Abstract
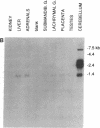
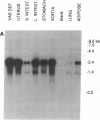
Genomic and cDNA clones, encoding a protein that is a member of the guanine nucleotide-binding regulatory protein (G protein)-coupled receptor superfamily, were isolated by screening rat genomic and thoracic aorta cDNA libraries with an oligonucleotide encoding a highly conserved region of the M1 muscarinic acetylcholine receptor. Sequence analyses of these clones showed that they encode a 343-amino acid protein (named RTA). The RTA gene is single copy, as demonstrated by restriction mapping and Southern blotting of genomic clones and rat genomic DNA. Sequence analysis of the genomic clone further showed that the RTA gene has an intron interrupting the region encoding the amino terminus of the protein. RTA RNA sequences are relatively abundant throughout the gut, vas deferens, uterus, and aorta but are only barely detectable (on Northern blots) in liver, kidney, lung, and salivary gland. In the rat brain, RTA sequences are markedly abundant in the cerebellum. RTA is most closely related to the mas oncogene (34% identity), which has been suggested to be a forebrain angiotensin receptor. We cannot detect angiotensin binding to the RTA protein after introducing the cognate cDNA or mRNA into COS cells or Xenopus oocytes, respectively, nor can we detect an electrophysiologic response in the oocyte after application of angiotensin peptides. We conclude that RTA is not an angiotensin receptor; to date, we have been unable to identify its ligand.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Young A. C., Brann M. R., Buckley N. J. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988 Jul;1(5):403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Burnham C. E., Hawelu-Johnson C. L., Frank B. M., Lynch K. R. Molecular cloning of rat renin cDNA and its gene. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5605–5609. doi: 10.1073/pnas.84.16.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cotecchia S., Schwinn D. A., Randall R. R., Lefkowitz R. J., Caron M. G., Kobilka B. K. Molecular cloning and expression of the cDNA for the hamster alpha 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D., Cifuentes F., Huidobro-Toro J. P., Vío C. P., Inestrosa N. C. Synthesis and expression of functional angiotensin II receptors in Xenopus oocytes injected with rat brain mRNA. Brain Res. 1987 Sep;388(3):268–270. doi: 10.1016/0169-328x(87)90034-9. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Bouvier M., Benovic J. L., Caron M. G., Lefkowitz R. J. The multiple membrane spanning topography of the beta 2-adrenergic receptor. Localization of the sites of binding, glycosylation, and regulatory phosphorylation by limited proteolysis. J Biol Chem. 1987 Oct 15;262(29):14282–14288. [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Lohse M. J., Kobilka B. K., Caron M. G., Lefkowitz R. J. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988 Sep 22;335(6188):358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Frielle T., Collins S., Daniel K. W., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hayward S. B., Stroud R. M. Projected structure of purple membrane determined to 3.7 A resolution by low temperature electron microscopy. J Mol Biol. 1981 Sep 25;151(3):491–517. doi: 10.1016/0022-2836(81)90007-3. [DOI] [PubMed] [Google Scholar]
- Healy D. P., Maciejewski A. R., Printz M. P. Localization of central angiotensin II receptors with [125I]-sar1, ile8-angiotensin II: periventricular sites of the anterior third ventricle. Neuroendocrinology. 1986;44(1):15–21. doi: 10.1159/000124615. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Blair L. A., Marshall J., Goedert M., Hanley M. R. The mas oncogene encodes an angiotensin receptor. Nature. 1988 Sep 29;335(6189):437–440. doi: 10.1038/335437a0. [DOI] [PubMed] [Google Scholar]
- Julius D., MacDermott A. B., Axel R., Jessell T. M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988 Jul 29;241(4865):558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Frielle T., Collins S., Yang-Feng T., Kobilka T. S., Francke U., Lefkowitz R. J., Caron M. G. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature. 1987 Sep 3;329(6134):75–79. doi: 10.1038/329075a0. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Matsui H., Kobilka T. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J., Regan J. W. Cloning, sequencing, and expression of the gene coding for the human platelet alpha 2-adrenergic receptor. Science. 1987 Oct 30;238(4827):650–656. doi: 10.1126/science.2823383. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Loosfelt H., Misrahi M., Atger M., Salesse R., Vu Hai-Luu Thi M. T., Jolivet A., Guiochon-Mantel A., Sar S., Jallal B., Garnier J. Cloning and sequencing of porcine LH-hCG receptor cDNA: variants lacking transmembrane domain. Science. 1989 Aug 4;245(4917):525–528. doi: 10.1126/science.2502844. [DOI] [PubMed] [Google Scholar]
- Lynch K. R., Simnad V. I., Ben-Ari E. T., Garrison J. C. Localization of preangiotensinogen messenger RNA sequences in the rat brain. Hypertension. 1986 Jun;8(6):540–543. doi: 10.1161/01.hyp.8.6.540. [DOI] [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- McFarland K. C., Sprengel R., Phillips H. S., Köhler M., Rosemblit N., Nikolics K., Segaloff D. L., Seeburg P. H. Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science. 1989 Aug 4;245(4917):494–499. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- Mendelsohn F. A., Quirion R., Saavedra J. M., Aguilera G., Catt K. J. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1575–1579. doi: 10.1073/pnas.81.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W., Morley S., Schwarz J., Richter D. Receptors for neuropeptides are induced by exogenous poly(A)+ RNA in oocytes from Xenopus laevis. Proc Natl Acad Sci U S A. 1988 Feb;85(3):714–717. doi: 10.1073/pnas.85.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Winslow J. W., Peterson G. L., Smith D. H., Ashkenazi A., Ramachandran J., Schimerlik M. I., Capon D. J. Primary structure and biochemical properties of an M2 muscarinic receptor. Science. 1987 May 1;236(4801):600–605. doi: 10.1126/science.3107123. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Bach A. W., Wozny M., Taleb O., Dal Toso R., Shih J. C., Seeburg P. H. Structure and functional expression of cloned rat serotonin 5HT-2 receptor. EMBO J. 1988 Dec 20;7(13):4135–4140. doi: 10.1002/j.1460-2075.1988.tb03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J. W., Kobilka T. S., Yang-Feng T. L., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning and expression of a human kidney cDNA for an alpha 2-adrenergic receptor subtype. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6301–6305. doi: 10.1073/pnas.85.17.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein R. C., Wong S. K., Ross E. M. The hydrophobic tryptic core of the beta-adrenergic receptor retains Gs regulatory activity in response to agonists and thiols. J Biol Chem. 1987 Dec 5;262(34):16655–16662. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Candelore M. R., Rands E., Hill W. S., Dixon R. A. Conserved aspartic acid residues 79 and 113 of the beta-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988 Jul 25;263(21):10267–10271. [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Register R. B., Candelore M. R., Rands E., Dixon R. A. Identification of residues required for ligand binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4384–4388. doi: 10.1073/pnas.84.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y., Sasai Y., Tanaka K., Fujiwara T., Tsuchida K., Shigemoto R., Kakizuka A., Ohkubo H., Nakanishi S. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989 Oct 25;264(30):17649–17652. [PubMed] [Google Scholar]
- Young D., O'Neill K., Jessell T., Wigler M. Characterization of the rat mas oncogene and its high-level expression in the hippocampus and cerebral cortex of rat brain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5339–5342. doi: 10.1073/pnas.85.14.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Waitches G., Birchmeier C., Fasano O., Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986 Jun 6;45(5):711–719. doi: 10.1016/0092-8674(86)90785-3. [DOI] [PubMed] [Google Scholar]