Abstract
Considerable evidence supports a contributory role for leukocyte-type 12/15 Lipoxygenase (L-12/15 LO) in mediating hippocampal and cortical neuronal injury in models of Alzheimer’s disease and stroke. Whether L-12/15 LO contributes to neuronal injury in a model of Huntington’s disease (HD) has yet to be determined. HD is characterized by marked striatal neuronal loss, which can be mimicked in humans and animals by inhibition of mitochondrial complex II using 3-Nitropropionic acid (3-NP). Herein, we compared histological and behavioral outcomes between mice that were wild-type or null for L-12/15 LO following systemic injection of 3NP. We found that mice deficient in L-12/15 LO had a higher incidence of striatal lesions coincident with an increase in morbidity as compared to their wild-type littermate controls. This could not be explained by differential metabolism of 3-NP as striatal succinate dehydrogenase activity was inhibited to the same extent in both genotypes. The present results show that deleting L-12/15 LO is detrimental to the striatum in the setting of chronic, systemic 3-NP exposure and are consistent with the overall conclusion that region-specific effects may determine the ultimate outcome of L-12/15 LO activation in the setting of brain injury.
Keywords: Huntington’s disease, oxidative stress, 3-nitropropionic acid, striatal injury, L-12/15- lipoxygenase
1. Introduction
Lipoxygenases (LOs) catalyze the oxidation of free and esterified phospholipid fatty acids generating bioactive lipid mediators and reactive oxygen species (ROS) [13, 42]. The leukocyte-type 12/15 LO (L-12/15 LO) is expressed throughout brain parenchyma in both neurons and glial cells of the cerebral cortex, basal ganglia, and hippocampus [33]. Physiologically, L-12/15 LO metabolites modulate several neuronal ion channel conductances [9, 29, 35, 47]. Pathophysiologically, L-12/15 LO activation is linked to neuronal cell death in animal models of both cerebral ischemia and Alzheimer’s disease [13, 22, 37, 46, 51] and in various in vitro models of oxidative stress [22, 26, 27, 32, 38, 54].
Although the exact mechanism(s) by which L-12/15 LO facilitates neuronal injury remain(s) to be fully elucidated, it is known to oxidatively modify lipoproteins and phospholipids [24, 44, 53] and to damage mitochondria [45]. Mitochondrial dysfunction is considered to be a common mediator of many acute and chronic neurological diseases, though it most closely and directly links to the pathophysiology of Huntington’s disease (HD), an adult-onset autosomal-dominant inherited neurodegenerative disease caused by a mutation in the short arm of human chromosome 4p16.3 [14, 28, 30]. Whether L-12/15 LO activation contributes to striatal damage in HD models has yet to be explored. 3-nitropropionic acid (3-NP) —a phyto/fungal toxin — irreversibly inhibits the electron transport enzyme succinate dehydrogenase (SDH) and produces striatal lesions and cognitive and motor dysfunction in rodents and primates that closely resemble that of human HD [5, 7, 34]. Thus, it was used herein to compare histological and behavioral outcomes of mice wild-type or null for alox15, the gene that encodes for the protein L-12/15 LO.
2. Materials and methods
2.1 Animal husbandry
Wild-type and mutant mice for study (male, 15–18 wks) were derived from F1 heterozygous (+/−) breeding units obtained by crossing Alox15 −/− (JAX, # 002778) male mice with wild-type C57BL/6J (+/+) female mice (JAX, #000664). F2 and F3 generations were used. Genotyping was performed via PCR analysis of tail genomic DNA samples: WT primers (266 bp) 5′-CGT GGT TGA AGA CTC TCA AGG -3′ (forward), 5′-CGA AAT CGC TGG TCT ACA GG -3′ (reverse); mutant primers (280 bp) 5′-CTT GGG TGG AGA GGC TAT TC -3′ (forward), 5′-AGG TGA GAT TAC AGG AGA TC -3′ (reverse). Mice were housed three to five per cage on a 12 hr light/dark schedule in an AALAC-accredited Animal Care facility. Mouse chow and water were provided ad libitum. Housing/breeding strategies were employed to control for genetic and environmental influences [49, 50].
