Abstract
Dysregulated repair of lung injury often results in lung fibrosis characterized by unremitting deposition of matrix components including the glycosaminoglycan hyaluronan (HA). HA is mainly produced by hyaluronan synthases (HAS) in mesenchymal cells. We previously demonstrated that over-expression of HAS2 in mesenchymal cells in mice regulates the invasiveness of fibroblasts and promotes severe lung fibrosis. The mechanisms that control the resolution of lung fibrosis are unknown. We propose that a critical step in resolving fibrosis is the induction of senescence in fibrotic fibroblasts and hyaluronan synthase 2 may regulate this process. We found that fibrotic fibroblasts developed the characteristics of replicative senescence in culture and that HAS2 expression was dramatically down-regulated. Furthermore, down-regulation of HAS2 initiated and regulated fibroblast senescence through a p27-CDK2-SKP2 pathway. Deletion of HAS2 in mouse mesenchymal cells increased the cellular senescence of fibroblasts in bleomycin-induced mouse lung fibrosis in vivo. These data suggest that HAS2 may be a critical regulator of the fate of pulmonary fibrosis and we propose a model where over-expression of HAS2 promotes an invasive phenotype resulting in severe fibrosis and down-regulation of HAS2 promotes resolution. Targeting HAS2 to induce fibroblast senescence could be an attractive approach to resolve tissue fibrosis.
Keywords: Fibrosis, lung fibrosis, fibrosis resolution, cell cycle, senescence, matrix
1. Introduction
Dysregulated tissue injury and repair often result in tissue fibrosis. Fibroblasts in fibrotic diseases are heterogeneous in morphological, secretory, and behavioral properties, possibly because of their cell origin, activation state, and local environment. Resolution of tissue fibrosis has not been adequately addressed. The fate of fibrotic fibroblasts is largely unknown. Liver fibrosis is often reversible [1], while idiopathic pulmonary fibrosis (IPF), a severe disease with a high mortality rate has proved irreversible to date [2]. The hypothesis has been put forth that fibroblasts from IPF patients are resistant to apoptosis leading to failure of resolution. For example, fibroblasts from IPF patients are resistant to FasL-induced apoptosis compared with normal fibroblasts [3]. A variety of extracellular matrix regulators such as CD44 [4], soluble fibronectin peptides [5], and PAI-1 [6] have also been shown to regulate fibroblast apoptosis. Collectively, these studies suggest there may be both intrinsic components of fibrotic fibroblasts as well as interactions with the surrounding matrix that may regulate apoptosis.
Senescence is a complex phenomenon related to cellular stress where cells lose their ability to proliferate [7]. Senescent cells irreversibly arrest in the G1 phase of cell cycle, develop a flattened, enlarged morphology, and demonstrate increased activity of senescence associated β-galactosidase (SA-β-gal) [7]. Various mechanisms including telomere erosion, chromatin disruption, DNA damage, oncogene activation, and oxidative stress are reported to trigger replicative or premature senescence [7]. Several pathways including p53, p16, and p27 are involved in initiating and regulating senescence [7]. Senescence is implicated in development [8], cancer [9], and tissue fibrosis [10]. The chronic inflammation caused by cellular senescence may be related to the pathogenesis of various chronic diseases [9, 11]. A few studies of senescent epithelial cells suggest a role in IPF [12, 13] and in bleomycin-induced lung fibrosis [14]. Insufficient autophagy may be responsible for accelerated cellular senescence [13]. In liver fibrosis models, hepatic myofibroblasts undergo senescence when fibrosis resolves [10]. The role of fibroblast senescence in lung fibrosis has not been investigated.
The matrix in which a cell resides has profound effects on cellular functions. Hyaluronan (HA) is one of the major components of extracellular matrix, which is mainly produced by mesenchymal cells [15]. All three hyaluronan synthase isoforms, designated as HAS1, HAS2 and HAS3, synthesize HA at the inner face of the cytoplasmic membrane in vertebrates [16]. HAS2 is the major isoform responsible for hyaluronan production in mesenchymal cells. HAS2 deficiency leads to embryonic lethality [17], and the vast majority of mice with targeted deletion of HAS2 in collagen-expressing mesenchymal cells died in utero [18]. Accumulation of HA is a characteristic of disorders that are associated with lung diseases [19] and progressive tissue fibrosis [20]. HA is able to modulate TGF-β induction of lung myofibroblasts and to control collagen deposition [21]. HAS2 has been implicated in cellular senescence since suppression of HAS2 in tumor cells inhibited cell proliferation by arresting cells in the G1 phase of the cell cycle [22]. Decreased cleavage of HA is associated with impaired dermal wound healing in aging [23]. Furthermore, myofibroblasts have been shown to be driven into senescence at the late stage of liver fibrosis, converting these extracellular matrix (ECM)-producing cells into ECM-degrading cells, thus imposing a self-limiting control on fibrogenesis [10]. Previous work from our laboratory has shown that over-expression of HAS2 in myofibroblasts promotes an invasive phenotype that causes severe fibrosis [18]. This invasive phenotype was dependent on HAS2 [18]. We hypothesized that HAS2 may be a critical determinant of the fate of fibrosis by either promoting invasion or senescence depending on the level of expression. We have found that down-regulation of HAS2 in fibrotic fibroblasts in vitro results in replicative senescence and targeted deletion of HAS2 in mesenchymal cells in vivo promotes senescence and the resolution of pulmonary fibrosis.
