Abstract
The present study investigated the effects of Vernonia cinerea (VC) on the reproductive function in streptozotocin (STZ)-induced diabetic male rats. Six-week-old male Sprague-Dawley rats were randomly divided into four groups: group 1, normal control rats; group 2, diabetic untreated rats; group 3, diabetic rats treated with VC (10 mg/kg); and group 4, diabetic rats treated with VC (40 mg/kg). Diabetes mellitus (DM) was induced by intraperitoneal injection of STZ (60 mg/kg). All animals were treated for 30 consecutive days. Body weight, blood glucose, food intake, epididymal sperm parameters, testicular microstructure and serum testosterone levels were evaluated. VC treatment significantly restored the sperm motility and testosterone concentration, and decreased the testicular histopathological changes in DM rats. Moreover, high-dose VC exhibited an antidibetic activity and significantly improved the sperm count. In conclusion, we found, for the first time, that administration of VC significantly restored the testicular function and testosterone concentration in diabetic male rats.
Keywords: diabetes mellitus, rat, reproductive performance, streptozotocin, Vernonia cinerea
Diabetes mellitus (DM) is a chronic metabolic disease that represents a major public health problem because of the current lifestyle and dietary habits [9, 24]. The prevalence of DM has increased rapidly. The World Health Organization (WHO) has estimated that the number of patients with DM is anticipated to progress to 366 million by 2030 [5, 26, 41]. DM can result in many systemic complications, such as complication in the heart, kidneys, peripheral nerves and reproductive system [5, 9]. DM has been associated with negative effects on the male sexual function in humans and animal models including reduced spermatogenesis, erectile dysfunction, ejaculation disorder, sexual behavior and endocrine system abnormalities [1, 6, 8, 13]. It is also associated with the overproduction of reactive oxygen species (ROS) and decreased efficiency of antioxidant defenses [12]. A recent study has suggested that oxidative stress is an important pathophysiological mechanism underlying male reproductive dysfunction [9, 13]. Spermatozoa are particularly susceptible to the damaging effects of ROS, because their cell membrane contains a large amount of unsaturated fatty acids and the cytoplasm contains only a small concentration ROS-neutralizing enzyme [42]. Other studies have indicated that antioxidant treatment could improve the testicular dysfunction and subsequently ameliorate fertility in diabetic patients [13].
Vernonia cinerea Less (VC) belongs to Family Asteraceae, which is widely distributed in South-East Asia, India, Bangladesh, Nepal and China [10, 43]. This plant has been used in Thai traditional medicine to treat fever, cancer, gastro-intestinal disorders, malaria and arthritis; cease smoking; and provide relief from asthma [7, 18, 36, 40, 43]. The bioactive compounds of VC have been characterized and quantified using gas chromatography-mass spectrometry (GC/MS). Various phytochemical substances, including sterols, flavonoids, triterpenoids, sesquiterpenes and tannins, have been isolated from VC by GC/MS [3, 21, 28, 37, 43, 44]. Other phytochemical substances have been identified by high-performance liquid chromatography (HPLC), including phenolic compounds, gallic acid, rutin, quercetin, caffeic acid and ferulic acid. In addition, trace elements, including Fe, Mn, Co, and Se were quantified by atomic absorption spectrometry (AAS) [31]. Some of these compounds have been shown to exhibit antioxidant and anti-inflammatory activities [22, 44]. Recent studies have indicated that VC supplement with exercise could reduce oxidative stress and beta-endorphin levels in smokers [18]. Another study showed that VC inhibited cytochrome P450 2A6 (CYP2A6); thus, it could be used in combination with the drug therapy for smoking cessation [27]. In addition, the ethanol extract of VC flowers was found to possess anti-inflammatory properties in rats with adjuvant-induced arthritis [17], and the methanol extract has been shown to exhibit anti-inflammatory activity in rat models [20]. However, there were no studies on the effects of VC on the reproductive function in diabetic animal models. The present study was conducted to determine the effect of VC on the reproductive function in streptozotocin (STZ)-induced diabetic male rats.
MATERIALS AND METHODS
Plant
VC was collected from Chiang Mai Province, Thailand. Air-dried and finely ground leaves and stem of VC (100 g) were extracted with 1 l of ethanol with daily shaking for 1 week, using a Buchner funnel and Whatmann no.1 filter paper. The whole process was repeated five times to ensure the maximum yield of the ethanol extract. The combined aqueous extract was concentrated at 37°C using a rotary evaporator and lyophilized using a freeze-dryer. The solid ethanol extract was stored at 4°C until use for bioassays.