2.2 3-nitropropionic acid (3-NP) Dosing
3-NP (Sigma) was dissolved in 0.9% saline at 25 mg/mL, adjusted to pH 7.4 using 5N NaOH, filter sterilized, and stored at 4°C for up to one week. Mice —who had been acclimated to handling for 5 days prior — received a total systemic dose of 780 mg/kg i.p. 3-NP (b.i.d 8–12 h intervals) given over nine days using an escalating dosing protocol as follows: 20 mg/kg × 2d, 30 mg/kg × 2d, 50 mg/kg × 1d, and 60 mg/kg × 4d. Mice completing the study were sacrificed ~12 hr after the final injection.
2.3 Behavioral Scoring
The severity scale for 3-NP-induced motor disorders was performed as described [15]. Hindlimb clasping, general locomotor activity, hindlimb dystonia, truncal dystonia and postural adjustment reflexes were assessed twice per day just prior to each injection by an observer blinded to genotype. Three scales were assigned corresponding to no abnormality, moderate or severe deficits. Any mouse attaining a cumulative behavioral score ≥ 9 or sustaining a weight loss ≥ 20% was immediately sacrificed. Five separate experiments were performed over 6 months.
2.4 Histology
Frozen brain sections (20 μm) cut serially through the rostro-caudal extent of each brain (+1.54 to −0.22 relative to bregma) were stained with 0.5% thionin essentially as described [12]. Images (1200 pixels) were captured by scanning (Epson Perfection 3170). The lesion area — denoted by pale thionin staining — as well as the total striatal area was measured using NIH ImageJ at three levels from bregma (+1.10, +0.86 and +0.50) by three individuals blind to the mouse’s genotype. For each level, the percent striatal damage was calculated as follows: (L/T) × 100, where L and T represent the lesioned area and total striatal area, respectively. Data are expressed as the mean lesion volume + SEM of all three levels derived from the mean calculated for all individuals.
2.5 Succinate dehydrogenase (SDH) assay
SDH activity was determined in crude brain mitochondrial preparations two hr following injection with either saline or 3-NP (200 mg/kg, i.p.) exactly as described [41].
2.6 Statistical analysis
