Abstract
Japan established a vaccine selection system, in which a committee evaluates veterinary influenza vaccines to determine if the vaccine should be updated. In 2013, it was concluded that the present equine influenza vaccine strains did not have to be updated, but clade 2 (Fc2) viruses of the Florida sublineage should be included. We collected three Fc2 viruses as candidates and conducted comparative tests. Results indicated that A/equine/Carlow/2011 (H3N8) is not suitable, because of its unstable antigenic characteristics. A comparison between A/equine/Richmond/1/2007 (H3N8) (Richmond/07) and A/equine/Yokohama/aq13/2010 (H3N8) (Yokohama/10) in eggs showed that they shared equal growth properties. Immunogenicity test in mice showed that Yokohama/10 induced higher HI antibody titers than Richmond/07. Therefore, we concluded that Yokohama/10 was the most suitable strain.
Keywords: efficacy, equine influenza, Florida sublineage clade 2 virus, veterinary vaccine
Equine influenza (EI) is a highly contagious respiratory disease accompanied by fever, harsh coughing and depression [7]. EI outbreaks have been reported worldwide with the exception of a few island countries, including New Zealand and Iceland [13]. After a hiatus of 36 years in Japan, there was a devastating outbreak of EI in 2007 [6, 16], which resulted in the cancellation of horse racings and other events. Hence, outbreaks of EI negatively impact horse racings and other events, import/export of horses as well as other activities, resulting in severe economic losses to the horse industry.
Vaccination is effective in the prevention and control of EI. The primary aim of vaccination is to reduce clinical manifestations and virus shedding. However, antigenic differences between vaccine strains and field viruses affect the EI vaccine efficacy [3, 4, 8]. Because the period from establishment of vaccine strains to registration with the regulatory authority requires several years and involves a significant cost, many vaccine manufacturers hesitate to update vaccine strains. As a result, the majority of current EI vaccines worldwide contain outdated strains [1, 2]. To rapidly update vaccine strains for veterinary influenza vaccine, the Ministry of Agriculture, Forestry and Fisheries, Japan (MAFF) established an influenza vaccine selection system [5]. In this system, MAFF establishes the vaccine strains and distributes them to market authorization holders (MAHs). Therefore, the MAHs are no longer required to develop new vaccine strains; prepare documents regarding the efficacy, safety and quality of the vaccine; or apply for registration. The MAHs only purchase the designated vaccine strains from MAFF. This system reduces the period of time required from development to marketing to approximately 1 year. To determine whether influenza vaccine strains should be updated and to select the most appropriate vaccine strains, the National Veterinary Assay Laboratory of the MAFF established a vaccine strains selection committee for veterinary influenza (selection committee), which consists of experts of influenza and biological products [5].
The World Organisation for Animal Health (OIE) Expert Surveillance Panel on Equine Influenza Vaccine Composition (OIE ESP) recommended that EI vaccines for the international market should contain both clade 1 (Fc1) and clade 2 (Fc2) viruses of the Florida sublineage [10,11,12]. The current Japanese inactivated EI vaccine, which does not include adjuvant, consists of A/equine/Avesta/1993 (H3N8) (Avesta/93, Eurasian lineage), A/equine/La Plata/1993 (H3N8) (La Plata/93, American Lineage) and A/equine/Ibaraki/1/2007 (H3N8) (Ibaraki/07, Fc1) [16]. Although the EI vaccine does not include Fc2, one vaccine strain, La Plata/93, shares similar antigenic characteristics with A/equine/Richmond/1/2007 (H3N8) (Richmond/07, Fc2) [17]. Therefore, the selection committee meeting held in 2011 and 2012 concluded that the current vaccine strains need not be updated at those times [5]. However, in the selection committee meeting held in 2013, it was reported that the antigenic characteristics of the current major prevalent Fc2 viruses isolated in the U.K. and Ireland had recently changed [18]. These field viruses showed lower reactivity to antiserum against La Plata/93 compared with previous Fc2 viruses, as demonstrated with virus neutralization test. The selection committee concluded that the current Japanese EI vaccine is still effective against Fc2 viruses and does not have to be updated rapidly [4]. However, the selection committee also suggested that considering the recent OIE ESP recommendation and that some countries, such as Australia, require that exporting countries should vaccinate horses with the most up-to-date EI vaccine prior to shipment (http://www.jlta.or.jp/requirements/horse/h_e_australia.pdf), Fc2 virus should be prepared for the next updated vaccine [5].