Animals and housing conditions
Thirty male Sprague-Dawley rats (6 weeks; 180–200 g) were obtained from the National laboratory animal center, Mahidol University, Thailand. The animals were housed singly in standard polypropylene cages and were maintained under controlled conditions of light/dark cycles (12-hr light/12-hr dark), room temperature (25 ± 2°C) and relative humidity (60–70%) with free access to water and rat chow. The animals were acclimatized to the laboratory conditions for at least 7 days before experimental procedures. All procedures were carried out in accordance with the Guide for the care and Use of Laboratory Animals of the National Institutes of Health, U.S.A., and were approved by the Animal use and care committee of Kasetsart University Research and Development Institute, Kasetsart University, Thailand (ID:ACKU02958).
Induction of DM
DM was induced by a single intraperitoneal injection of STZ that was freshly prepared in ice-cold citrate buffer (0.1 M, pH 4.5) at a dose of 60 mg/kg. Three days after STZ injection, DM induction was confirmed by measuring blood glucose levels in blood samples from the tail vein using a blood glucose meter (Accu-Check Active, Roche Diagnostic, Germany). Only animals exhibiting a fasting glucose level 250 mg/dl were included in this study.
Experimental design
Animals were randomly divided into four groups, six animals in each group. Group 1 included control nondiabetic animals, whereas animals in groups 2–4 were diabetic. Group 2 included untreated diabetic animals, whereas rats in groups 3 and 4 were treated with a crude extract of VC at 10 and 40 mg/kg, respectively. The extract was suspended in distilled water with few drops of Tween 80 to prepare a 1% suspension. Drug administration was continued for 30 consecutive days using an oral gavage tube.
Blood glucose, body weight and food intake of animals
Blood glucose and body weight were monitored once weekly during the experiment. Food consumption was monitored daily by weighing at 11:00; a visual check was made for any food dropped on the floor of the cage. Food intake of each rat was measured by weighing the remaining chow.
Sperm collection and analysis
At the end of the experiment, all animals were sacrificed by a lethal dose of pentobarbital sodium. The testes and epididymides were collected and weighed. The testes were fixed for histological examination. The spermatozoa were collected by mincing the caudal part of the epididymis into small pieces and mixing it in 1 ml of Hank’s balance salt solution, which was prewarmed at 37°C. Sperm parameters, such as sperm count, motility and viability, were examined microscopically according to the method described by Raji et al. (2003). Sperm count was determined using Neubauer chamber cell counting under10 magnifications. Sperm viability, as well as the percentage of morphologically normal and abnormal sperms, was accessed by the one-step eosin-nigrosin staining technique. Non-stained cells were considered living, whereas dead cells were stained orang-red because the stain could penetrate through the membrane. In addition, sperm morphology was evaluated.
Serum testosterone levels
Blood samples were collected from the posterior vena cava and centrifuge at 2,200 g for 15 min at 4°C. The serum was stored at −35°C for further analyses. Serum testosterone level was measured by an enzyme-linked immunosorbent assay (ELISA) Kit (Testosterone ELISA Kit, Abcam, Cambidge, U.K.) according to the manufacturer’s instructions.
Histological examination
The testes were collected and fixed in 10% buffered formalin. The fixed tissue samples were processed according to the paraffin embedding technique and cut into 5-µm thick sections. The samples were stained with hematoxylin-eosin (HE). The seminiferous tubules and interstitial space were examined under a light microscope to evaluate basal membrane thickening, vascular changes, Leydig cells and spermatogenesis.
Statistical analysis
Data are expressed as the mean ± standard error of mean (SEM). Statistical analyses were performed by one-way ANOVA followed by Tukey post hoc test using R Project Statistical Computing package (R Core Team, 2016) [29]. P-values <0.05 were considered statistically significant.
RESULTS
Effects of VC on body weight, food intake and blood glucose
The STZ-induced diabetic rats showed a low body weight, increased food consumption, and high blood glucose level (Table 1). VC treatment did not prevent weight loss in diabetic rats and did not affect the food consumption. However, high-dose of VC (40 mg/kg) significantly decreased blood glucose level in STZ-induced diabetic rats.
