Abstract
The main factors affecting the outcome of Trueperella pyogenes (T. pyogenes) mastitis were examined through a survey of diagnostic data and interviews relating to the occurrence of T. pyogenes mastitis in 83 quarters from 82 Holstein cows between August 2012 and April 2014. Ultimately, one cow was sold during the examination, and 82 quarters from 81 cows were used for analysis on prognosis. T. pyogenes mastitis occurred year round in both lactating and dry cows. The incidence of T. pyogenes mastitis did not significantly differ by month or show seasonality in either lactating or dry cows. Therefore, the occurrence of T. pyogenes mastitis also differed from that of summer mastitis. The 1-month survival rate of infected cows was 64.6% (53/82), and the recovery rate of quarters with T. pyogenes mastitis was 14.6% (12/82). Bivariate logistic regression analysis was performed with survival and culling of infected cows as objective variables and with recovery and non-recovery of quarters with T. pyogenes mastitis as objective variables. The severe cases were significantly culled (odds ratio, 16.30) compared to mild cases, and the status of quarters didn’t recover (odds ratio, 6.50). The results suggest that mild to moderate symptom severity at the time of onset are the main factors affecting outcomes in cows and recovery of quarters infected with T. pyogenes mastitis. Further, high level of NAGase activity also suggested the potential use as an indicator of culling of cows with T. pyogenes mastitis.
Keywords: bovine mastitis, logistic regression analysis, NAGase activity, severity, Trueperella pyogenes
Trueperella pyogenes (T. pyogenes) was previously classified with the genera Corynebacterium, Actinomyces and Arcanobacterium, but a change in the classification has placed it in the genus Trueperella [23]. T. pyogenes forms pyogenic lesions that cause mastitis in cattle [10] and is one of the main bacterial causes of summer mastitis, along with the anaerobic bacterium Peptococcus indolicus [3, 7, 8, 17, 21]. Summer mastitis occurs in heifers and dry cows and is characterized by malodorous, purulent milk and detection of anaerobic bacteria [3, 7, 8, 17, 19, 21]. In recent large-scale surveys, T. pyogenes has been identified as one of the main pathogenic bacteria causing mastitis in lactating cows [2, 4, 5]. Reported symptoms include high somatic cell count [16], notable reduction in milk yield [5], high rate of mammary gland dysfunction [18] and tendency toward culling [2, 6]. T. pyogenes mastitis causes immense damage to the dairy industry [1], and it is essential to reduce the extent of this damage.
The occurrence and outcome of summer mastitis have been well studied, but there has been little research into T. pyogenes mastitis in lactating cows. An epidemiological feature of summer mastitis is transmission of the pathogenic bacteria by Hydrotaea irritans, an insect closely related to the housefly [3], and this is the reason that the disease onset commonly occurs during the summer [8, 21]. Summer mastitis is also caused by damage to the teat [18]. Hirvonen et al. [9] reported a high recovery rate (40%) of summer mastitis if treatment starts at an early stage, but in general, the recovery rate is considered to be low [18, 22]. However, much is still unknown with regard to the occurrence of T. pyogenes mastitis in cows, as well as recovery and culling.
Proper understanding of the occurrence of T. pyogenes mastitis would enable preventive measures to be established. Understanding which cows recover and which are culled would lead to more efficient treatment and reduced medical expenses. The present study surveyed the occurrence of T. pyogenes mastitis in cows and investigated the main factors affecting the outcome in order to clarify the current status of the disease.
MATERIALS AND METHODS
We conducted a survey of 83 quarters from 82 Holstein cows on 65 farms in Chiba Prefecture and southwestern Ibaraki Prefecture between August 2012 and April 2014. Cows included in the survey had clinical T. pyogenes mastitis. The therapy of T. pyogenes mastitis was performed based on the symptoms by local and systemic therapy with antibiotics in all cases. After the start of examination, one cow was sold during the examination, and the outcome of 1 quarter from the cow was not obtained.
Milk samples
Milk at the onset from 83 quarters from 82 cows diagnosed with T. pyogenes mastitis was classified. Quarter milk samples were collected into sterilized tubes and stored at 4°C after sampling until laboratory measurement.