In this study, we collected three Fc2 viruses as candidate vaccine strains, conducted comparative tests, such as growth properties in embryonated chicken eggs, stability of antigenic characteristics by serial passage in embryonated chicken eggs and immunogenicity in mice in two vaccine manufacturers, and selected the most appropriate Fc2 virus as the vaccine strain.
Growth properties in embryonated chicken eggs were evaluated as follows, each candidate vaccine strain was serially diluted 10-fold, and 0.2 ml of each serial dilutions (101–1010) were inoculated into five allantoic cavities of 11-day-old, specific pathogen free (SPF), embryonated chicken eggs. After incubation at 34°C for 48 hr, the allantoic fluids were collected and examined for hemagglutination (HA) activity. The 50% egg infectious dose (EID50)/0.2 ml were calculated using the Reed and Muench method [15]. The allantoic fluids of the concentrations from 10-fold up to 1,000-fold of all eggs that tested HA -positive were mixed at equal volumes and passaged.
Stability of antigenic characteristics by serial passages in embryonated chicken eggs was evaluated as follows. Each candidate vaccine strain was inoculated into the allantoic cavities of 11-day-old SPF embryonated chicken eggs and incubated at 34°C for 48 hr. The each obtained allantoic fluid was inactivated by treatment with 0.05% formalin at 4°C for 7 days and was immunized into 30 ddY mice of 4-week-old via the intraperitoneal route. Two weeks after immunization, all mice were bled, and obtained sera were pooled. Using the sera, hemagglutination inhibition (HI) test was conducted with the first, fifth and tenth egg-passaged each virus, which was obtained as described in section of growth properties in embryonated chicken eggs. HI test was performed as previously described with some modifications using 96-well V-bottomed microplates [14]. In brief, 0.2 ml of the test serum was mixed with 0.6 ml of receptor-destroying enzyme (Denka Seiken, Tokyo, Japan) and incubated overnight at 37°C to remove nonspecific inhibitors. This step was followed by heat inactivation in a water bath at 56°C for 1 hr and cooled in ice water. Then, 0.05 ml of RBC suspension was added; the mixture was incubated at room temperature for 1 hr and centrifuged. The supernatants were used as 4-fold-diluted serum. A 25 µl aliquot of the serum was diluted 2-fold with phosphate-buffered saline and mixed with 25 µl of HA antigen adjusted to 8 HA units in 96-well V-bottomed plate, and the plate was incubated for 1 hr at 37°C. Then, 50 µl of 0.5% of chicken RBC suspension was added to each well, and the plate was incubated for 1 hr. HI antibody titers were expressed as the highest dilution of the serum showing complete HI.
Immunogenicity of candidate vaccine strains in mice was conducted according to the potency test for inactivated EI vaccine, as described in the “Minimum Requirements for Veterinary Biological Products” (MAFF Notice No. 1567, Series of 2002) with some modifications. In brief, formalin-inactivated sixth egg-passaged virus culture was adjusted to 100 and 200 chicken cell agglutination (CCA)/ml. Four groups of five ddY mice were immunized with each CCA concentration via the intraperitoneal route. Two weeks after immunization, all mice were bled. The obtained sera were pooled for each group, and the HI titers were measured. The criteria demand that at least two of four groups must have an HI titer against homologous strain ≥1:8. Differences of HI titer of each CCA content among the strains were evaluated by Bonferroni’s method. The level of P values at <0.016 was considered significant in this study.
These animal experiments were conducted by Nisseiken Co., Ltd. (Nisseiken, Tokyo, Japan) and The Chemo-Sero-Therapeutic Research Institute (Kaketsuken, Kumamoto, Japan) and approved by the animal experimentation ethical committees (approved number: 13seizou-016 (Nisseiken), A13-150 (Kaketsuken)).
Three Fc2 viruses were selected as candidate vaccine strains; namely, Richmond/07, which is recommended by OIE ESP as a vaccine strain [10,11,12] and provided by the Animal Health Trust (Kentford, U.K.), A/equine/Carlow/2011 (H3N8) (Carlow/11) [18], which was selected because of showing different antigenic characteristics compared with previous isolated strains and was provided by the Irish Equine Center (Kildare, Ireland), and A/equine/Yokohama/aq13/2010 (H3N8)(Yokohama/10) [9], which was isolated in the Animal Quarantine Station, MAFF (Yokohama, Japan) and was selected, because it was the most recent Fc2 isolate available in Japan.