Table 1. Effect of V.cinerea extract on body weight, food intake and blood glucose levels.
| Group | Body weight (g) | Food intake (g/day) | Blood glucose (mg/dl) |
|---|---|---|---|
| NC | 348.83 ± 19.79 a) | 20.39 ± 1.51 a) | 90.5 ± 11.05 a) |
| DMC | 235.2 ± 25.11 b) | 25.71 ± 4.07 b) | 300.5 ± 14.48 b) |
| DM+VC10 | 248.66 ± 33.42 b) | 25.32 ± 2.41 b) | 198.25 ± 11.38 b) |
| DM+VC40 | 290.33 ± 33.74 b) | 25.78 ± 2.71 b) | 114.4 ± 9.55 a) |
Data represent the mean ± standard error of mean (SEM). Different superscript letters indicate significant difference between these groups (P<0.05). NC, normal control rat; DMC, diabetic control rats; DM+VC10, diabetic rats treated with V. cineria (10 mg/kg); DM+VC40, diabetec rats treated with V. cinerea (40 mg/kg).
Effects of VC on epididymal sperm characteristics
Table 2 shows the epididymal sperm characteristics. STZ-induced diabetic rats displayed a significant decrease in sperm count, motility and viability. However, administration of VC (40 mg/kg) improved the sperm count (P<0.05), and VC (10 and 40 mg/kg) significantly increased the sperm motility and decreased sperm abnormalities in diabetic rats (P<0.05).
Table 2. Eipididymal sperms parameters.
| Group | Epididymal sperm parameter |
|||
|---|---|---|---|---|
| Sperm count (million/ml) | Sperm motility (%) | Sperm viabillity (%) | Sperm abnormality (%) | |
| NC | 47.4 ± 0.27 a) | 96 ± 2.66 a) | 92.6 ± 2.12 a) | 0.5 ± 0.39 a) |
| DM | 18.4 ± 0.59 b) | 62.06 ± 1.43 b) | 78.01 ± 4.89 b) | 1.8 ± 0.39 b) |
| DM+VC10 | 19.7 ± 1.35 b) | 71.6 ± 2.20 c) | 77.08 ± 4.77 b) | 1.17 ± 0.33 a) |
| DM+VC40 | 26.78 ± 0.95 c) | 75.6 ± 3.46 c) | 70.6 ± 6.42 b) | 0.9 ± 0.60 a) |
Data represent the mean ± standard error of mean (SEM). Different superscript letters indicate significant difference between these groups (P<0.05). NC, normal control rat; DMC, diabetic control rats; DM+VC10, diabetic rats treated with V. cineria (10 mg/kg); DM+VC40, diabetec rats treated with V. cinerea (40 mg/kg).
Effects of VC on reproductive organ weight and serum testosterone level
The testis and epididymis weight did not differ between the groups (Table 3). The serum testosterone level was significantly lower in the STZ-induced diabetic rats. However, treatment with VC at 10 and 40 mg/kg significantly increased serum testosterone level in diabetic rats (P<0.05; Table 4).
Table 3. Weight of testis and epididymis.
| Group | Organ weight (g) |
|
|---|---|---|
| Testes | Epididymis | |
| NC | 3.82 ± 0.54 | 1.22 ± 0.66 |
| DM | 3.04 ± 1.36 | 0.84 ± 0.37 |
| DM+VC10 | 3.05 ± 1.25 | 0.96 ± 0.39 |
| DM+VC40 | 3.65 ± 1.63 | 1.14 ± 0.52 |
Data represent the mean ± standard error of mean (SEM).
Table 4. Serum testosterone level.
| Groups | Testosterone level (ng/ml) |
|---|---|
| NC | 10.24 ± 2.8 a) |
| DM | 1.27 ± 1.11 b) |
| DM+VC10 | 7.81 ± 3.55 a) |
| DM+VC40 | 7.96 ± 3.24 a) |
Data represent the mean ± standard error of mean (SEM). Different superscript letters indicate significant difference between these groups (P<0.05). NC, normal control rat; DMC, diabetic control rats; DM+VC10, diabetic rats treated with V. cineria (10 mg/kg); DM+VC40, diabetec rats treated with V. cinerea (40 mg/kg).