Examination of milk culture
To identify T. pyogenes, 10 µl of milk was inoculated onto 5% sheep blood agar and cultivated aerobically for 24 hr at 37°C. The colonies thus obtained were pure cultured and identified with BD BBL CRYSTAL GP (Becton, Dickinson and Co., Frankline Lakes, NJ, U.S.A.). At the same time, 10 µl of milk was inoculated onto 5% sheep blood agar and cultivated anaerobically for 24 hr at 37°C. The resulting colonies were checked to ensure no growth under aerobic conditions and identified using BD BBLCRYSTAL ANR (Becton, Dickinson and Co.).
Somatic cell count
Somatic cell count (SCC) in the milk of cows at the time of onset of T. pyogenes mastitis was determined using the DeLaval cell counter (DCC: DeLaval International AB, Tumba, Sweden) based on the method of Kawai et al. [13].
Lactoferrin concentration
Lactoferrin (LF) concentration in the milk of cows at the time of onset of T. pyogenes mastitis was determined using the bovine lactoferrin plate (The Institute for Metabolic Ecosystem, Osaki, Japan).
N-Acetyl-β-D-glucosaminidase activity
N-Acetyl-β-D-glucosaminidase (NAGase) activity in the milk of cows at the time of onset of T. pyogenes mastitis was determined using the β-N-Acetylglucosaminidase Assay Kit (Sigma-Aldrich Co. LLC., St. Louis, MO, U.S.A.). Milk samples were centrifugalized at 3,000 rpm for 10 min at 20°C, and resultant whey was provided to determine. NAGase activity was calculated from difference between absorbance value of whey sample and absorbance value of substrate unreacted same whey sample (back ground control) to avoid effect of whey color.
Overview of affected cattle and occurrence of disease
Data collected included age in days at onset of symptoms, number of days in lactation, onset in summer (July to September; yes/no), body temperature (≥39.5°C/ <39.5°C), tie stall barn (yes/no), dry therapy (yes/no), isolation of anaerobic bacteria (yes/no), severity (mild-moderate/severe), teat trauma (yes/no), mammary irrigation regimen (yes/no), period (lactation/dry), concurrent disease (yes/no) and history of blind quarter (yes/no). Symptom severity (mild: abnormal milk, moderate: abnormal udder and severe: exhibit systemic symptoms) was classified using the method of Roberson [20]. The experiment was done based on the ethical code for animal welfare of the Azabu University.
Interviews
Farmers were interviewed concerning the outcomes of affected cattle and infected quarters approximately 1 month after milk collection. Infected quarters with a sufficient reduction in somatic cell count and forwarding milk were defined as “recovered.” Non-shipment of milk or blind quarter was defined as “non-recovered.”
Statistics
The rate of occurrence of T. pyogenes mastitis by month was analyzed using Fisher’s exact test. Outcomes of diseased cows and recovery of quarters were compared using a test of normality for 5 numerical variables (age, days in mlik, log SCC, log LF and log NAGase), and the differences between the 2 groups were subsequently investigated. For numerical variables that showed normality, analysis of variance was performed using an F test. Student’s t test was performed for items showing equality of variance, and Welch’s t test was used for items showing inequality of variance. The Wilcoxon rank-sum test was performed on numerical variables that did not show normality. A univariate analysis using Fisher’s exact test was used to analyze the relationship between outcome and 11 categorical variables (onset in summer, body temperature, tie stall barn, dry therapy, isolation of anaerobic bacteria, severity, teat trauma, mammary irrigation regimen, period, concurrent disease and history of blind quarter). For items with P<0.10 in the univariate analysis, presence of the item was scored as 1 and absence as 0. Bivariate logistic regression analysis was performed by backward stepwise selection using P values, with recovery of the infected quarter as the objective variable and other items as explanatory variables. Numeric items were also inserted into the model as covariates. The number of days in lactation, which did not have normality, was excluded. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (the R foundation for Statistical Computing, Vienna, Austria). This is a modified version of R commander for the application of statistical functions frequently used in biostatistics [11].