CCA test was performed according as described by Miller & Stanley [8] in the “Minimum Requirements for Veterinary Biological Products” (MAFF Notice No. 1567, Series of 2002). In brief, test samples were diluted 2-fold, added by the same volume of RBC suspension, mixed well and incubated at 25°C for 75 min. Optical density (OD) of the mixture was measured at 540 nm. CCA value was calculated by plugging the OD value in Miller-Stanley’s monogram.
As shown in Table 1, results of the vaccine manufacturers demonstrated that all candidate vaccine strains had stable growth properties and seemed to adjust to embryonated chicken eggs by serial passage, as shown by higher titers with successive passages. All the strains showed similar growth properties.
Table 1. Growth properties of candidate vaccine strains in embryonated chicken eggs.
Table 2 showed the results of stability of antigenic characteristics by passages in embryonated chicken eggs. Results obtained from vaccine manufacturer B showed that both Richmond/07 and Yokohama/10 had stable HI antibody titers without variation until 10 passages (Table 2b). However, because HI antibody titer decreased by 4-fold, antigenic characteristics of Carlow/11 seemed to be unstable compared with the other strains (Table 2b). On the other hand, results obtained from vaccine manufacturer A showed that all the candidate vaccine strains had stable HI antibody titers with variation of less than 4-fold until 10 passages, indicating stable antigenic characteristics of all candidate viruses in embryonated chicken eggs (Table 2a). In this experiment, the candidate vaccine strains included in allantoic fluid were immunized into mice to obtain antiserum. Ingredients from embryonated chicken eggs might have played a role as adjuvant and might not have made clear the differences of antigenic characteristics.
Table 2. Stability of antigenic characteristics by passages in embryonated chicken eggs.
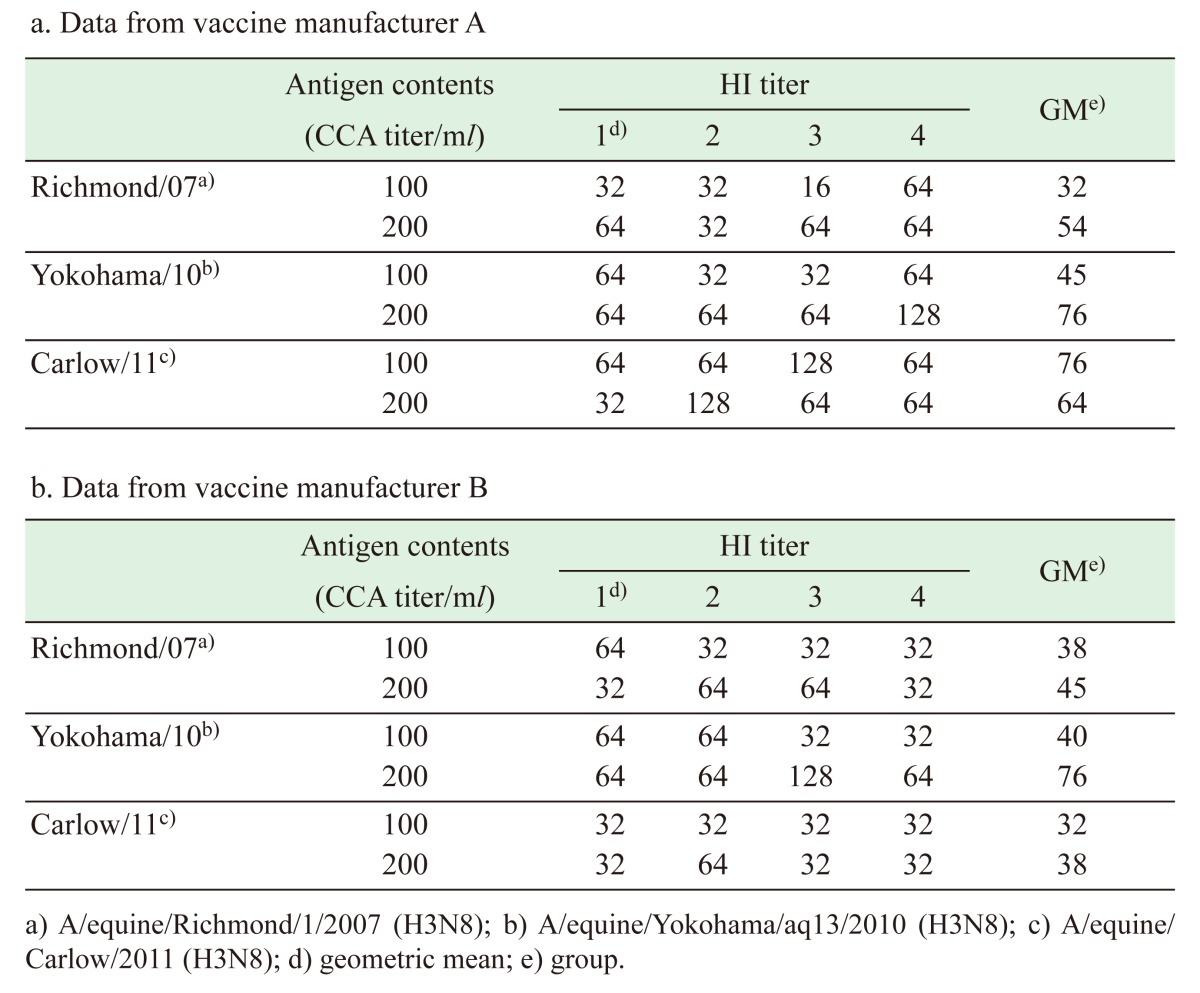
As shown in Table 3, HI antibody titers in mouse serum after immunization with the antigen contents (100 and 200 CCA/ml) of each candidate vaccine strain showed dose-dependent increases and met the criterion of HI titer ≥1:8. There were not any significant differences among the strains with each vaccine manufacturer’s datum (Bonferroni’s method; P values <0.016). Results obtained from vaccine manufacturer A showed that Carlow/11 induced the highest HI antibody titer, followed