Effects of VC on testicular histological structure
The control group showed a normal testicular structure with normal seminiferous tubules, interstitial structure and spermatogenesis. However, diabetic groups exhibited degenerated seminiferous tubules with remarkable reduction in germ cells (spermatogonia, spermatocytes, spermatids and Sertoli cells), premature detachment of germ cells and an evident increase in the extracellular matrix in the interstitial areas. Administration of VC to diabetic rats improved the testicular structure and interstitial structure, and increased germinal epithelium and all levels of spermatogenic cells. The control group exhibited Leydig cells with normal morphology. However, there was a decrease in the number of Leydig cells, as well as an eosinophilic congested fluid in the interstitial space of the untreated diabetic rats. After administration of VC, Leydig cells restored their normal structure (Figs. 1 and 2).
Fig. 1.
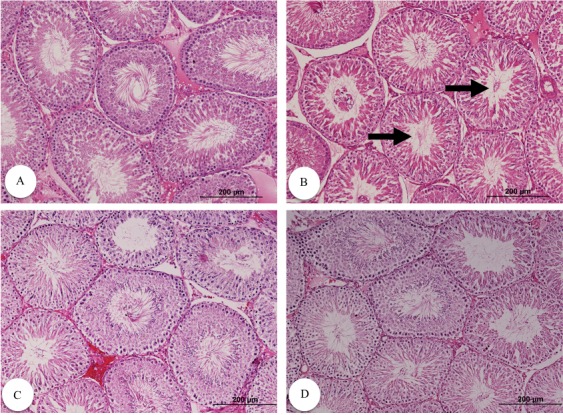
Testicular histological structure different groups stained with H&E (magnification: 100). Normal (A), diabetic (B), diabetic with 10 mg/kg VC (C) and diabetic with 40 mg/kg VC (D). Arrows indicate marked decrease in spermatozoon in the lumen of the tubules.
Fig. 2.
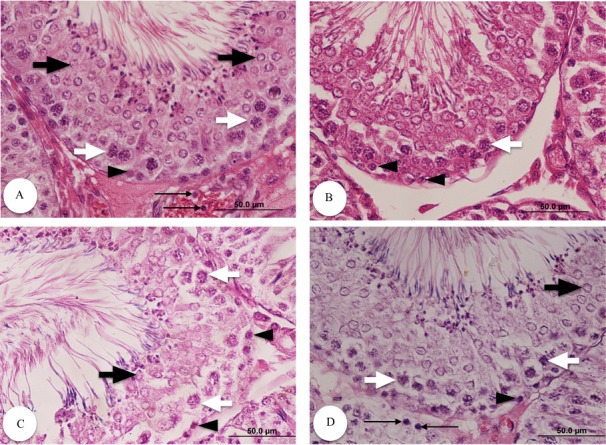
Testicular histological structure of different groups stained with H&E (magnification: 400). Normal (A), diabetic (B), diabetic with 10 mg/kg VC (C) and diabetic with 40 mg/kg VC (D). Sections show spermatogonia (arrowheads), spermatocyte (white arrows), spermatid (black arrows) and Leydig cells (thin arrows).
DISCUSSION
The present study demonstrated that administration of VC (40 mg/kg) to STZ-induced diabetic rats daily for 30 consecutive days improved blood glucose levels. Our results are in agreement with the previous findings, which showed that the leaf extract of VC had antidiabetic activities in alloxan-induced diabetic rats [10] In addition, previous studies showed that the acetone extract of V. colorata leaves exhibited a hypoglycemic and antidiabetic activity in normoglycemic and alloxan-induced diabetic rats, suggesting that flavonoids showed hypoglycemic and antidiabetic activity [38, 39], whereas another study found that the terpenoid, leupeol acetate had an antihyperglycemic activity [38]. A previous study suggested that flavonoids exerted the antidiabetic effects via action on various molecular targets, including regulation of glucose metabolism in hepatocytes, increasing glucose uptake by the skeletal muscle and white adipose tissue [4]. Another study revealed that phytochemical compounds, such as garlic acid and resveratrol, decreased pancreatic β-cell damage in STZ-induced diabetic rats and suggested that the antidiabetic mechanism might invlove stimulation of insulin secretion or release of bound insulin [15]. However, VC supplementation in diabetic rats did not alleviate the body weight and food consumption. Many previous studies reported weight loss in diabetic rats and suggested that diabetes caused a decrease in fatty acid metabolism in the liver [1, 11].