RESULTS
Summary of infected cows and infected quarters
Onset of T. pyogenes mastitis occurred in 40 quarters from 39 cows during dry period and in 43 quarters from 43 cows during lactation period. The outcome for these cows 1 month after onset was survival in 53 cows (64.6%), death in 5 cows (6.1%) and culling in 24 cows (29.3%). The outcome of diseased quarters was recovery in 12 quarters (14.6%) and non-recovery (lactation ceased or milk discarded) in 70 quarters (85.4%), with an unclear outcome in 1 quarter. Among the survived 53 cows, the outcome of diseased quarters was recovery in 11 quarters (20.8%) and non-recovery in 42 quarters (79.2%).
Results of anaerobic culture of milk with T. pyogenes
Porphyromonas endodontalis was detected in 2 samples (2.4%) at March and April, Fusobacterium sp. and Bacteroides vulgatus were each detected in 1 sample (1.2%) at December and November, and the remaining 79 samples (95.2%) were negative for anaerobic bacterial growth (Table 1). All the cows detected anaerobic bacteria were lactating cows.
Table 1. Results of anaerobic culture of mastitic milk from which Trueperella pyogenes was isolated.
| Bacteria | Number | % |
|---|---|---|
| Porphyromonas endodontalis | 2 | 2.4 |
| Fusobacterium sp. | 1 | 1.2 |
| Bacteroides vulgatus | 1 | 1.2 |
| No growth | 79 | 95.2 |
The generation status of cows with T. pyogenes mastitis
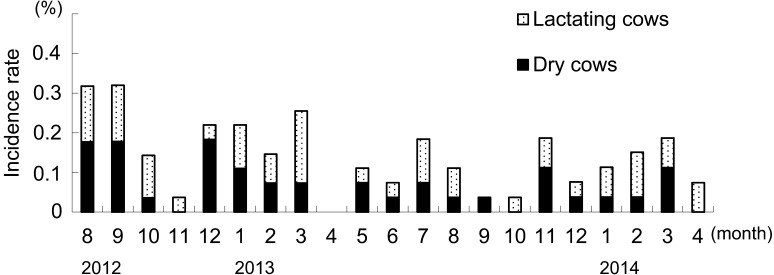
The incidence of cows with T. pyogenes mastitis per month is shown in Fig. 1. T. pyogenes mastitis occurred throughout the year in both lactating and dry cows. The incidence of T. pyogenes mastitis was not significantly different among months, and no seasonality was found. 76.7% (33/43) of cows at dry stage and within 3 days after parturition showed severe symptoms.
Fig. 1.
Incidence rate of cows with T. pyogenes mastitis by month. Incidence rate showed proportion of number of incidence to monthly number of breeding cows. The incidence rate of T. pyogenes mastitis was not significantly different among months, and no seasonality was found.
Comparison of outcome of infected cows (culling/survival) and quarter recovery (no/yes) for numerical and categorical variables
Among mastitic cows, the culled group had significantly higher NAGase activity (P<0.01). For quarter recovery, the recovered group had significantly lower SCC (P<0.05) and significantly higher LF concentration (P<0.05) (Table 2).
Table 2. Comparison of numerical variables between outcome and quarter recovery.
| Variables | Outcome |
Quarter recovery |
||||
|---|---|---|---|---|---|---|
| Survival | Culling | P value | Yes | No | P value | |
| Age (years) | 5.17 ± 2.17 | 5.95 ± 2.27 | 0.128 b) | 5.27 ± 2.28 | 5.47 ± 2.24 | 0.781 b) |
| Days in milk (days) | 62.2 ± 131.0 | 33.9 ± 78.2 | 0.124 a) | 97.5 ± 126.5 | 45.9 ± 113.5 | 0.097 a) |
| logSCC (×104/ml) | 2.95 ± 0.73 | 3.23 ± 0.69 | 0.0874 b) | 2.65 ± 0.58 | 3.12 ± 0.73 | 0.047 b) |
| logLF (μg/ml) | 3.50 ± 0.31 | 3.63 ± 0.36 | 0.121 b) | 3.69 ± 0.15 | 3.53 ± 0.35 | 0.042 c) |
| logNAGase (nmol/ml/min) | 1.37 ± 0.41 | 1.66 ± 0.27 | 0.0005 c) | 1.24 ± 0.45 | 1.50 ± 0.38 | 0.075 b) |
Data were expressed as mean ± SD. a) Wilcoxon rank sum test, b) Student’s t-test, c) Welch’s t-test.
