Abstract
This study aimed at characterizing fecal microbiota of three captive carnivore species of leopard cats Prionailurus bengalensis, Eurasian otters Lutra lutra and raccoon dogs Nyctereutes procyonoides. We used DNA barcoding sequencing to analyze 16S rRNA genes of uncultured bacteria in the feces collected in the Seoul Zoo. The sequencing analyses revealed that: 1) Firmicutes was the most dominant phylum for all three animals; 2) bacterial genus-rank compositions were distinct across species of the animals; and 3) bacterial community memberships were different across species of the studied animals. We expect such baseline information is useful for better understanding of these endangered species and future management of their health in zoos.
Keywords: 16S rDNA, gastrointestinal tract, gut, microbiome, microflora
Gut microbiota is important in shaping health in human [2, 15]. Similarly, gut microbiota is important for health in animals, such as cats [12], dogs [12, 13] and monkeys [9]. Indeed, a study reported decreases in Blautia, Faecalibacterium and Turicibacter, and increases in Sutterella and Clostridium in feces of dogs with acute hemorrhagic diarrhea as compared to healthy dogs [13]. Another study reported changes in gut microbiota in the rhesus monkeys with chronic enterocolitis [9]. Thus, gut microbiota is thought to be a useful marker for health in animals in the same way in human [3]. Meanwhile, gut microbiota is known to differ among types of animals [8, 9]. Thereby, it is crucial to characterize baseline patterns of healthy gut microbiota of each animal species, if it is used as a marker of animal health.
This study aimed at characterizing fecal microbiota of three carnivore species inhabiting Korea and captive in the Seoul Zoo. Feces were collected from each cage or enclosure of healthy leopard cats Prionailurus bengalensis (n=2), Eurasian otters Lutra lutra (n=3) and raccoon dogs Nyctereutes procyonoides (n=4). These medium-sized carnivore species were selected, because they inhabit South Korea and are subject to conservation, with otters and leopard cats listed as classes I and II endangered species by the Ministry of Environment of Korea, respectively. Studies are limited about variations in gut microbiota across such carnivore species. We anticipate such information is useful for better understanding of the endangered species and managing their health in zoos. These animals were reared in cages or enclosed by species. A pair of otters was exhibited in a 80-m2 outdoor enclosure with a pool and an indoor room. The other female otter was exhibited in a 24-m2 indoor enclosure with a pool and a den. These otters were fed twice a day with live Chinese muddy loach Misgurnus mizolepis and thawed Japanese horse mackerel Trachurus japonicus. Two adult female leopard cats were housed in a cage with a size of 2.6 m (W) × 7.5 m (L) × 2.1 m (H) and fed with chicken and beef once a day. One of the leopard cats was rescued in the wild, and the other was born in the Seoul Zoo. Two male and two female adult raccoon dogs born in the Seoul Zoo were housed in two different cages by sex. These cages have an identical structure to the cage for leopard cats. The raccoon dogs were fed twice a day with beef, chicken and the Omnivore Diet Dry® (ZuPreem, Shawnee Mission, KS, U.S.A.). Additional 150 g of Chinese muddy loaches were included for leopard cats and raccoon dogs for the first 4 weeks. About 30 to 40 g of each fresh fecal sample was collected in a 50 ml tube from February 15 to June 1, 2015. The samples were stored at −20°C until DNA extraction conducted on June 8 and 9, 2015. The study was approved by the Institutional Animal Care and Use Committee at Seoul Zoo (SEOUL 2015-002) and by the Institutional Animal Care and Use Committees at Seoul National University (SNU-150122-7).