This is the first study to report the effect of VC on the male reproductive function in STZ-induced diabetic rats. Our results confirmed the previous experimental findings that show that the deleterious effects of diabetes on the male reproductive functions involve multiple levels including altered spermatogenesis, degenerative changes of the testes, reduced testosterone synthesis and secretion, altered glucose metabolism, ejaculation dysfunction and decrease libido [8, 13, 19, 23, 35]. Oxidative stress has been considered the primary mechanism responsible for diabetes-induced testicular damage. This may cause peroxidation of fatty acids in the cell membrane resulting in atrophy of Leydig cells, degeneration of spermatogenic cells and diminished number of germ cells [14, 31, 33]. Our experiment showed that diabetic rats exhibited a significantly lower sperm concentration, sperm motility and serum testosterone levels; in addition, the microstructure of the testes showed widened intertubular space and distorted shape of seminiferous tubules. Antioxidants play an important role in preventing the deleterious effects of oxidative stress. Several studies have shown that phytochemical constituents of VC including flavonoids, triterpenoids, sesquiterpene lactones and trace elements exhibit antioxidant and anti-inflammatory properties [2, 14, 16, 30, 33, 36]. Our result showed that VC treatment could improve sperm concentration and motility, increase serum testosterone concentration, decrease the percentage of abnormal spermatozoa and restore the normal testicular structure. Previous studies have reported that some phytochemicals including gallic acid has the potential to protect against oxidative stress in the testes and to reversed sperm abnormalities [25, 34]. Another study showed that selenium had beneficial effects on male infertility, wherein it improved the sperm count and motility, increased serum testosterone levels and alleviated the histopathological changes in the testes [3, 32, 33]. Furthermore, our results are in agreement with those of previous studies, showing that VC treatment in diabetic rats increased sperm concentration and motility, increased serum testosterone levels and improve the testicular microstructure. Leydig cells or interstitial cells are important for testosterone synthesis and secretion. Testosterone is essential for maintenance of the structure and function of the testis and accessory organ as well as spermatogenesis. In the present study, serum testosterone levels decreased in diabetic rats, which concurrently showed significant decrease in sperm count. However, VC administration improved the sperm concentration, possibly owing to the increased testosterone secretion from Leydig cells. In addition, VC treatment in STZ-induced diabetic rats significantly increased testosterone concentration, because it prevented the negative complication of DM on the testicular function.
Our results showed the aphrodisiac properties of VC in STZ-induced diabetic rats. VC exhibited an antidiabetic activity, improved the sperm count and motility, and increased testosterone level. Additional studies are needed to clarify the mechanism underlying the antidiabetic and aphrodisiac properties of the phytochemical compounds in VC including flavonoids, triterpenoids and sesquiterpenes, and to investigate their effects on other complications of DM.
Acknowledgments
We express our appreciation to the Kasetsart University Research and Development Institute for financial support. The authors are grateful to Ondum Paweekorn and Chawanrungroj Chareonpong for assistance with laboratory techniques.
REFERENCES