The recovery rate for each item is shown by category in Table 3. Body temperature ≥39.5°C (P<0.05, odds ratio=0.30), severity (severe) (P<0.01, odds ratio=0.09) and presence of concurrent disease (P<0.01, odds ratio=0.20) were significantly associated with survival. For quarter recovery, severity (severe) (P<0.01, odds ratio=0.12) and presence of concurrent disease (P<0.05, odds ratio=0.13) were significantly associated with recovery (Table 3).
Table 3. Univariate analyses of risk factors for outcome and quarter recovery.
| Variables | Category | Outcome |
Quarter recovery |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Survival | Culling | Odds ratio | P value | Yes | No | Odds ratio | P value | ||
| Onset in summer | Yes | 25 | 13 | 1.17 | 0.82 | 5 | 32 | 1.02 | 1 |
| No | 28 | 17 | Reference | 6 | 39 | Reference | |||
| Body temperature | ≥39.5°C | 27 | 23 | 0.30 | 0.03 | 4 | 46 | 0.28 | 0.09 |
| <39.5°C | 24 | 6 | Reference | 7 | 22 | Reference | |||
| Tie stall barn | Yes | 45 | 23 | 1.70 | 0.38 | 9 | 59 | 0.92 | 1 |
| No | 8 | 7 | Reference | 2 | 12 | Reference | |||
| Dry therapy | Yes | 39 | 23 | 0.85 | 0.80 | 8 | 53 | 0.91 | 1 |
| No | 14 | 7 | Reference | 3 | 18 | Reference | |||
| Isolation of anaerobic bacteria | Yes | 2 | 2 | 0.55 | 0.62 | 0 | 4 | 0.00 | 1 |
| No | 51 | 28 | Reference | 11 | 67 | Reference | |||
| Severity | Severe | 28 | 28 | 0.09 | <0.001 | 3 | 53 | 0.12 | <0.01 |
| Mild-moderate | 24 | 2 | Reference | 8 | 17 | Reference | |||
| Teat trauma | Yes | 7 | 6 | 0.72 | 0.76 | 0 | 13 | 0.00 | 0.34 |
| No | 39 | 24 | Reference | 8 | 54 | Reference | |||
| Mammary irrigation regimen | Yes | 10 | 7 | 0.73 | 0.58 | 2 | 15 | 0.77 | 1 |
| No | 41 | 21 | Reference | 9 | 52 | Reference | |||
| Period | Lactation | 31 | 12 | 2.09 | 0.12 | 8 | 35 | 2.71 | 0.20 |
| Dry | 22 | 18 | Reference | 3 | 36 | Reference | |||
| Concurrent disease | Yes | 13 | 19 | 0.20 | <0.001 | 1 | 31 | 0.13 | <0.05 |
| No | 39 | 11 | Reference | 10 | 39 | Reference | |||
| History of blind quarter | Yes | 8 | 4 | 1.15 | 1 | 2 | 10 | 1.35 | 0.66 |
| No | 45 | 26 | Reference | 9 | 61 | Reference | |||
Bivariate logistic regression analysis of main factors relating to outcome of infected cows and infected quarter recovery
Five items that were log SCC, log NAGase, body temperature (≥39.5°C/ <39.5°C), severity (mild-moderate/severe) and concurrent disease (yes/no) were selected for bivariate logistic regression analysis with outcome (culling/survival). Seven items that were days in milk, log SCC, log LF, log NAGase, body temperature (≥39.5°C/ <39.5°C), severity (mild-moderate/severe) and concurrent disease (yes/no) were selected for bivariate logistic regression analysis with quarter recovery (no/yes). In each case, backward stepwise selection was performed using P values as selection criteria. Severity (mild-moderate/severe) was selected as an independent predictor associated with outcome (culling/survival) (P=0.009, odds ratio=16.30) and quarter recovery (no/yes) (P=0.021, odds ratio=6.50), respectively (Tables 4 and 5).