DNA was extracted from the samples and sequenced according to the methods reported elsewhere [17]. Briefly, DNA was extracted from about 0.5 g of each sample by the PowerSoil DNA Isolation Kit (Mobio Laboratory, Inc., Carsbad, CA, U.S.A.). In addition to the Mobio’s Power Beads, 0.1-mm diameter glass beads (300 mg) and 0.5-mm diameter glass beads (100 mg) were supplemented for the 3-min physical disruption step by the mini beadbeater-24 (Biospec Products, Inc., Bartlesville, OK, U.S.A.) [6]. The extracted DNA was PCR-amplified by universal bacterial primers for Escherichia coli numbering region of 341 to 805 of 16S rRNA genes [7]. The amplicons were purified, and the index PCR was performed using the Nextera XT Index kit (Illumina, San Diego, CA, U.S.A.). The index-tagged amplicons were normalized, pooled and sequenced by Illumina MiSeq. The read 1 and 2 sequences were joined with a minimum allowed overlap of 10 bp. The sequences shorter than 200 bp were excluded. The chimeric sequences were further removed by checking against the reference Ribosomal Database Project (RDP) database [16]. The numbers of the remaining high-quality sequences ranged from 257 to 37,324 sequences per library. Taxonomic assignments were performed by the RDP Naïve Bayesian Classifier [16]. For the diversity analyses, two libraries of R150310 and L150310 with less than 1,200 sequences were excluded, and 1,200 sequences were sub-sampled from each of the remaining libraries and binned into operational taxonomic units (OTUs) at 97% sequence identity. The α diversity metrics of Shannon indices and Chao 1 estimators were calculated. For the β diversity analyses, the Jaccard indices and the Yue and Clayton theta similarity coefficients were calculated. Raw sequences were uploaded to the Sequence Read Archive of NCBI under the accession number SRP080781.
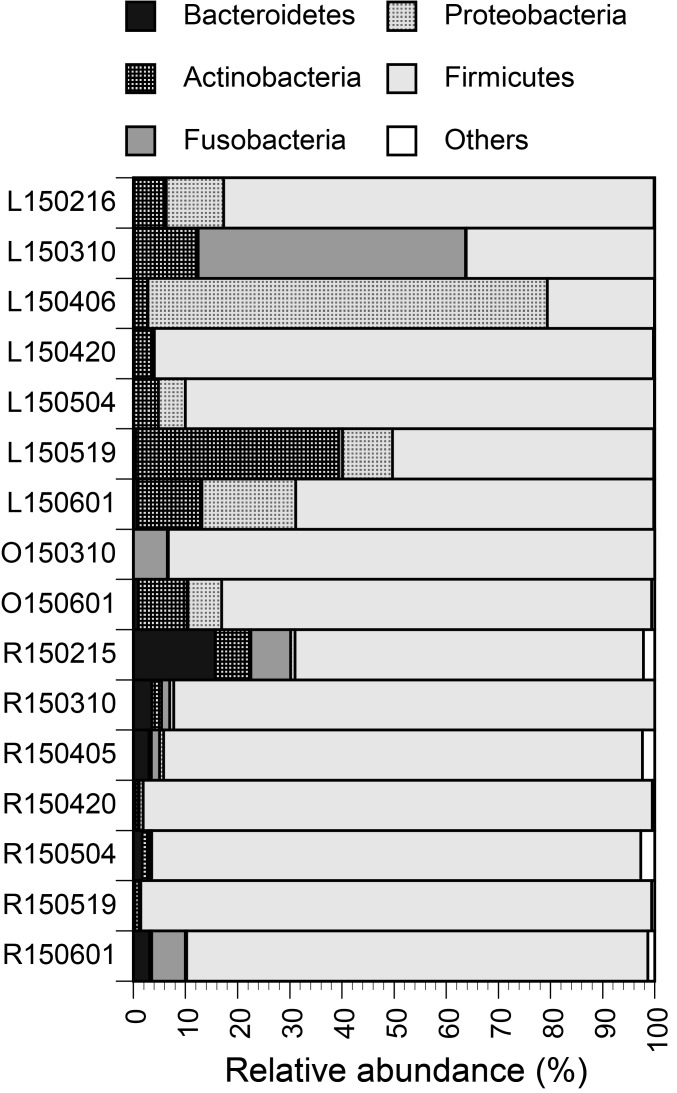
Firmicutes was the most dominant phylum for all three tested animals (Fig. 1), with mean relative abundances of 63% for leopard cats, 88% for otters and 90% for raccoon dogs. Firmicutes is known to be the most dominant phylum in feces of humans [1] and animals [8]. In feces of leopard cats, Actinobacteria and Proteobacteria were also rich, with mean relative abundances of 11% and 17%, respectively. The tendency appears to be different from microbiota in feces of domestic cats, with the reported three most abundant phyla of Bacteroidetes (68%), Firmicutes (13%) and Proteobacteria (6%) [14]. No study has been reported about fecal microbiota of leopard cats, while Shehzad et al. [11] analyzed feces of leopard cats based on high-throughput sequencing and demonstrated rodents as their main diets in the wild. In feces of raccoon dogs, Bacteroidetes and Fusobacteria were more abundant than in feces of leopard cats and otters, with respective mean relative abundances of 4.0% and 2.6%.
Fig. 1.