by Yokohama/10 and then Richmond/07 for 100 CCA/ml immunization and that Yokohama/10 induced the highest HI antibody titer, followed by Carlow/11 and then Richmond/07 for 200 CCA/ml immunization (Table 3a); whereas, results obtained from vaccine manufacturer B showed that Yokohama/10 induced the highest HI antibody titer, followed by Richmond/07 and then Carlow/11 (Table 3b).
Table 3. Immunogenicity of candidate vaccine strains in mice.
From the results of comparative tests, it was demonstrated that there were not significant differences of antigenic properties among the candidate vaccine strains. Comparing differences among the strains, the stability of antigenic characteristics by serial passages in embryonated chicken eggs showed that Carlow/11 antigenically seemed to be less stable than the other strains, which might come from that Carlow/11 is a recently isolated variant and thus may not be suitable as a vaccine strain. The growth properties of Richmond/07 and Yokohama/10 in embryonated chicken eggs had almost equal EID50, CCA and HA titers in the fifth to eighth passage, which are commonly used as the vaccine seed. Immunogenicity analysis of candidate vaccine strains in mice showed a tendency that Yokohama/10 induces higher HI antibody titers than does Richmond/07. Overall, we concluded that Yokohama/10 was the most suitable Fc2 virus as the updated vaccine strain.
In this study, we assessed immunogenicity and stability of candidate vaccine strains in mice. Although we did not use horses to assess them, Yamanaka et al. reported immunogenicity of candidate vaccine strains in horses, cross virus neutralization test and cross HI test [18, 19]. It showed that Yokohama/10 and Carlow/11 had almost equal immunogenicity in horses and that candidate vaccine strains had similar antigenic reactivity, which agreed with our data in mice (some data not shown).
In 2014, the selection committee designated Yokohama/10 to be included in the updated Japanese inactivated EI vaccine. As a result, the selection committee added a vaccine composition that included Yokohama/10 and Ibaraki/07 (Fc1), in accordance with the 2014 OIE ESP recommendations [4, 12].
Acknowledgments
We thank Dr. Debra Elton, Animal Health Trust (Kenford, U.K.), and Dr. Ann Cullinane, Irish Equine Center (Kildare, Ireland), for providing us with viruses, and Dr. Takashi Yamanaka, Japan Racing Association, Nisseiken and Kaketsuken, for the cooperation with this study.