Statistical analyses were performed using GraphPad Prism, Version 6.03 as described in each figure legend. Percent data were transformed (arcsin square root) prior to analysis. Weight data were analyzed via two-way repeated measures ANOVA. Behavioral data were analyzed by two-way repeated measures ANOVA following log transformation of the raw data [Y = log (Y+1)].
3. Results
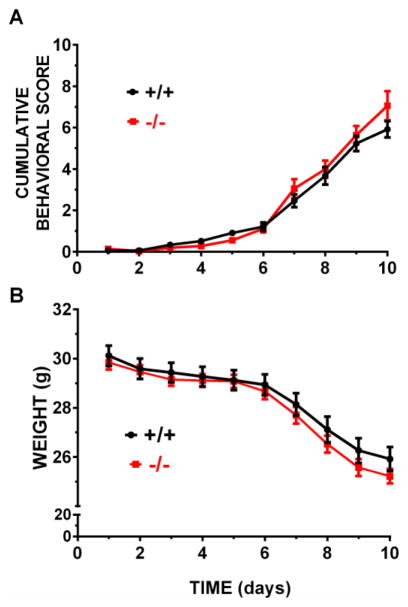
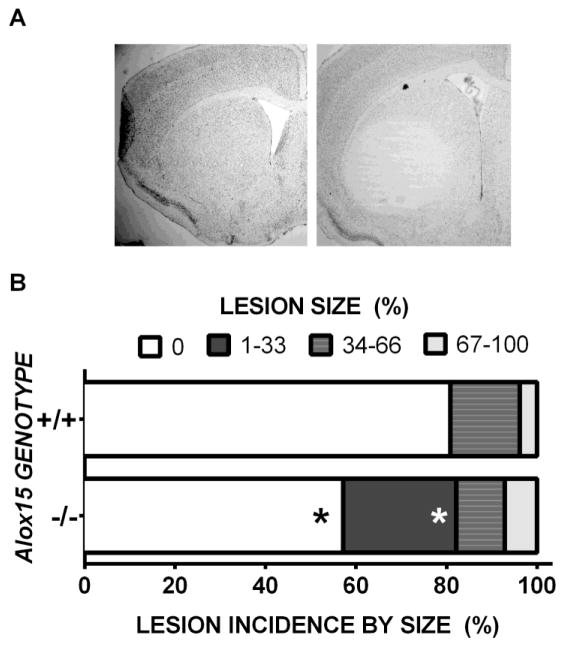
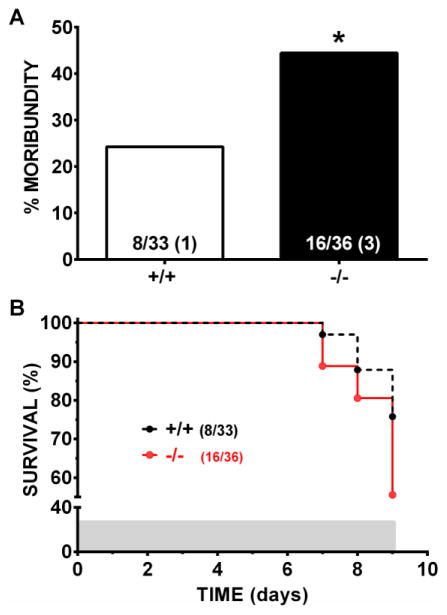
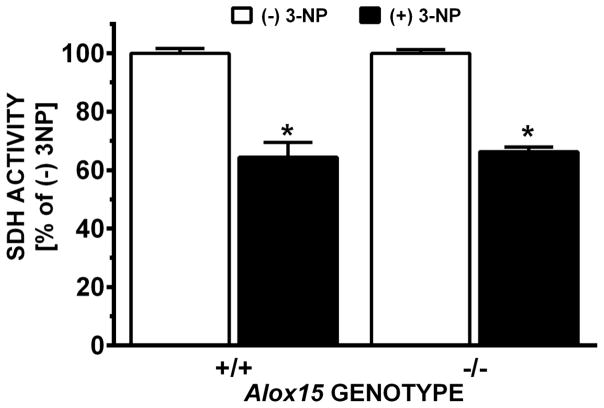
Alox15 +/+ and Alox15 −/− were used to examine how loss of function of L-12/15 LO would affect 3-NP-induced motor system impairment and striatal toxicity. After the first four injections of 3-NP, mice did not display any visible neurological deficit symptoms (Figure 1). Motor symptoms increased progressively thereafter in both genotypes similarly with increased days of dosing with 3-NP (Fig. 1A, p < 0.0001 for day and p = 0.827 for genotype) as did the expected reduction in gross body weight (Fig. 1B, p < 0.0001 for day and p = 0.469 for genotype). Despite this, not all mice, regardless of genotype, developed histologically-identifiable lesions (Fig. 2). However, the incidence of lesions between genotypes did differ (Fig. 2B). Only 19% of Alox15+/+ mice showed 3-NP induced striatal neurodegeneration, whereas the incidence of lesions in Alox15 −/− mice more than doubled to 43% (Fig. 2B, p=0.031). This increase was dominated by a high proportion of smaller lesions with a mean size of 20 ± 4% of the total striatal area (Fig. 2B, p = 0.006). Coincident with this was a genotype-dependent increase in morbundity with 44.4% of the Alox15 −/− attaining a score of ≥ 9 compared to just 24.2% of Alox15 +/+ littermate controls (Fig. 3A, p = 0.039). However, the rate at which the mice in each genotypic group were removed —graphed as % survival — did not differ (Fig. 3B, p=0.086). To rule out the possibility that this underlying sensitivity to 3-NP was due to genotypic differences striatal SDH inhibition, we quantified striatal SDH activity after an acute systemic injection of 3-NP (200mg/kg, i.p.). SDH activity in Alox15+/+ and Alox15−/− was equally reduced (64.4% vs 66.4%, respectively) (Fig. 4).