2. RESULTS
2.1. Fibrotic fibroblasts demonstrate senescent characteristics in vitro
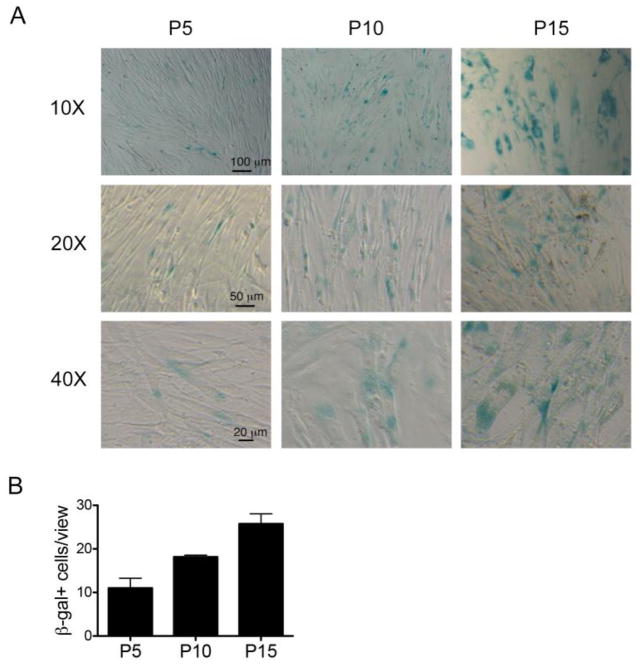
We first determined whether human lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis underwent senescence when cultured without extracellular matrix [18]. One of the key features of cellular senescence is increased SA-β-gal activity [24]. We found that fibrotic fibroblasts from IPF patients demonstrated increased SA-β-gal activity when they reached late passages in culture in vitro (Fig. 1A, B).
Fig. 1.
(A). Replicative senescence in fibrotic fibroblasts. Fibrotic fibroblasts at passage (p) 5, 10 and 15 cultured in 15% FBS-DMEM were stained for SA-β-gal. n = 5 patients. Scale bars are shown. (B). Number of SA-β-gal positive cells per view per view with 20X magnification. 4–6 random views were counted each passage of cells.
2.2. Reduced expression of HAS2 in senescent lung fibroblasts
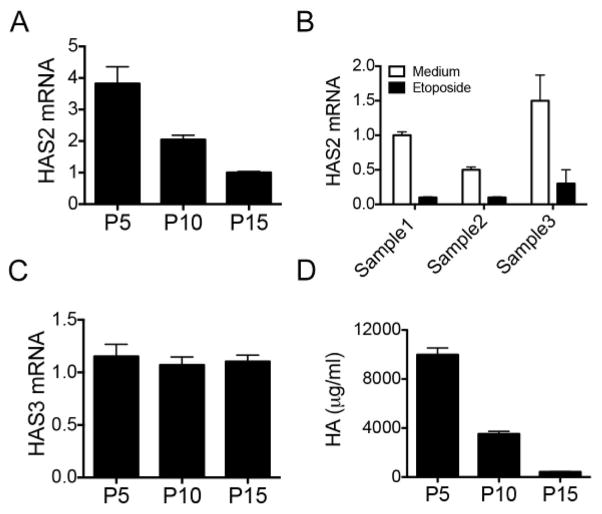
We previously demonstrated that overexpression of HAS2 in mesenchymal cells promoted an invasive fibroblast phenotype leading to severe fibrosis and increased mortality in mice [18]. Since the targeted deletion of HAS2 in mesenchymal cells abrogated bleomycin-induced pulmonary fibrosis, we sought to determine if HAS2 could regulate fibroblast senescence and promote the resolution of fibrosis. We previously found that fibroblasts from IPF patients demonstrated increased HAS2 mRNA expression when they were invading matrigel [18]. We then examined the expression of HAS2 in IPF fibroblasts when they were not in the context of matrix and cultured for repeated passages on plastic. We found that the expression of HAS2 was decreased in replicate-induced (Fig. 2A) or DNA damage-induced senescent fibrotic fibroblasts (Fig. 2B). Interestingly, expression of HAS3 remained the same level in different passage of the cells (Fig. 2C). HAS1 expression was too low to be detected in all passages of fibroblasts (data not shown). HA production was dramatically reduced in replicate-induced senescent fibroblasts (Fig. 2D). These data suggest a correlation between fibroblast senescence and the expression of HAS2.