Table 4. Bivariate logistic regression model for culling within a month.
| Variable | Category | Odds ratio | 95% confidence interval |
P value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Severity | Mild/moderate | Reference | |||
| Severe | 16.30 | 2.00 | 133.00 | 0.009 | |
Table 5. Bivariate logistic regression model for marketable milk loss of quarter within a month.
| Variable | Category | Odds ratio | 95% confidence interval |
P value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Severity | Mild/moderate | Reference | |||
| Severe | 6.50 | 1.32 | 31.90 | 0.021 | |
DISCUSSION
The present study indicates that cows with severe mastitis symptoms or severe general condition at the time of onset of T. pyogenes mastitis tend to be culled without recovery of the infected quarter. The occurrence of T. pyogenes mastitis also differed from that of summer mastitis.
Although 6.1% (5/82) of cows with T. pyogenes mastitis died within 1 month, ultimately 29.3% (24/82) were culled, and the recovery rate of diseased quarters was only 14.6% (12/82). It is known that SCC increases at the onset of mastitis and LF is released from activated neutrophils in the inflammatory response [12, 15]. Further, NAGase activity increases by the relation to tissue damage of mammary gland [14]. When comparing the outcomes of cows with T. pyogenes mastitis and quarter recovery, mastitic cows that were culled had significantly higher NAGase activity values, whereas recovered quarters had significantly lower SCC and significantly higher LF concentration at the time of onset. This suggests that recovered quarters were in cows at an early stage of inflammation, when SCC is comparatively low and little damage has been done to the mammary tissue, in which LF carries out migration functions. It also suggests that NAGase activity is high in cows in which infection has progressed far enough to cull the cow and the mammary gland has become considerably damaged. When comparing the outcomes of diseased cows and quarter recovery, the fact that severity was significantly associated with quarter recovery suggested a relationship with disease progress. It would appear that the tendency of lactating cows with T. pyogenes mastitis to be culled [2, 6] is greatly influenced by the decrease in milk yield resulting from the non-recovery of the infected quarters.
Cows with high NAGase activity values in their milk when mastitis was found already had advanced tissue damage. In these cases, the infection must be discovered even earlier. However, T. pyogenes mastitis often occurs in dry cows or immediately after parturition [18]. The infection may progress and cause considerable damage to the mammary tissue, because it is difficult to discover abnormalities in milk during the early stages of infection.
In the bivariate logistic regression analyses, the model for culling of infected cows and non-recovery of infected quarters used severity (severe) as the explanatory variable, respectively. The results show that infected cows with severe clinical symptom at the time of onset are 16.30 times more likely to be culled and 6.50 times more likely to not recover of quarter than those with mild-moderate clinical symptom. Therefore, recovery from T. pyogenes mastitis is more difficult for cows with advanced infection. The results also suggest the possibility that NAGase activity could be used to determine whether infected cows are likely to recover. Hirvonen et al. [9] were unable to determine an absolute threshold level of NAGase activity above which heifer quarters with summer mastitis do not recover, but the present results suggested that high level of NAGase activity showed the potential use as an indicator of culling of cows with T. pyogenes mastitis in cows. T. pyogenes is able to colonize many different host tissues and cause a diverse range of diseases. However, most aspects of the pathogenesis of infections caused by this important opportunistic pathogen remain poorly characterized [10]. The pathogenesis of summer mastitis involves transmission of pathogenic bacteria by H. irritans, a species closely related to the housefly [3]. High rates of detection of anaerobic bacteria, such as P. indolicus and Fusobacterium necrophorum (along with T. pyogenes), have also been reported [8, 18, 21]. In a survey of mastitis among heifers in Hokkaido, the disease occurred from May to October, with the greatest incidence in August. Anaerobic bacteria, such as P. indolicus, were found in 75.7% (56/74) of cases [21]. In the present survey of T. pyogenes mastitis in cows, disease occurrence was year round in both lactating and dry cows, and seasonality (higher incidence in summer) was not found. In the anaerobic culture of mastitic milk, Porphyromonas endodontalis was isolated from 2 samples (2.4%), Fusobacterium sp. and Bacteroides vulgatus were each isolated from 1 sample (1.2%), and 79 samples (95.2%) tested negative for anaerobic bacteria growth. Successful infection of T. pyogenes alone is reported to be common in lactating and dry cows, but occurrence of clinical mastitis to be rare in lactating cows [7]. Also, according to Hirvonen et al. [9], anaerobic bacteria are associated with an increase in symptom severity in summer mastitis, but detection of anaerobic bacteria falls markedly at 96 hr post-inoculation of T. pyogenes, P. indolicus and F. necrophorum. In the present study, all cases were clinical mastitis, and many of the infected cows had a high degree of severity and did not respond successfully to treatment. The low detection of anaerobic bacteria could have been caused by a drop in anaerobic bacterial growth in many cows, because a considerable time had passed since the infection of causative bacteria. However, this point needs further elucidation as it seems likely that the transmission, route of infection and course of disease of T. pyogenes mastitis in cows differ from those of summer mastitis.