Figure 1. Comparision of behavioral motor deficits and weight loss between wild-type and L-12/15 LO deficient mice.
L-12/15 LO null mutant mice (−/−, n = 36) and their wild-type littermates (+/+, n = 33) were injected twice daily with 3-NP as described in methods. (A) Behavioral motor scores were recorded just prior to each 3-NP injection. The 2nd score for each animal was used to generate the graph (mean ± SEM). (B) Each mouse was weighed daily prior to the first injection and the collective mass reported as mean ± SEM. There is no statistically significant difference between genotypes in either behavior or weight as assessed by two-way ANOVA.
Figure 2. Comparison of lesion incidence between wild-type and L-12/15 LO deficient mice.
(A) Representative photomicrographs depicting striatal outcomes of 3-NP exposure in wild-type (left) and L-12/15 LO null mutant mice (right) at +0.50 from the bregma. (B) Comparison of lesion incidence between +/+ (n=26) and −/− (n=28) mice. The graph depicts the percent of mice from each genotype in 4 defined groups based on lesion size (0, 1–33%, 34–66% and 67–100%) determined by dividing the number in each group by the total number of mice analyzed. The black asterisk (*) denotes a significant difference in overall lesion incidence (p = 0.031), whereas a white asterisk depicts a between-group size difference (p = 0.006), both determined by Chi-squared test. Sample sizes differ from those described in Fig. 1 as some slices were lost during procurement and/or staining. Mice found dead were also excluded from analysis.
Figure 3. Toxicity of 3-NP is more severe in L-12/15 LO null mutant mice.
(A) The number of L-12/15 LO +/+ and −/− mice determined to be moribund are expressed as the percent of the total number of mice subjected to the systemic injection paradigm (fraction within bars). Moribund is defined as any mouse found dead (number shown in parentheses within the bars) or mice sacrificed due to excessive weight lost (>20%) or severe motor behavioral deficits (score ≥ 9). (*) significantly different from +/+ group (p = 0.039, Chi-squared test). (B) A Kaplan–Meier survival curve depicts the rate at which the mice were removed from study. The shaded region represents the 9-day dosing paradigm. No genotypic differences in rate of removal was determined by Mantel-COX log-rank test.
Figure 4. Striatal SDH activity after acute 3-NP injection.
L-12/15 LO null mutant mice (−/−,) and their wild type littermates (+/+) were injected with 200 mg/kg 3-NP or saline (n = 3 each). Two hr later, SDH activities were measured as described in methods. Data were normalized to each genotype’s control group (− 3-NP) and expressed as mean + SEM. Significant between-group differences (*;+/− 3-NP treatment), but no within-group (i.e., genotype) differences were found by two-way ANOVA followed by Bonferroni’s t test.
Discussion
Herein, we demonstrate that mice deficient in L-12/15 LO are more sensitive to 3-NP-induced toxicity although a substantial individual variability in striatal lesion size in response to 3-NP in both genotypes was observed. This variability is not atypical; several studies demonstrate similar results [5, 6, 8]. Despite this, behavioral outcomes did not differ between genotypes. A caveat to this analysis is that mice null for the Alox15 gene demonstrated a 100% increase in behavioral morbidity when compared to their littermate controls, necessitating removal from the study. Additionally, we did not observe any differences in 3-NP-induced weight loss with nearly identical numbers of mice from each genotype [n = 6 (+/+) and 7 (−/−)] being removed for excessive weight loss, a phenomenon that also occurs in HD patients [4]. These findings support the idea that this is a consequence of inhibition of peripheral tissue mitochondrial function [3, 18].