- 1.Abbasi Z., Tabatabaei S. R., Mazaheri Y., Barati F., Morovvati H.2013. Effects of sesame oil on the reproductive parameters of diabetes mellitus-induced male rats. World J. Mens Health 31: 141–149. doi: 10.5534/wjmh.2013.31.2.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abirami P., Rajendran A.2012. GC-MS analysis of methanol extracts of Vernonia cinerea. Euro. J. Exp. Bio. 2: 9–12. [Google Scholar]
- 3.Ansar S., Abudawood M., Hamed S. S., Aleem M. M.2017. Sodium selenite protects against silver nanoparticle-induced testicular toxicity and inflammation. Biol. Trace Elem. Res. 175: 161–168. [DOI] [PubMed] [Google Scholar]
- 4.Babu P. V., Liu D., Gilbert E. R.2013. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 24: 1777–1789. doi: 10.1016/j.jnutbio.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahmanzadeh M., Vahidinia A., Mehdinejadiani S., Shokri S., Alizadeh Z.2016. Dietary supplementation with astaxanthin may ameliorate sperm parameters and DNA integrity in streptozotocin-induced diabetic rats. Clin. Exp. Reprod. Med. 43: 90–96. doi: 10.5653/cerm.2016.43.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caiaffo V., de Oliveira B. D., de Sá F. B., Neto J. E., da Silva Júnior V. A.2016. Diabetes mellitus, testicular damages and seafood: a curious relationship. Curr. Diabetes Rev. 12:(in press). [DOI] [PubMed] [Google Scholar]
- 7.Chea A., Hout S., Long C., Marcourt L., Faure R., Azas N., Elias R.2006. Antimalarial activity of sesquiterpene lactones from Vernonia cinerea. Chem. Pharm. Bull. (Tokyo) 54: 1437–1439. doi: 10.1248/cpb.54.1437 [DOI] [PubMed] [Google Scholar]
- 8.da Costa C. F., Gobbo M. G., Taboga S. R., Pinto-Fochi M. E., Góes R. M.2016. Melatonin intake since weaning ameliorates steroidogenic function and sperm motility of streptozotocin-induced diabetic rats. Andrology 4: 526–541. doi: 10.1111/andr.12158 [DOI] [PubMed] [Google Scholar]
- 9.Giribabu N., Kumar K. E., Rekha S. S., Muniandy S., Salleh N.2014. Chlorophytum borivilianum (Safed Musli) root extract prevents impairment in characteristics and elevation of oxidative stress in sperm of streptozotocin-induced adult male diabetic Wistar rats. BMC Complement. Altern. Med. 14: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haque Md. A., Abdullah, C.S., Romana, B., Rafique, Md. B., Zia-ul-Juda, G. Md., Hossain, S.F. and Begum, B. 2013. Evaluation of anti-diarrheal and anti-diabetic activities of the stem, barks and leaves of the plants Vernonia cinerea (Family: Asteraceae). J. Appl. Pharm. Sci. 3: 69–72. [Google Scholar]
- 11.Hassan A. A., Hassouna M. M., Taketo T., Gagnon C., Elhilali M. M.1993. The effect of diabetes on sexual behavior and reproductive tract function in male rats. J. Urol. 149: 148–154. [DOI] [PubMed] [Google Scholar]
- 12.Heeba G. H., Hamza A. A.2015. Rosuvastatin ameliorates diabetes-induced reproductive damage via suppression of oxidative stress, inflammatory and apoptotic pathways in male rats. Life Sci. 141: 13–19. doi: 10.1016/j.lfs.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 13.Jangir R. N., Jain G. C.2014. Diabetes mellitus induced impairment of male reproductive functions: a review. Curr. Diabetes Rev. 10: 147–157. doi: 10.2174/1573399810666140606111745 [DOI] [PubMed] [Google Scholar]
- 14.Kara Ö., Sari E., Akşit H., Yay A., Akşit D., Dönmez M. I.2016. Effects of selenium on ischaemia-reperfusion injury in a rat testis model. Andrologia 48: 1267–1273. doi: 10.1111/and.12571 [DOI] [PubMed] [Google Scholar]
- 15.Kaur G., Padiya R., Adela R., Putcha U. K., Reddy G. S., Reddy B. R., Kumar K. P., Chakravarty S., Banerjee S. K.2016. Garlic and resveratrol attenuated diabetic complications, loss of β-cells, pancreatic and hepatic oxidative stress in streptozotocin-induced diabetic rats. Front. Pharmacol. 7: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar P. P., Kuttan G.2009. Vernonia cinerea L. scavenges free radicals and regulates nitric oxide and proinflammatory cytokines profile in carrageenan induced paw edema model. Immunopharmacol. Immunotoxicol. 31: 94–102. doi: 10.1080/08923970802438391 [DOI] [PubMed] [Google Scholar]