REFERENCES
- 1.Blowey R., Edmondson P.2010. Summer Mastitis. pp. 215–219. In: Mastitis Control in Dairy Herds, 2nd ed., CAB International, Oxfordshire. [Google Scholar]
- 2.Cha E., Hertl J. A., Schukken Y. H., Tauer L. W., Welcome F. L., Gröhn Y. T.2013. The effect of repeated episodes of bacteria-specific clinical mastitis on mortality and culling in Holstein dairy cows. J. Dairy Sci. 96: 4993–5007. doi: 10.3168/jds.2012-6232 [DOI] [PubMed] [Google Scholar]
- 3.Chirico J., Jonsson P., Kjellberg S., Thomas G.1997. Summer mastitis experimentally induced by Hydrotaea irritans exposed to bacteria. Med. Vet. Entomol. 11: 187–192. doi: 10.1111/j.1365-2915.1997.tb00312.x [DOI] [PubMed] [Google Scholar]
- 4.Ericsson Unnerstad H., Lindberg A., Persson Waller K., Ekman T., Artursson K., Nilsson-Ost M., Bengtsson B.2009. Microbial aetiology of acute clinical mastitis and agent-specific risk factors. Vet. Microbiol. 137: 90–97. doi: 10.1016/j.vetmic.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 5.Gröhn Y. T., Wilson D. J., González R. N., Hertl J. A., Schulte H., Bennett G., Schukken Y. H.2004. Effect of pathogen-specific clinical mastitis on milk yield in dairy cows. J. Dairy Sci. 87: 3358–3374. doi: 10.3168/jds.S0022-0302(04)73472-4 [DOI] [PubMed] [Google Scholar]
- 6.Gröhn Y. T., González R. N., Wilson D. J., Hertl J. A., Bennett G., Schulte H., Schukken Y. H.2005. Effect of pathogen-specific clinical mastitis on herd life in two New York State dairy herds. Prev. Vet. Med. 71: 105–125. doi: 10.1016/j.prevetmed.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 7.Hillerton J. E., Bramley A. J.1989. Infection following challenge of the lactating and dry udder of dairy cows with Actinomyces pyogenes and Peptostreptococcus indolicus. Br. Vet. J. 145: 148–158. doi: 10.1016/0007-1935(89)90097-3 [DOI] [PubMed] [Google Scholar]
- 8.Hillerton J. E., Bramley A. J., Watson C. A.1987. The epidemiology of summer mastitis: a survey of clinical cases. Br. Vet. J. 143: 520–530. doi: 10.1016/0007-1935(87)90041-8 [DOI] [PubMed] [Google Scholar]
- 9.Hirvonen J., Pyörälä S., Heinäsuo A., Jousimies-Somer H.1994. Penicillin G and penicillin G-tinidazole treatment of experimentally induced summer mastitis--effect on elimination rates of bacteria and outcome of the disease. Vet. Microbiol. 42: 307–315. doi: 10.1016/0378-1135(94)90062-0 [DOI] [PubMed] [Google Scholar]
- 10.Jost B. H., Billington S. J.2005. Arcanobacterium pyogenes: molecular pathogenesis of an animal opportunist. Antonie van Leeuwenhoek 88: 87–102. doi: 10.1007/s10482-005-2316-5 [DOI] [PubMed] [Google Scholar]