Our findings of enhanced striatal sensitivity are in contrast with what has been previously demonstrated in models of stroke and Alzheimer’s disease. Increased L-12/15 LO immunostaining was demonstrated in the peri-infarct area of mice subjected to experimental ischemia [46] as well as in post-mortem brain sections from human stroke patients [53]. The finding that Alox15 −/− mice or Alox15 +/+ mice treated with a novel selective inhibitor of L-12/15-LO had less cerebral ischemic damage when compared to untreated Alox15 +/+ mice [53] indicated that this increase contributes to pathology. L-12/15 LO immunoreactivity was also increased in post-mortem AD brains when compared with age-matched controls, in a manner that correlated with the extent of lipid peroxidation [37, 52]. Genetic deletion of L-12/15 LO in the Tg2576 model of AD resulted in a significant reduction in the production and deposition of Aβ in the hippocampus that correlated with improvement of cognitive deficits [51], whereas genetic over-expression of L-12/15 LO in this same mouse strain, led to increased brain Aβ levels and worsened memory impairments [11]. Finally, 3xTg mice receiving systemic administration of a selective inhibitor of L-12/15 LO showed significant reductions in Aβ deposition, phosphorylation of tau, and improvements of memory over those receiving placebo [10]. Together, these data have led to the suggestion that inhibition of L-12/15 LO may be a viable therapeutic strategy for the treatment of stroke [53] and AD [21].
Important differences in the function of the enzyme and/or the susceptibility of the different brain areas studied might explain the differing results found in our study. For instance, the stroke and AD studies focus on cortex and hippocampus, respectively, whereas herein the focus in on the striatum. Given that systemic injection of 3-NP causes similar inhibition of hippocampal, cortical and striatal SDH levels (our unpublished observations), a brain area specific mechanism seems plausible. Disparate cellular effects of L-12/15 LO also have been reported. Whereas its enzymatic products might act as pro-inflammatory factors in some settings [37, 51–53], studies also point to its role in suppressing inflammation via production of a class of eicosanoids called lipoxins [19, 39, 40]. Thus,. loss of L-12/15 LO result in unmitigated inflammation following 3-NP treatment [1, 2]. Enhanced inflammatory gene expression, including increased peripheral expression of interleukin-1β, in L-12/15 LO deficient mice has been reported [23]. We have shown that IL-1β potentiates neuronal cell death under conditions of energy deprivation [16, 17, 20]. Should a similar result occur in brain, it would likely be deleterious. Finally, loss of L-12/15 LO could result in the build-up of arachidonic acid — which itself can be deleterious to brain [31, 48] — or could result in enhanced production of other, perhaps toxic, eicosanoids (e.g., cyclooxygenase [COX] or 5-Lipoxygenase [5-LO] derived metabolites) via substrate diversion [36, 43]. Of note, administration of licofelon, a dual inhibitor of COX/5-LO, significantly reduced 3-NP-induced HD-like symptoms in rats [25], suggesting either one or both enzymes are involved in 3-NP-induced neurotoxicity.
Conclusion
In brain, L-12/15 LO can be either beneficial or detrimental. In striatum, our data suggest that L-12/15 LO signaling may be part of an adaptive response that protects against insults resulting from altered bioenergetics or primary mitochondrial dysfunction. More broadly, present results are consistent with the conclusion that brain region-specific effects may determine the ultimate outcome of L-12/15 LO activation.
Highlights.
Increased incidence of 3-NP-mediated striatal lesions in mice deficient in L-12/15 lipoxygenase (L-12/15 LO) as compared to wild-type mice.
L-12/15 LO deficient mice show enhanced morbidity to systemic 3-NP treatment as compared to their wild-type littermate controls.
L-12/15 LO is beneficial in the setting of chronic, systemic 3-NP.