Fig. 2.
HAS2 is suppressed in senescent fibroblasts. (A). HAS2 mRNA levels in fibrotic fibroblasts grown in 15% FBS-DMEM for 5 passages (p5), 10 passages (p10), and 15 passages (p15) were measured using real-time PCR. The experiments were performed three times. (B). HAS2 mRNA levels in fibrotic fibroblasts treated with etoposide at 100 μM for 24 h were measured using real-time PCR. The data shown is one representative of two separate experiments with similar results. Fibroblasts from three different persons were used each time. (C) HAS3 expressions of p5, p10, and p15 fibroblasts were quantitated with q-PCR. The relative expression of the target genes was calculated by using the 2−ΔΔCT method and expressed as fold-change. (D). HA concentration in 24-h culture medium of p5, p10, and p15 fibroblasts.
2.3. HAS2 depletion induced fibroblast senescence
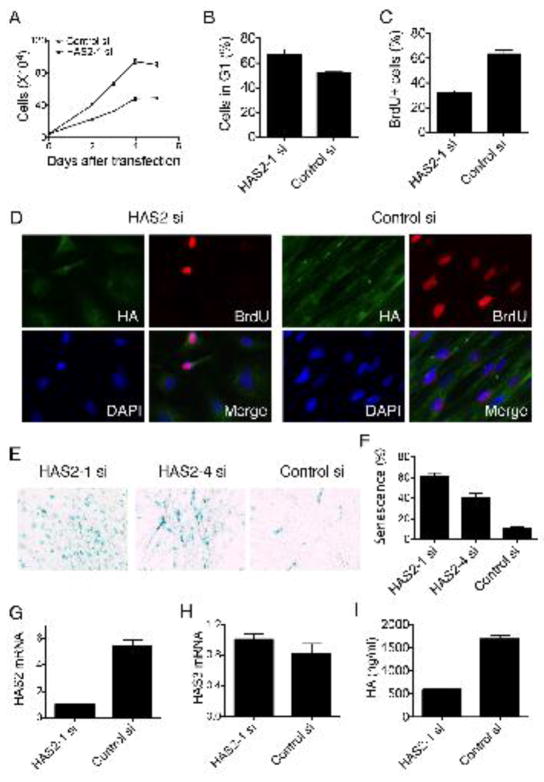
We therefore explored the potential roles of HAS2 on fibroblast proliferation and senescence. To approach this, we first decreased HAS2 expression by knocking down HAS2 in fibrotic fibroblasts and found that deletion of HAS2 markedly suppressed the proliferation of fibrotic fibroblasts (Fig. 3A). The effects of HAS2 depletion on cell cycle progression were further investigated. Flow cytometry analysis revealed that HAS2 depletion significantly increased the proportion of cells in G1 phase (Fig. 3B). Consistent with the results, there was less BrdU incorporation in HAS2 siRNA transfected fibroblasts (Fig. 3C, D). Most interestingly, the proportion of cells with positive SA-β-gal staining was dramatically increased following HAS2 depletion (Fig. 3E, F). While HAS2 expression (Fig. 3G) and HA production (Fig. 3I) of the cells were dramatically decreased, there was no significant change of HAS3 expression in Has2 depleted fibroblasts (Fig. 3H). Has1 expression was too low to be detected (data not shown). These results suggested that the depletion of HAS2 is sufficient to trigger senescence. We then sought to determine if the fibroblast senescent phenotype that emerged with inhibition of HAS2 could be reversed by exogenous administration of HA. Exogenous HA failed to rescue the phenotype suggesting the regulatory processes were intrinsic to the fibroblasts (data not show).
Fig. 3.