- 11.Kanda Y.2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48: 452–458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai K., Hagiwara S., Anri A., Nagahata H.1999. Lactoferrin concentration in milk of bovine clinical mastitis. Vet. Res. Commun. 23: 391–398. doi: 10.1023/A:1006347423426 [DOI] [PubMed] [Google Scholar]
- 13.Kawai K., Hayashi T., Kiku Y., Chiba T., Nagahata H., Higuchi H., Obayashi T., Itoh S., Onda K., Arai S., Sato R., Oshida T.2013. Reliability in somatic cell count measurement of clinical mastitis milk using DeLaval cell counter. Anim. Sci. J. 84: 805–807. doi: 10.1111/asj.12136 [DOI] [PubMed] [Google Scholar]
- 14.Kitchen B. J., Middleton G., Durward I. G., Andrews R. J., Salmon M. C.1980. Mastitis diagnostic tests to estimate mammary gland epithelial cell damage. J. Dairy Sci. 63: 978–983. doi: 10.3168/jds.S0022-0302(80)83035-9 [DOI] [PubMed] [Google Scholar]
- 15.Lash J. A., Coates T. D., Lafuze J., Baehner R. L., Boxer L. A.1983. Plasma lactoferrin reflects granulocyte activation in vivo. Blood 61: 885–888. [PubMed] [Google Scholar]
- 16.Malinowski E., Lassa H., Kłlossowska A., Smulski S., Markiewicz H., Kaczmarowski M.2006. Etiological agents of dairy cows’ mastitis in western part of Poland. Pol. J. Vet. Sci. 9: 191–194. [PubMed] [Google Scholar]
- 17.Packer R. A.1977. Bovine mastitis produced by corynebacteria. J. Am. Vet. Med. Assoc. 170: 1164–1165. [PubMed] [Google Scholar]
- 18.Pyörälä S., Jousimies-Somer H., Mero M.1992. Clinical, bacteriological and therapeutic aspects of bovine mastitis caused by aerobic and anaerobic pathogens. Br. Vet. J. 148: 54–62. doi: 10.1016/0007-1935(92)90067-B [DOI] [PubMed] [Google Scholar]
- 19.Quinn A. K., Vermunt J. J., Twiss D. P.2002. Arcanobacterium pyogenes mastitis in a 18-month-old heifer. N. Z. Vet. J. 50: 167–168. doi: 10.1080/00480169.2002.36305 [DOI] [PubMed] [Google Scholar]
- 20.Roberson J. R.2012. Treatment of clinical mastitis. Vet. Clin. North Am. Food Anim. Pract. 28: 271–288. doi: 10.1016/j.cvfa.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 21.Seno N., Azuma R.1983. A study on heifer mastitis in Japan and its causative microorganisms. Natl. Inst. Anim. Health Q. (Tokyo) 23: 82–91. [PubMed] [Google Scholar]
- 22.Waage S., Skei H. R., Rise J., Rogdo T., Sviland S., Odegaard S. A.2000. Outcome of clinical mastitis in dairy heifers assessed by reexamination of cases one month after treatment. J. Dairy Sci. 83: 70–76. doi: 10.3168/jds.S0022-0302(00)74857-0 [DOI] [PubMed] [Google Scholar]
- 23.Yassin A. F., Hupfer H., Siering C., Schumann P.2011. Comparative chemotaxonomic and phylogenetic studies on the genus Arcanobacterium Collins et al. 1982 emend. Lehnen et al. 2006: proposal for Trueperella gen. nov. and emended description of the genus Arcanobacterium. Int. J. Syst. Evol. Microbiol. 61: 1265–1274. doi: 10.1099/ijs.0.020032-0 [DOI] [PubMed] [Google Scholar]