Acknowledgments
This study was supported by NIH/NINDS R01 NS036812-16.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahuja M, Bishnoi M, Chopra K. Protective effect of minocycline, a semi-synthetic second-generation tetracycline against 3-nitropropionic acid (3-NP)-induced neurotoxicity. Toxicology. 2008;244:111–122. doi: 10.1016/j.tox.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja M, Chopra K, Bishnoi M. Inflammatory and neurochemical changes associated with 3-nitropropionic Acid neurotoxicity. Toxicology mechanisms and methods. 2008;18:335–339. doi: 10.1080/15376510701563738. [DOI] [PubMed] [Google Scholar]
- 3.Alston TA, Mela L, Bright HJ. 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proc Natl Acad Sci U S A. 1977;74:3767–3771. doi: 10.1073/pnas.74.9.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aziz NA, van der Burg JM, Landwehrmeyer GB, Brundin P, Stijnen T, Group ES, Roos RA. Weight loss in Huntington disease increases with higher CAG repeat number. Neurology. 2008;71:1506–1513. doi: 10.1212/01.wnl.0000334276.09729.0e. [DOI] [PubMed] [Google Scholar]
- 5.Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13:4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binienda Z, Virmani A, Przybyla-Zawislak B, Schmued L. Neuroprotective effect of L-carnitine in the 3-nitropropionic acid (3-NPA)-evoked neurotoxicity in rats. Neuroscience letters. 2004;367:264–267. doi: 10.1016/j.neulet.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Brouillet E, Jacquard C, Bizat N, Blum D. 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. Journal of Neurochemistry. 2005;95:1521–1540. doi: 10.1111/j.1471-4159.2005.03515.x. [DOI] [PubMed] [Google Scholar]
- 8.Brouillet E, Jenkins BG, Hyman BT, Ferrante RJ, Kowall NW, Srivastava R, Roy DS, Rosen BR, Beal MF. Age-dependent vulnerability of the striatum to the mitochondrial toxin 3-nitropropionic acid. J Neurochem. 1993;60:356–359. doi: 10.1111/j.1471-4159.1993.tb05859.x. [DOI] [PubMed] [Google Scholar]
- 9.Buttner N, Siegelbaum SA, Volterra A. Direct modulation of Aplysia S-K+ channels by a 12-lipoxygenase metabolite of arachidonic acid. Nature. 1989;342:553. doi: 10.1038/342553a0. [DOI] [PubMed] [Google Scholar]
- 10.Chu J, Li JG, Giannopoulos PF, Blass BE, Childers W, Abou-Gharbia M, Pratico D. Pharmacologic blockade of 12/15-lipoxygenase ameliorates memory deficits, Abeta and tau neuropathology in the triple-transgenic mice. Molecular psychiatry. 2015;20:1329–1338. doi: 10.1038/mp.2014.170. [DOI] [PubMed] [Google Scholar]
- 11.Chu J, Zhuo JM, Pratico D. Transcriptional regulation of beta-secretase-1 by 12/15-lipoxygenase results in enhanced amyloidogenesis and cognitive impairments. Ann Neurol. 2012;71:57–67. doi: 10.1002/ana.22625. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Claycomb RJ, Hewett SJ, Hewett JA. Prophylactic, prandial rofecoxib treatment lacks efficacy against acute PTZ-induced seizure generation and kindling acquisition. Epilepsia. 2011;52:273–283. doi: 10.1111/j.1528-1167.2010.02889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cyrus T, Praticò D, Zhao L, Witztum JL, Rader DJ, Rokach J, FitzGerald GA, Funk CD. Absence of 12/15-Lipoxygenase Expression Decreases Lipid Peroxidation and Atherogenesis in Apolipoprotein E–Deficient Mice. Circulation. 2001;103:2277–2282. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- 14.Damiano M, Diguet E, Malgorn C, D’Aurelio M, Galvan L, Petit F, Benhaim L, Guillermier M, Houitte D, Dufour N, Hantraye P, Canals JM, Alberch J, Delzescaux T, Deglon N, Beal MF, Brouillet E. A role of mitochondrial complex II defects in genetic models of Huntington’s disease expressing N-terminal fragments of mutant huntingtin. Human molecular genetics. 2013;22:3869–3882. doi: 10.1093/hmg/ddt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernagut PO, Diguet E, Stefanova N, Biran M, Wenning GK, Canioni P, Bioulac B, Tison F. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57Bl/6 mice: behavioural and histopathological characterisation. Neuroscience. 