Depletion of HAS2 expression induces fibroblast senescence. Fibroblasts transfected with HAS2 siRNA and control siRNA. (A). Cell numbers were counted at indicated time after transfection. Data shown is the mean of triplicates ± s. d. of one representative from 3 separate experiments. (B). Forty-eight hours after transfection, fibrotic fibroblasts were harvested for the cell cycle profile with FACS assay. Bar graphs depict the percentage of cells in G1 phase after HAS2 knock down (N = 3, *P = 0.022). Data represented 1 of 3 separate experiments. (C). Fibrotic fibroblasts transfected with HAS2 siRNA or control siRNA were plated for BrdU incorporation assay. Ten randomly selected fields were counted. Bar graph represented the percentage of BrdU positive cells after transfection. The experiments repeated 3 times. (D). Fibroblasts transfected with HAS2 siRNA or control siRNA were plated for BrdU incorporation assay. Cells were stained with Biotin-HABP and anti-BrdU. Microscopic photos were taken at a 400 × magnification. The experiments repeated 3 times. (E). At 72 h after transfected with HAS2 siRNA (HAS2-1 siRNA and HAS2-4 siRNA) or control siRNA, fibroblasts were plated for SA-β-gal assay. Microscopic photos were taken at a 20X magnification. The experiments repeated 3 times. (F). Bar graph represents the percentage of SA-β-gal positive cells counted in 5 randomly selected fields. (G) HAS2 expressions in fibroblasts transfected with HAS2 siRNA and control siRNA. (H) HAS3 expressions in fibroblasts transfected with HAS2 siRNA and control siRNA. The relative expression of the target genes was calculated by using the 2−ΔΔCT method and expressed as fold-change. (I) HA concentration in 48h culture medium of fibroblasts transfected with HAS2 siRNA and control siRNA. n=3–7. Experiments were repeated with fibroblasts isolated from three patients.
2.4. Senescence induced by HAS2 deletion is dependent on p27
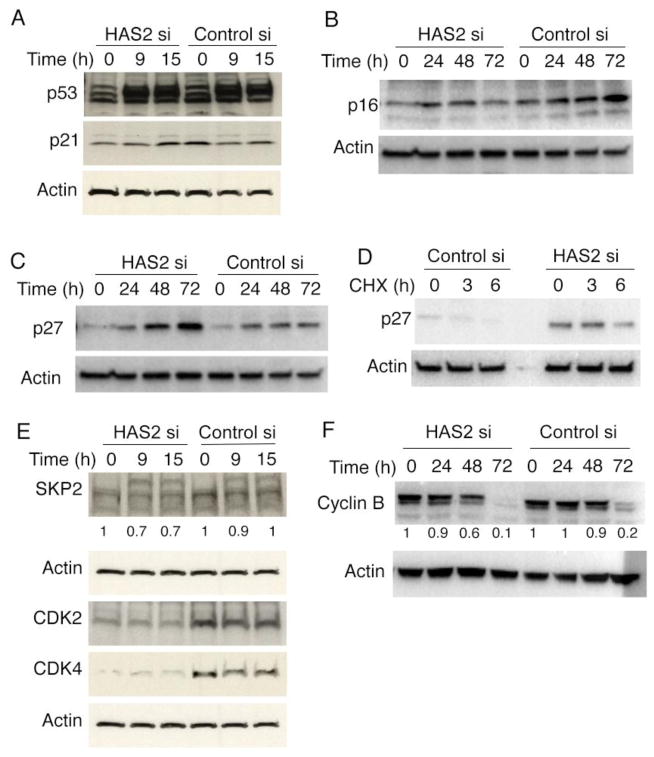
To determine the molecular mechanisms downstream of HAS2 associated with senescence, we first explored the effects of HAS2 knock down on classical senescence regulatory pathways. Unexpectedly, p53 and p21 expression were not changed during HAS2 depletion-induced senescence (Fig. 4A). Furthermore, p16 was not up-regulated in HAS2 depleted cells (Fig. 4B).
Fig. 4.