Relative abundances of bacterial phyla. The numbers in the sample names indicate sampling days (YYMMDD). The alphabets indicate L for leopard cats, O for otters and R for raccoon dogs.
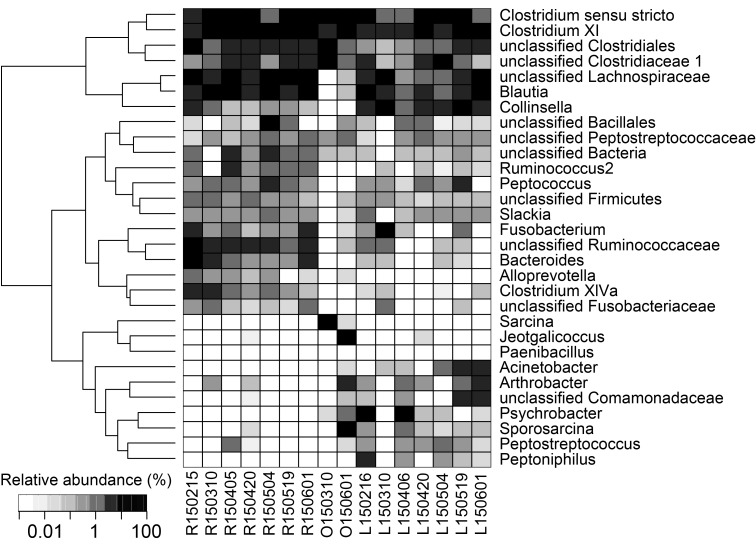
At the lower taxonomic ranks, bacteria in the order Clostridiales were the most dominant for all three animals tested in this study (Fig. 2). Mean relative abundances of a bacterial group of Clostridium sensu stricto, Clostridium XI, unclassified Clostridiales and unclassified Clostridiaceae 1 were 41% for leopard cats, 52% for otters and 56% for raccoon dogs. Clostridium was abundantly detected from the feces of the Eurasian otters in Portugal in the wild [10]. Interestingly, Psychrobacter, Peptostreptococcus, Peptoniphilus and Acinetobacter were abundant in the feces of leopard cats, with respective mean relative abundances of 12.3%, 0.3%, 1.2% and 1.5%, but these genera were undetected or detected with much lower abundances from the feces of raccoon dogs. In contrast, Alloprevotella and Fusobacteriaceae were more abundant in the feces of raccoon dogs, i.e., 0.4% and 0.4%, respectively, than in the feces of leopard cats, i.e., 0.0% and 0.1%, respectively. A culture-dependent study [18] has been reported about fecal flora of raccoon dog, which is difficult to be compared with the finding of the present culture-independent study. Meanwhile, a pyrosequencing-based study [4] showed Fusobacteria were more abundant in domestic dogs than in domestic cats, which might be analogous to the relationship of fecal microbiota of raccoon dogs and leopard cats observed in this study.
Fig. 2.
Relative abundances of the 30 most abundant taxa. The numbers in the sample names indicate sampling days (YYMMDD). The alphabets indicate L for leopard cat, O for otter and R for raccoon dog.
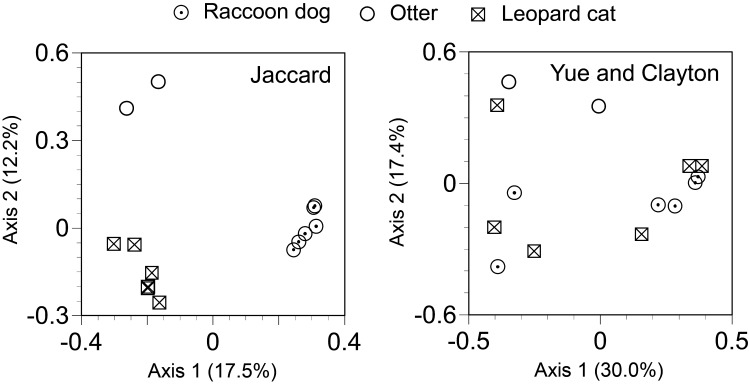
The β diversity analysis confirmed differences of fecal microbiota across species of the studied animals (Fig. 3). Although no significant difference was observed based on the Yue and Clayton theta similarity coefficient, which is a measure of community structure, a significant difference was observed between the fecal microbiota of leopard cats and raccoon dogs based on the Jaccard index, which is a measure of community membership (P<0.05, Parsimony test). The result suggests types of bacterial taxa consisting of fecal microbiota are different between leopard cats and raccoon dogs. Meanwhile, no significant difference was observed in the α diversity metrics of Chao1 estimator, which is a measure of species richness, and Shannon indices, which is a measure of species evenness, across types of the studied animals (P>0.05, Wilcoxon signed rank tests) (Table 1). The results suggest no difference neither in the numbers of detected taxa nor in the closeness of the numbers of detected taxa across species of the studied animals.