2002;114:1005–1017. doi: 10.1016/s0306-4522(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 16.Fogal B, Hewett JA, Hewett SJ. Interleukin-1beta potentiates neuronal injury in a variety of injury models involving energy deprivation. J Neuroimmunol. 2005;161:93–100. doi: 10.1016/j.jneuroim.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Fogal B, Li J, Lobner D, McCullough LD, Hewett SJ. System x(c)- activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury. J Neurosci. 2007;27:10094–10105. doi: 10.1523/JNEUROSCI.2459-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Echeagaray E, Gonzalez N, Ruelas A, Mendoza E, Rodriguez-Martinez E, Antuna-Bizarro R. Low doses of 3-nitropropionic acid in vivo induce damage in mouse skeletal muscle. Neurol Sci. 2011;32:241–254. doi: 10.1007/s10072-010-0394-2. [DOI] [PubMed] [Google Scholar]
- 19.Hersberger M. Potential role of the lipoxygenase derived lipid mediators in atherosclerosis: leukotrienes, lipoxins and resolvins. Clin Chem Lab Med. 2010;48:1063–1073. doi: 10.1515/CCLM.2010.212. [DOI] [PubMed] [Google Scholar]
- 20.Jackman NA, Melchior SE, Hewett JA, Hewett SJ. Non-cell autonomous influence of the astrocyte system xc- on hypoglycaemic neuronal cell death. ASN neuro. 2012;4 doi: 10.1042/AN20110030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi YB, Giannopoulos PF, Pratico D. The 12/15-lipoxygenase as an emerging therapeutic target for Alzheimer’s disease. Trends in pharmacological sciences. 2015;36:181–186. doi: 10.1016/j.tips.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna S, Roy S, Slivka A, Craft TK, Chaki S, Rink C, Notestine MA, DeVries AC, Parinandi NL, Sen CK. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 2005;36:2258–2264. doi: 10.1161/01.STR.0000181082.70763.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kronke G, Katzenbeisser J, Uderhardt S, Zaiss MM, Scholtysek C, Schabbauer G, Zarbock A, Koenders MI, Axmann R, Zwerina J, Baenckler HW, van den Berg W, Voll RE, Kuhn H, Joosten LA, Schett G. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J Immunol. 2009;183:3383–3389. doi: 10.4049/jimmunol.0900327. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn H, Belkner J, Suzuki H, Yamamoto S. Oxidative modification of human lipoproteins by lipoxygenases of different positional specificities. J Lipid Res. 1994;35:1749–1759. [PubMed] [Google Scholar]
- 25.Kumar P, Kalonia H, Kumar A. Role of LOX/COX pathways in 3-nitropropionic acid-induced Huntington’s disease-like symptoms in rats: protective effect of licofelone. British journal of pharmacology. 2011;164:644–654. doi: 10.1111/j.1476-5381.2011.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebeau A, Terro F, Rostene W, Pelaprat D. Blockade of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by beta-amyloid peptide. Cell Death Differ. 2004;11:875–884. doi: 10.1038/sj.cdd.4401395. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald ME, Ambrose CM, Duyao Mabel P, Myers Richard H, Lin Carol, Srinidhi Lakshmi, Barnes Glenn, Taylor Sherryl A, James Marianne, Groot Nicolet, MacFarlane Heather, Jenkins Barbara, Anderson Mary Anne, Wexler Nancy S, Gusella JF. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 29.Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999;19:6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McQuade LR, Balachandran A, Scott HA, Khaira S, Baker MS, Schmidt U. Proteomics of Huntington’s disease-affected human embryonic stem cells reveals an evolving pathology involving mitochondrial dysfunction and metabolic disturbances. Journal of proteome research. 2014;13:5648–5659. doi: 10.1021/pr500649m. [DOI] [PubMed] [Google Scholar]
- 31.Miller B, Sarantis M, Traynelis SF, Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992;355:722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- 32.Nagasawa K, Kakuda T, Higashi Y, Fujimoto S. Possible involvement of 12-lipoxygenase activation in glucose-deprivation/reload-treated neurons. Neuroscience letters. 2007;429:120–125. doi: 10.1016/j.neulet.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 33.Nishiyama M, Okamoto H, Watanabe T, Hori T, Hada T, Ueda N, Yamamoto S, Tsukamoto H, Watanabe K, Kirino T. Localization of arachidonate 12-lipoxygenase in canine brain tissues. J Neurochem. 1992;58:1395–1400. doi: 10.1111/j.1471-4159.1992.tb11355.x. [DOI] [PubMed] [Google Scholar]