HAS2 depletion induced fibroblast senescence is associated with increased p27-CDK2-SKP2 expression. Fibroblasts were transfected with HAS2 siRNA or control siRNA. Cell lysates were harvested at various time points after transfection and subjected to Western blots (A–C, E, and F). (A). p53 and p21 expression at indicated time points. (B). p16 expression at indicated time points. (C). p27 expression at indicated time points. (D) Fibroblasts were transfected with HAS2 siRNA or control siRNA. 48h after transfection, the cells were treated with cycloheximide, and the cell lysates were harvested at 0h, 3h, and 6h after cycloheximide treatment. The cell lysates were subjected to Western blot analysis for p27. (E). Fibroblasts were transfected with HAS2 siRNA or control siRNA. Cell lysates were harvested at various time points after transfection and subjected to Western blots analysis with antibodies against SKP2, CDK2 and CDK4. (F) Fibroblasts were transfected with HAS2 siRNA or control siRNA. Cell lysates were harvested at various time points after transfection and subjected to Western blots analysis with antibodies against cyclin B. Densitometry quantification for SKP2 and Cyclin B was included. The experiments were performed at least three times.
p27 regulates cell progression from G1 to the S phase by mediating G1 arrest by inhibiting cyclin/CDK-complex activities in response to growth inhibitory signals [25, 26]. When we explored the effects of HAS2 deletion on p27 expression, we found that p27 protein levels were significantly increased in HAS2 deficient fibrotic fibroblasts at various time points after HAS2 siRNA transfection (Fig. 4C). Characterization of p27 in the presence of cycloheximide revealed that down-regulation of HAS2 increased p27 protein stability (Fig. 4D).
2.5. Senescence induced by HAS2 deletion is dependent on CDK2-SKP2
We then examined the expression of S-phase kinase associated protein-2 (SKP2), which is the substrate-targeting subunit of SCF ubiquitin E3 ligase complex involved in p27 degradation. We observed a decrease (~30%) in SKP2 expression in HAS2 deficient fibrotic fibroblasts (Fig. 4E) compared with control transfectants. These data suggested that HAS2 deletion-induced accumulation of p27 is likely caused by the specific inhibition of SKP2 expression.
p27 inhibits cell proliferation by regulating CDK2 activity [26]. Studies have shown that CDK2 phosphorylates p27 on threonine 187, which is essential for the subsequent ubiquitin-mediated degradation of p27 [25]. Therefore, we further examined CDK2 expression levels. We found that CDK2 protein levels were dramatically down-regulated upon HAS2 knock down in fibrotic fibroblasts (Fig. 4E), which supported the hypothesis that HAS2 deletion-induced proliferative inhibition was through p27 accumulation and its effector pathway. CDK4 (Fig. 4E) and cyclin B (Fig. 4F) levels were also decreased in HAS2 deficient fibrotic fibroblasts, suggesting the involvement of additional regulatory proteins on cellular proliferation in HAS2 deleted cells.
2.6. HAS2 deletion enhances cell stress responses
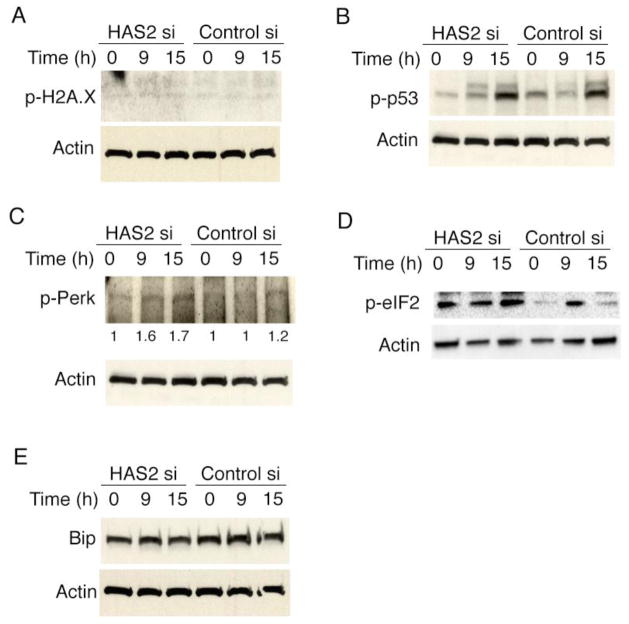
DNA damage, oxidative damage and oncogenic insult-induced cell stresses are associated with premature senescence, characterized by the increase in phosphorylated histone H2AX [27]. We then determined whether HAS2 deficiency could induce DNA damage or cell stress. We found no convincing evidence of DNA-damage-response activation in HAS2 deficient cells as determined by the levels of phosphorylated histone H2AX (γH2AX) [27] (Fig. 5A) and phospho-p53 (Fig. 5B). However, we observed that endoplasmic reticulum (ER) stress proteins such as Perk (Fig. 5C) and elF2α (Fig. 5D) were phosphorylated upon HAS2 deletion. The ER stress chaperone protein GRP78 (Bip) did not increase in the HAS2 deleted cells compared with that in control cells (Fig. 5E).
Fig. 5.
