., Chow S.2001. Population structure of finless porpoises (Neophocaena phocaenoides) in coastal waters of Japan based on mitochondrial DNA sequences. J. Mammal. 82: 123–130. [Google Scholar]