Fig. 3.
Principal coordinates analysis plots of the Jaccard and Yue and Clayton distances based on operational taxonomic units (OTUs) at 97% sequence identity. The libraries with less than 1,200 sequences were not included for the analyses.
Table 1. Alpha diversity measures of fecal microbiota of each test animal a).
| α diversity measure | Animal | Number of fecal samples | Mean | Standard deviation |
|---|---|---|---|---|
| Chao1 | Leopard cat | 6 | 259 | 112 |
| Otter | 2 | 269 | n.d. | |
| Raccoon dog | 6 | 371 | 126 | |
| Shannon | Leopard cat | 6 | 2.59 | 0.66 |
| Otter | 2 | 2.65 | n.d. | |
| Raccoon dog | 6 | 3.19 | 0.57 |
a) Based on operational taxonomic units (OTUs) at 97% sequence identity. 1,200 sequences were sub-sampled from each library to calculate the α diversity measures. The libraries with less than 1,200 sequences were not included for the analyses. Abbreviation: n.d., not determined due to small sample sizes.
In conclusion, this study revealed differences in healthy fecal microbiota of three captive carnivore species of leopard cats, Eurasian otters and raccoon dogs. Ley et al. [8] indicate gut microbiota of animals shaped by factors, such as diet, host phylogeny and gut morphology. Although the reasons are unknown, the different fecal microbiota observed in this study might be attributable to species-specific variations in such factors. We foresee baseline information of fecal microbiota of the three carnivore species obtained by this study is useful for better understanding of these endangered species and future management of their health in zoos in ways that gut microbiota is proposed as a diagnostic marker for diseases, such as metabolic and gastrointestinal disorders in human [3] and in companion animals, such as domestic dogs [5].
Acknowledgments
We thank Y.M. Lim and J.H. Bae who are the staffs of the Seoul Zoo to help us collecting fecal samples.
References
- 1.Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D. R., Fernandes G. R., Tap J., Bruls T., Batto J. M., Bertalan M., Borruel N., Casellas F., Fernandez L., Gautier L., Hansen T., Hattori M., Hayashi T., Kleerebezem M., Kurokawa K., Leclerc M., Levenez F., Manichanh C., Nielsen H. B., Nielsen T., Pons N., Poulain J., Qin J., Sicheritz-Ponten T., Tims S., Torrents D., Ugarte E., Zoetendal E. G., Wang J., Guarner F., Pedersen O., de Vos W. M., Brunak S., Doré J., Antolín M., Artiguenave F., Blottiere H. M., Almeida M., Brechot C., Cara C., Chervaux C., Cultrone A., Delorme C., Denariaz G., Dervyn R., Foerstner K. U., Friss C., van de Guchte M., Guedon E., Haimet F., Huber W., van Hylckama-Vlieg J., Jamet A., Juste C., Kaci G., Knol J., Lakhdari O., Layec S., Le Roux K., Maguin E., Mérieux A., Melo Minardi R., M’rini C., Muller J., Oozeer R., Parkhill J., Renault P., Rescigno M., Sanchez N., Sunagawa S., Torrejon A., Turner K., Vandemeulebrouck G., Varela E., Winogradsky Y., Zeller G., Weissenbach J., Ehrlich S. D., Bork P., MetaHIT Consortium. 2011. Enterotypes of the human gut microbiome. Nature 473: 174–180. doi: 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisgaard H., Li N., Bonnelykke K., Chawes B. L. K., Skov T., Paludan-Müller G., Stokholm J., Smith B., Krogfelt K. A.2011. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 128: 646–52.e1, 5. doi: 10.1016/j.jaci.2011.04.060 [DOI] [PubMed] [Google Scholar]
- 3.Daniels L., Budding A. E., de Korte N., Eck A., Bogaards J. A., Stockmann H. B., Consten E. C., Savelkoul P. H., Boermeester M. A.2014. Fecal microbiome analysis as a diagnostic test for diverticulitis. Eur. J. Clin. Microbiol. Infect. Dis. 33: 1927–1936. doi: 10.1007/s10096-014-2162-3 [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Mazcorro J. F., Lanerie D. J., Dowd S. E., Paddock C. G., Grützner N., Steiner J. M., Ivanek R., Suchodolski J. S.2011. Effect of a multi-species synbiotic formulation on fecal bacterial microbiota of healthy cats and dogs as evaluated by pyrosequencing. FEMS Microbiol. Ecol. 78: 542–554. doi: 10.1111/j.1574-6941.2011.01185.x [DOI] [PubMed] [Google Scholar]