Stress responses mediate HAS2 deficiency-induced senescence. Fibroblasts were transfected with HAS2 siRNA or control siRNA. Cell lysates were harvested at various time points after transfection and subjected to Western blots. The expression of proteins involving in DNA damage response pathway including phospho-H2A.X (A), phospho-p53 (B), and proteins involving in stress response pathway including phospho-perk (C), phospho-elF2α (D), and Bip (E) were analyzed. Densitometry quantification for phospho-perk was included in C. The experiments were performed three times.
2.7. HAS2 expression correlates with senescence in vivo
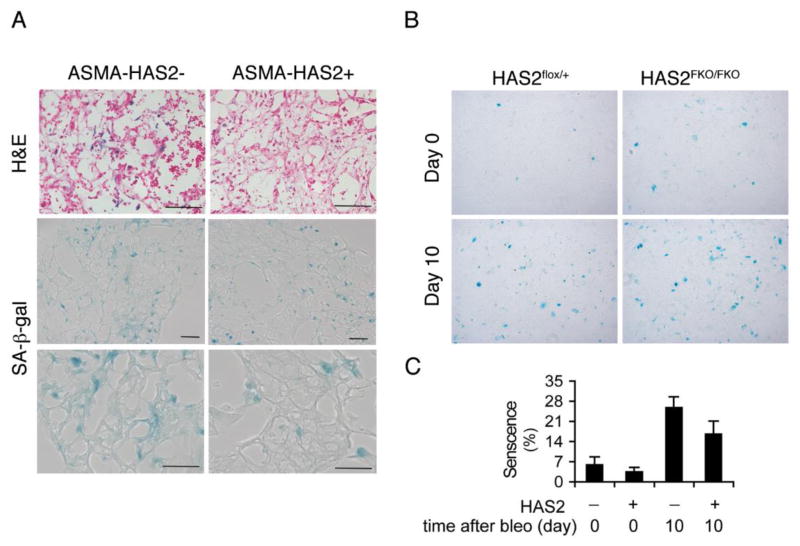
To examine the roles of HA on mesenchymal cell proliferation and senescence in vivo, we utilized ASMA-HAS2 transgenic mice and HAS2 conditional knock out mice with or without bleomycin treatment as described previously [18]. ASMA-HAS2 transgenic mice overexpress HAS2 in myofibroblasts (19). We found that after bleomycin treatment SA-β-gal positive cells with elongated-shape accumulated in the lung interstitium, where myofibroblasts reside (Fig. 6A).
Fig. 6.
HAS2 expression levels correlate with mouse fibrotic fibroblast senescence. (A). SA-β-gal staining in lung sections of ASMA-HAS2+ and wild type control mice at day 14 after bleomycin treatment (n = 6 – 8 per group). The experiments were performed twice. X20. (B, C). Mouse fibroblasts isolated from HAS2FKO/FKO and control HAS2flox/+ mice treated without or with bleomycin for 10 days were stained for SA-β-gal expression (B), and quantified using NIH image J program (C). The experiments were performed twice. n = 4 per group. Scale bars, 100 μm.
We then isolated fibroblasts from mouse lung with or without bleomycin treatment. Fibrotic fibroblasts from bleomycin treated mouse lung exhibited increased senescent cells compared with control cells (Fig. 6B). Greater senescence was observed in fibroblasts isolated from HAS2 conditional knock out mice with targeted deletion of HAS2 in mesenchymal cells after bleomycin injury (Fig. 6B, C). Taken together, these data suggest that reduced expression of HAS2 may induce ER stress, which leads to fibroblast senescence through the inhibition of the p27-SKP2-CDK2 pathway.
3. Discussion
The purpose of this study was to examine the role of HAS2 in regulating fibroblast senescence in vitro and in vivo in murine fibrosis induced by bleomycin and fibroblasts from patients with IPF. IPF is a progressive fibrotic lung disease leading to death with 5 years of diagnosis in most patients [28]. The fibroblast is the key effector cell by producing extracellular matrix in regions of gas exchange and generating a fibrodestructive process [2]. We provide evidence to support that fibroblast senescence was regulated by expression of HAS2, through a p27-SKP2-CDK2 pathway. The study suggests that targeting HAS2 and downstream pathways to induce fibroblast senescence could be an attractive way to promote fibrosis resolution.