- 17.Latha R. M., Geetha T., Varalakshmi P.1998. Effect of Vernonia cinerea Less flower extract in adjuvant-induced arthritis. Gen. Pharmacol. 31: 601–606. doi: 10.1016/S0306-3623(98)00049-4 [DOI] [PubMed] [Google Scholar]
- 18.Leelarungrayub D., Pratanaphon S., Pothongsunun P., Sriboonreung T., Yankai A., Bloomer R. J.2010. Vernonia cinerea Less. supplementation and strenuous exercise reduce smoking rate: relation to oxidative stress status and beta-endorphin release in active smokers. J. Int. Soc. Sports Nutr. 7: 21–30. doi: 10.1186/1550-2783-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C. Y., Hsu Y. J., Chien Y. W. E., Cha T. L., Tsao C. W.2016. Dietary resistant maltodextrin ameliorates testicular function and spermatogenesis in streptozotocin-nicotinamide-induced diabetic rats. Andrologia 48: 363–373. doi: 10.1111/and.12454 [DOI] [PubMed] [Google Scholar]
- 20.Mazumder U. K., Gupta M., Manikandan L., Bhattacharya S., Haldar P. K., Roy S.2003. Evaluation of anti-inflammatory activity of Vernonia cinerea Less. extract in rats. Phytomedicine 10: 185–188. doi: 10.1078/094471103321659915 [DOI] [PubMed] [Google Scholar]
- 21.Merfort I.2011. Perspectives on sesquiterpene lactones in inflammation and cancer. Curr. Drug Targets 12: 1560–1573. doi: 10.2174/138945011798109437 [DOI] [PubMed] [Google Scholar]
- 22.Mishra T. N., Singh R. S., Upadhyay J., Srivastava R.1984. Chemical constituents of Vernonia cinerea. Part I Isolation and spectral studies of triterpenes. J. Nat. Prod. 47: 368–372. doi: 10.1021/np50032a023 [DOI] [Google Scholar]
- 23.Mulholland J., Mallidis C., Agbaje I., McClure N.2011. Male diabetes mellitus and assisted reproduction treatment outcome. Reprod. Biomed. Online 22: 215–219. doi: 10.1016/j.rbmo.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 24.Oliveira P. F., Tomás G. D., Dias T. R., Martins A. D., Rato L., Alves M. G., Silva B. M.2015. White tea consumption restores sperm quality in prediabetic rats preventing testicular oxidative damage. Reprod. Biomed. Online 31: 544–556. doi: 10.1016/j.rbmo.2015.06.021 [DOI] [PubMed] [Google Scholar]
- 25.Olusoji M. J., Oyeyemi O. M., Asenuga E. R., Omobowale T. O., Ajayi O. L., Oyagbemi A. A.2016. Protective effect of Gallic acid on doxorubicin-induced testicular and epididymal toxicity. Andrologia [DOI] [PubMed] [Google Scholar]
- 26.Piryaei A., Najar A., Bayat M.2015. Effects of pentoxifylline administration on histomorphological parameters of streptozotocin-induced diabetic rat testes. Lab. Anim. Res. 31: 111–116. doi: 10.5625/lar.2015.31.3.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasopthum A., Pouyfung P., Sarapusit S., Srisook E., Rongnoparut P.2015. Inhibition effects of Vernonia cinerea active compounds against cytochrome P450 2A6 and human monoamine oxidases, possible targets for reduction of tobacco dependence. Drug Metab. Pharmacokinet. 30: 174–181. doi: 10.1016/j.dmpk.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 28.Pratheeshkumar P., Kuttan G.2012. Modulation of cytotoxic T lymphocyte, natural killer cell, antibody-dependent cellular cytotoxicity, and antibody-dependent complement-mediated cytotoxicity by Vernonia cinerea L. and vernolide-A in BALB/c mice via enhanced production of cytokines IL-2 and IFN-γ. Immunopharmacol. Immunotoxicol. 34: 46–55. doi: 10.3109/08923973.2011.574703 [DOI] [PubMed] [Google Scholar]
- 29.R Core Team2016. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. URL https://www.R-project.org.