- 5.Hooda S., Minamoto Y., Suchodolski J. S., Swanson K. S.2012. Current state of knowledge: the canine gastrointestinal microbiome. Anim. Health Res. Rev. 13: 78–88. doi: 10.1017/S1466252312000059 [DOI] [PubMed] [Google Scholar]
- 6.Hospodsky D., Yamamoto N., Peccia J.2010. Accuracy, precision, and method detection limits of quantitative PCR for airborne bacteria and fungi. Appl. Environ. Microbiol. 76: 7004–7012. doi: 10.1128/AEM.01240-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glöckner F. O.2013. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41: e1. doi: 10.1093/nar/gks808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley R. E., Hamady M., Lozupone C., Turnbaugh P. J., Ramey R. R., Bircher J. S., Schlegel M. L., Tucker T. A., Schrenzel M. D., Knight R., Gordon J. I.2008. Evolution of mammals and their gut microbes. Science 320: 1647–1651. doi: 10.1126/science.1155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenna P., Hoffmann C., Minkah N., Aye P. P., Lackner A., Liu Z., Lozupone C. A., Hamady M., Knight R., Bushman F. D.2008. The macaque gut microbiome in health, lentiviral infection, and chronic enterocolitis. PLoS Pathog. 4: e20. doi: 10.1371/journal.ppat.0040020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira M., Sales-Luís T., Duarte A., Nunes S. F., Carneiro C., Tenreiro T., Tenreiro R., Santos-Reis M., Tavares L., Vilela C. L.2008. First assessment of microbial diversity in faecal microflora of Eurasian otter (Lutra lutra Linnaeus, 1758) in Portugal. Eur. J. Wildl. Res. 54: 245–252. doi: 10.1007/s10344-007-0137-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shehzad W., Riaz T., Nawaz M. A., Miquel C., Poillot C., Shah S. A., Pompanon F., Coissac E., Taberlet P.2012. Carnivore diet analysis based on next-generation sequencing: application to the leopard cat (Prionailurus bengalensis) in Pakistan. Mol. Ecol. 21: 1951–1965. doi: 10.1111/j.1365-294X.2011.05424.x [DOI] [PubMed] [Google Scholar]
- 12.Suchodolski J. S.2011. Companion animals symposium: microbes and gastrointestinal health of dogs and cats. J. Anim. Sci. 89: 1520–1530. doi: 10.2527/jas.2010-3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suchodolski J. S., Markel M. E., Garcia-Mazcorro J. F., Unterer S., Heilmann R. M., Dowd S. E., Kachroo P., Ivanov I., Minamoto Y., Dillman E. M., Steiner J. M., Cook A. K., Toresson L.2012. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 7: e51907. doi: 10.1371/journal.pone.0051907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tun H. M., Brar M. S., Khin N., Jun L., Hui R. K. H., Dowd S. E., Leung F. C. C.2012. Gene-centric metagenomics analysis of feline intestinal microbiome using 454 junior pyrosequencing. J. Microbiol. Methods 88: 369–376. doi: 10.1016/j.mimet.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbaugh P. J., Hamady M., Yatsunenko T., Cantarel B. L., Duncan A., Ley R. E., Sogin M. L., Jones W. J., Roe B. A., Affourtit J. P., Egholm M., Henrissat B., Heath A. C., Knight R., Gordon J. I.2009. A core gut microbiome in obese and lean twins. Nature 457: 480–484. doi: 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q., Garrity G. M., Tiedje J. M., Cole J. R.2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73: 5261–5267. doi: 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu S., An C., Kim S., Lee S., Lee K., Yamamoto N.2016. Effects of the biocides on the culturable house dust-borne bacterial compositions and diversities. Hum. Ecol. Risk Assess. 22: 1133–1146. doi: 10.1080/10807039.2016.1143769 [DOI] [Google Scholar]
- 18.Zhang H., Cao X., Chen L.2012. Composition and diversity of Canidae fecal flora. Acta Ecol. Sin. 32: 253–257. doi: 10.1016/j.chnaes.2012.07.008 [DOI] [Google Scholar]