Resolution of lung fibrosis has not been adequately investigated. The fate of fibrotic fibroblasts/myofibroblasts and the pathways that regulate fate are unknown. Liver fibrosis can be reversible [29], while it is usually considered that IPF is irreversible [2]. IPF fibroblasts have been suggested to be resistant to apoptosis. For example, low to absent staining of Fas was found in fibroblastic cells of fibroblast foci in lung tissues from IPF patients [30]. Fibroblasts from IPF patients are resistant to FasL-induced apoptosis compared with normal fibroblasts [3]. Fibroblast apoptosis can also be regulated by prostaglandin E2 [31], TNFα [32], CD44 [4], soluble fibronectin peptides [5], and plasminogen activator inhibitor-1 [6]. Therefore, strategies to enhance fibroblast apoptosis would be an attractive approach to treat patients with progressive lung fibrosis. Our data are consistent with a study in liver fibrosis that fibroblast senescence was suggested as a self-limiting mechanism leading to fibrosis resolution [10]. Senescent fibroblasts were found in granulation tissues of healing cutaneous wounds, and the matricellular protein CCN1 acts through integrin-mediated cell adhesion to promote fibroblast senescence as a self-limiting mechanism in skin fibrogenesis [33]. However, a recent study suggesting that senescent myofibroblasts accumulate in lung tissues from the patients with IPF [34]. The senescence phenotype was regulated by the reactive oxygen species-generating enzyme Nox4 in aging mice [34]. The possible reasons for this seeming discrepancy may be related to experimental conditions, different stages of fibroblasts and fibrosis, and the ages of the mice. This also highlights the complexities of fibroblast senescence during fibrogenesis. We hypothesized that fibroblasts from patients with progressive lung fibrosis may develop a senescence phenotype when removed from the extracellular matrix milieu. Our data show that deletion or reduced expression of HAS2 results in induction of senescence in fibrotic fibroblasts from patients with IPF. These data suggest that fibroblasts/myofibroblasts acquire different phenotypes such as senescence and apoptosis, which may serve as a means to target fibrosis progression. The progressive nature of tissue fibrosis may be the result of a weakened force of resolution, potentially as a failure to down regulated HAS2 expression.
Our recent work demonstrated that overexpression of HAS2 in fibroblasts promoted an invasive fibroblast phenotype leading to severe fibrosis in mice [18]. This observation led us to determine if inhibition of HAS2 could promote a distinct phenotype associated with senescence and resolution of fibrosis. We found that reduced expression of HAS2 is sufficient to trigger fibroblast senescence, characterized by enhanced SA-β-gal staining, an increase in the G1 phase of the cell cycle, and reduced cell proliferation. HA and HAS2 has been suggested to have a role in fibroblast senescence. Compared with young fibroblasts, reduced expression of HAS2 was found in aged fibroblasts [35]. Aged human fibroblasts undergo replicative senescence [36]. Fibroblasts isolated from mice harboring a mutated versican with reduced HA binding showed an increase in expression of senescence markers p53, p21, and p16 [37]. Moreover, a recent report suggests that miRNA-23a-3p targets HAS2 leading to dermal aging and senescence [38]. In addition, HA fragmentation during injury may also have a role in fibroblast senescence. For example, HA oligomers lead to tumor cell senescence [39], and diminished versican deposition increases free HA fragments, leading to cellular senescence [37].
Senescence triggers include telomere shortening, activated oncogenes, DNA damage, and oxidative stress. For example, oncogenic ras drives cellular senescence [40] that can be mediated by ER-associated unfolded protein response in tumor cells [41]. How fibrotic fibroblasts undergo cellular senescence is unclear. Oncogene activation is unlikely involved in resolution of tissue fibrosis. Telomerase and telomere shortening may contribute in the process, since telomerase mutations and telomere shortening are associated with IPF [42]. However, telomere erosion is usually a protracted process. Our results with siRNA knockdown experiments did not support HAS2 deletion inducing DNA damage as measured by phospho-p53 and phospho-γ-H2ax levels. We cannot exclude the possibility that telomere shortening contributes to fibroblast senescence in vivo. Many proliferative cell types exposed to subcytotoxic stresses undergo stress-induced premature senescence [43]. Our data support the notion that HAS2 knock down alters ER stress proteins in a manner that promotes cellular senescence [41].