- 30.Rajamurugan R., Selvaganabathy N., Kumaravel S., Ramamurthy C., Sujatha V., Suresh Kumar M., Thirunavukkarasu C.2011. Identification, quantification of bioactive constituents, evaluation of antioxidant and in vivo acute toxicity property from the methanol extract of Vernonia cinerea leaf extract. Pharm. Biol. 49: 1311–1320. doi: 10.3109/13880209.2011.604334 [DOI] [PubMed] [Google Scholar]
- 31.Raji Y., Udoh U. S., Mewoyeka O. O., Ononye F. C., Bolarinwa A. F.2003. Implication of reproductive endocrine malfunction in male antifertility efficacy of Azadirachta indica extract in rats. Afr. J. Med. Med. Sci. 32: 159–165. [PubMed] [Google Scholar]
- 32.Ren X. M., Wang G. G., Xu D. Q., Luo K., Liu Y. X., Zhong Y. H., Cai Y. Q.2012. The protection of selenium on cadmium-induced inhibition of spermatogenesis via activating testosterone synthesis in mice. Food Chem. Toxicol. 50: 3521–3529. doi: 10.1016/j.fct.2012.07.021 [DOI] [PubMed] [Google Scholar]
- 33.Rezvanfar M. A., Rezvanfar M. A., Shahverdi A. R., Ahmadi A., Baeeri M., Mohammadirad A., Abdollahi M.2013. Protection of cisplatin-induced spermatotoxicity, DNA damage and chromatin abnormality by selenium nano-particles. Toxicol. Appl. Pharmacol. 266: 356–365. doi: 10.1016/j.taap.2012.11.025 [DOI] [PubMed] [Google Scholar]
- 34.Saygin M., Asci H., Ozmen O., Cankara F. N., Dincoglu D., Ilhan I.2015. Impact of 2.45 GHz microwave radiation on the testicular inflammatory pathway biomarkers in young rats: The role of gallic acid. Environ. Toxicol. 31: 1771–1784. doi: 10.1002/tox.22179 [DOI] [PubMed] [Google Scholar]
- 35.Shrilatha B., Muralidhara Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: its progression and genotoxic consequences. Reprod. Toxicol. 23: 578–587. doi: 10.1016/j.reprotox.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 36.Sonibare M. A., Aremu O. T., Okorie P. N.2016. Antioxidant and antimicrobial activities of solvent fractions of Vernonia cinerea (L.) Less leaf extract. Afr. Health Sci. 16: 629–639. doi: 10.4314/ahs.v16i2.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suhaiman L., Carlos de-Rosas J., Sartor T., Palmada N., Giordano O. S., Lopez L. A.2011. Effect of dehydroleucodine on the reproductive tract of male mice. Andrologia 43: 297–302. doi: 10.1111/j.1439-0272.2010.01053.x [DOI] [PubMed] [Google Scholar]
- 38.Sy G. Y., Nongonierma R. B., Sarr M., Cissé A., Faye B.2004. Antidiabetic activity of the leaves of Vemonia colorata (Wilid.) Drake (Composees) in alloxan-induced diabetic rats. Dakar Méd. 49: 36–39 (in French). [PubMed] [Google Scholar]
- 39.Sy G. Y., Cissé A., Nongonierma R. B., Sarr M., Mbodj N. A., Faye B.2005. Hypoglycaemic and antidiabetic activity of acetonic extract of Vernonia colorata leaves in normoglycaemic and alloxan-induced diabetic rats. J. Ethnopharmacol. 98: 171–175. doi: 10.1016/j.jep.2005.01.024 [DOI] [PubMed] [Google Scholar]
- 40.Toyang N. J., Wabo H. K., Ateh E. N., Davis H., Tane P., Sondengam L. B., Bryant J., Verpoorte R.2013. Cytotoxic sesquiterpene lactones from the leaves of Vernonia guineensis Benth. (Asteraceae). J. Ethnopharmacol. 146: 552–556. doi: 10.1016/j.jep.2013.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ugarte M., Brown M., Hollywood K. A., Cooper G. J., Bishop P. N., Dunn W. B.2012. Metabolomic analysis of rat serum in streptozotocin-induced diabetes and after treatment with oral triethylenetetramine (TETA). Genome Med. 4: 35–50. doi: 10.1186/gm334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walczak-Jedrzejowska R., Wolski J. K., Slowikowska-Hilczer J.2013. The role of oxidative stress and antioxidants in male fertility. Cent. European J. Urol. 66: 60–67. doi: 10.5173/ceju.2013.01.art19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youn U. J., Miklossy G., Chai X., Wongwiwatthananukit S., Toyama O., Songsak T., Turkson J., Chang L. C.2014. Bioactive sesquiterpene lactones and other compounds isolated from Vernonia cinerea. Fitoterapia 93: 194–200. doi: 10.1016/j.fitote.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youn U. J., Park E. J., Kondratyuk T. P., Simmons C. J., Borris R. P., Tanamatayarat P., Wongwiwatthananukit S., Toyama O., Songsak T., Pezzuto J. M., Chang L. C.2012. Anti-inflammatory sesquiterpene lactones from the flower of Vernonia cinerea. Bioorg. Med. Chem. Lett. 22: 5559–5562. doi: 10.1016/j.bmcl.2012.07.010 [DOI] [PubMed] [Google Scholar]