- 34.Palfi S, Ferrante RJ, Brouillet E, Beal MF, Dolan R, Guyot MC, Peschanski M, Hantraye P. Chronic 3-nitropropionic acid treatment in baboons replicates the cognitive and motor deficits of Huntington’s disease. J Neurosci. 1996;16:3019–3025. doi: 10.1523/JNEUROSCI.16-09-03019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piomelli D, Volterra A, Dale N, Siegelbaum SA, Kandel ER, Schwartz JH, Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987;328:38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- 36.Poeckel D, Zemski Berry KA, Murphy RC, Funk CD. Dual 12/15- and 5-lipoxygenase deficiency in macrophages alters arachidonic acid metabolism and attenuates peritonitis and atherosclerosis in ApoE knock-out mice. J Biol Chem. 2009;284:21077–21089. doi: 10.1074/jbc.M109.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM. 12/15-lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am J Pathol. 2004;164:1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins, leukotrienes and essential fatty acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Serhan CN, Reardon E. 15-Hydroxyeicosatetraenoic acid inhibits superoxide anion generation by human neutrophils: relationship to lipoxin production. Free radical research communications. 1989;7:341–345. doi: 10.3109/10715768909087960. [DOI] [PubMed] [Google Scholar]
- 41.Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;280:22925–22936. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- 43.Sun D, Funk CD. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J Biol Chem. 1996;271:24055–24062. [PubMed] [Google Scholar]
- 44.Takahashi Y, Glasgow WC, Suzuki H, Taketani Y, Yamamoto S, Anton M, Kuhn H, Brash AR. Investigation of the oxygenation of phospholipids by the porcine leukocyte and human platelet arachidonate 12-lipoxygenases. Eur J Biochem. 1993;218:165–171. doi: 10.1111/j.1432-1033.1993.tb18362.x. [DOI] [PubMed] [Google Scholar]
- 45.van Leyen K, Duvoisin RM, Engelhardt H, Wiedmann M. A function for lipoxygenase in programmed organelle degradation. Nature. 1998;395:392–395. doi: 10.1038/26500. [DOI] [PubMed] [Google Scholar]
- 46.van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-Lipoxygenase in the Ischemic Brain. Stroke. 2006;37:3014–3018. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- 47.Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- 48.Wieloch T, Siesjo BK. Ischemic brain injury: the importance of calcium, lipolytic activities, and free fatty acids. Pathologie-biologie. 1982;30:269–277. [PubMed] [Google Scholar]
- 49.Wolfer DP, Crusio WE, Lipp HP. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends in neurosciences. 2002;25:336–340. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
- 50.Wolfer DP, Lipp HP. Dissecting the behaviour of transgenic mice: is it the mutation, the genetic background, or the environment? Experimental physiology. 2000;85:627–634. [PubMed] [Google Scholar]
- 51.Yang H, Zhuo JM, Chu J, Chinnici C, Pratico D. Amelioration of the Alzheimer’s disease phenotype by absence of 12/15-lipoxygenase. Biological psychiatry. 2010;68:922–929. doi: 10.1016/j.biopsych.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Yao Y, Clark CM, Trojanowski JQ, Lee VM, Pratico D. Elevation of 12/15 lipoxygenase products in AD and mild cognitive impairment. Ann Neurol. 2005;58:623–626. doi: 10.1002/ana.20558. [DOI] [PubMed] [Google Scholar]
- 53.Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N, Smirnova N, Gazaryan I, Ratan RR, Witztum JL, Montaner J, Holman TR, Lo EH, van Leyen K. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Annals of Neurology. 2013;73:129–135. doi: 10.1002/ana.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Wang H, Li J, Jimenez DA, Levitan ES, Aizenman E, Rosenberg PA. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J Neurosci. 2004;24:10616–10627. doi: 10.1523/JNEUROSCI.2469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]