REFERENCES
- 1.Cooke G.2013. Vaccination for equine influenza: the sports regulator’s viewpoint. Equine Vet. J. 45: 770–771. doi: 10.1111/evj.12151 [DOI] [PubMed] [Google Scholar]
- 2.Cullinane A., Elton D., Mumford J.2010. Equine influenza - surveillance and control. Influenza Other Respi. Viruses 4: 339–344. doi: 10.1111/j.1750-2659.2010.00176.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daly J. M., Yates R. J., Browse G., Swann Z., Newton J. R., Jessett D., Davis-Poynter N., Mumford J. A.2003. Comparison of hamster and pony challenge models for evaluation of effect of antigenic drift on cross protection afforded by equine influenza vaccines. Equine Vet. J. 35: 458–462. doi: 10.2746/042516403775600433 [DOI] [PubMed] [Google Scholar]
- 4.Daly J. M., Yates P. J., Newton J. R., Park A., Henley W., Wood J. L., Davis-Poynter N., Mumford J. A.2004. Evidence supporting the inclusion of strains from each of the two co-circulating lineages of H3N8 equine influenza virus in vaccines. Vaccine 22: 4101–4109. doi: 10.1016/j.vaccine.2004.02.048 [DOI] [PubMed] [Google Scholar]
- 5.Gamoh K., Nakamura S.2015. Introduction of an update system for vaccine strains of veterinary influenza vaccines in Japan. Biologicals 43: 150–152. doi: 10.1016/j.biologicals.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 6.Ito M., Nagai M., Hayakawa Y., Komae H., Murakami N., Yotsuya S., Asakura S., Sakoda Y., Kida H.2008. Genetic analyses of an H3N8 influenza isolate, causative strain of the outbreak of equine influenza at the Kanazawa racecourse in Japan in 2007. J. Vet. Med. Sci. 70: 899–906. doi: 10.1292/jvms.70.899 [DOI] [PubMed] [Google Scholar]
- 7.Landolt G. A.2014. Equine influenza virus. Vet. Clin. North Am. Equine Pract. 30: 507–522. doi: 10.1016/j.cveq.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 8.Miller G. L., Stanley W. M.1944. Quantitative aspects of the red blood cell aggulutination test for influenza virus. J. Exp. Med. 79: 185–195. doi: 10.1084/jem.79.2.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motoshima M., Okamatsu M., Asakura S., Kuribayashi S., Sengee S., Batchuluun D., Ito M., Maeda Y., Eto M., Sakoda Y., Sodnomdarjaa R., Kida H.2011. Antigenic and genetic analysis of H3N8 influenza viruses isolated from horses in Japan and Mongolia, and imported from Canada and Belgium during 2007−2010. Arch. Virol. 156: 1379–1385. doi: 10.1007/s00705-011-1000-5 [DOI] [PubMed] [Google Scholar]
- 10. O. I. E. Bulletin. Expert surveillance panel of equine influenza vaccine composition. OIE headquarters, Paris; 2012: February 27, 2012, pp. 46–47. [Google Scholar]
- 11. O. I. E. Bulletin. Expert surveillance panel of equine influenza vaccine composition. OIE headquarters, Paris; 2013: March 4, 2013, pp. 44–45. [Google Scholar]
- 12. O. I. E. Bulletin. Equine influenza vaccine composition. OIE headquarters, Paris; 2014: March 4, 2014, pp. 77–78. [Google Scholar]
- 13. O. I. E. Terrestrial Manual. 2015. Chapter 2.5.7. Equine Influenza, p.1. [Google Scholar]
- 14.Park A. W., Wood J. L., Daly J. M., Newton J. R., Glass K., Henley W., Mumford J. A., Grenfell B. T.2004. The effects of strain heterology on the epidemiology of equine influenza in a vaccinated population. Proc. Biol. Sci. 271: 1547–1555. doi: 10.1098/rspb.2004.2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed L. J., Muench H.1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27: 493–497. [Google Scholar]
- 16.Yamanaka T., Niwa H., Tsujimura K., Kondo T., Matsumura T.2008. Epidemic of equine influenza among vaccinated racehorses in Japan in 2007. J. Vet. Med. Sci. 70: 623–625. doi: 10.1292/jvms.70.623 [DOI] [PubMed] [Google Scholar]
- 17.Yamanaka T., Bannai H., Nemoto M., Tsujimura K., Kondo T., Matsumura T.2011. Antibody responses induced by Japanese whole inactivated vaccines against equine influenza virus (H3N8) belonging to Florida sublineage clade2. J. Vet. Med. Sci. 73: 483–485. doi: 10.1292/jvms.10-0408 [DOI] [PubMed] [Google Scholar]
- 18.Yamanaka T., Cullinane A., Gildea S., Bannai H., Nemoto M., Tsujimura K., Kondo T., Matsumura T.2015. The potential impact of a single amino-acid substitution on the efficacy of equine influenza vaccines. Equine Vet. J. 47: 456–462. doi: 10.1111/evj.12290 [DOI] [PubMed] [Google Scholar]
- 19.Yamanaka T., Nemoto M., Bannai H., Tsujimura K., Kondo T., Matsumura T., Gildea S., Cullinane A.2016. Assessment of antigenic difference of equine influenza virus strains by challenge study in horses. Influenza Other Respi. Viruses 10: 536–539. doi: 10.1111/irv.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]