Several regulatory proteins such as p53 and p16-pRb have been shown to regulate senescence processes [7]. We found that HAS2 deletion does not change expression of p53, p21, and p16, but significantly increases p27 expression. p27 is a cyclin-dependent kinase inhibitor in the G1 phase arrest of cell cycle [25, 26]. p27 negatively regulates protein kinase CDK2, which is necessary for G1-S progression [25]. The increase in p27 was concomitant with decreases in expression of SKP2, CDK2, and CDK4 after HAS2 knock down. SKP2 has been implicated in cellular senescence through an increase in ARF4, p27, and p21 [44]. Cdk2 deficiency or pharmacological inhibition of Cdk2 causes cellular senescence under conditions of oncogenic stress and oxygen-induced culture shock [45]. These findings support the concept that senescence caused by HAS2 deletion in lung fibroblasts occurs through a p27-SKP2-CDK2 pathway. As HAS2 is a multi-pass membrane-bound enzyme [46], the regulation of p27-SKP2-CDK2 pathway by HAS2 is likely an indirect effect. HAS2 may interact with other cytoplasmic proteins [47], such as protein kinase C (PKC) [48] to influence down-stream signaling mediating cellular senescence. Furthermore, HAS2-mediated senescence may be through CD44-HA interactions. CD44 is known to play a role in aging [49, 50] and cellular senescence [51]. It was showed that CD44 can complex with EGF receptor upon TGF-β activation in young fibroblasts [35], and HA-dependent CD44-EGF receptor interaction is lost in aged fibroblasts [35]. Moreover, HA is known to promote the interactions between CD44 and several Rho-specific guanine nucleotide exchange factors [52], and both Rac1 and RhoA regulate cell senescence in tumor cells [53, 54].
We recently demonstrated that fibroblasts from patients with IPF show an invasive property when compared with fibroblasts from normal individuals [18]. HAS2 expression was correlated well with fibroblast phenotype. Deletion of HAS2 in fibroblasts showed a reduction in ASMA staining and in hydroxyproline content in the lung tissue [18]. HAS2 is regulated by TGF-β [21, 55, 56] and other cytokines [57]. It is well known that TGF-β plays a key role in fibrogenesis [58]. TGF-β drives HAS2 transcription and stimulates HA production [55, 56], and myofibroblast differentiation [55]. Therefore, the regulation of fibroblast senescence by HAS2 may very well be related to TGF-β signaling. In this study, we demonstrated that under-expression of HAS2 in fibroblasts determined cellular fate. Collectively, these data support the concept that HAS2 works as a master regulatory switch such that over expression promotes fibroblast invasion to enhance fibrogenesis, while under expression leads to senescence favoring the resolution of fibrosis. We also found that the addition of exogenous HA could not reverse the senescent phenotype and restore cell growth in primary fibroblasts, suggesting that the loss of HAS2 expression in fibroblasts alters the cell gene program and cellular properties. The latter may not be readily mimicked by elevation of HA levels in the extracellular milieu.
The current study is a first step in understanding the role of senescence in the resolution of lung fibrosis and we recognize that much more work needs to be performed. First, demonstration of fibroblast senescence in the lungs of the patients with IPF or other fibrosing lung diseases, such as nonspecific interstitial pneumonia and hypersensitivity is needed. It would be interesting to determine if the reversible nature of the fibrosis in some of these disease entities was related to enhanced fibroblast senescence. Due to technical difficulties, staining lung sections with SA-β-gal is challenging. Second, it would be very interesting to determine if and how TGF-β is involved in the process. TGF-β plays a central role in fibrogenesis [58] and controls fibroblast apoptosis [59]. Moreover, TGF-β induces p21 in lung tissue [60]. Third, the contribution of CD44 to fibroblast senescence should be explore since CD44 is the major HA receptor and anti-CD44 antibodies induced fibroblast apoptosis [4]. Lastly, determination of the effect of fibroblast senescence in inflammation is also needed. In liver fibrosis, senescent fibroblasts not only down-regulated ECM production, but also regulated immune surveillance genes and recruited natural killer cells to remove senescent myofibroblasts [10]. It would be of importance to determine if immune properties exist in senescent fibroblasts from patients with IPF.
In summary, we observed that fibrotic lung fibroblasts acquire senescent features in both human fibroblasts from disease and in mice following fibrotic injury. HAS2 expression level regulates mouse and human mesenchymal cell senescence and the extent of senescence inversely correlates with the extent of fibrosis. Strategies designed to enhance senescence, potentially by targeting HAS2 may lead to new therapeutic approaches for severe and progressive lung fibrosis.
4. Experimental Procedures
4.1. Fibroblasts isolation and culture
Human lung fibroblasts were isolated from surgical lung biopsies or lung transplant explants obtained from patients with idiopathic pulmonary fibrosis as previously described [18]. The specimens were obtained under the auspices of IRB-approved protocols. Fibroblasts were cultured in complete medium (15% FBS-DMEM). Unless specified, the cells of passage 5 – 7 were used for experiments. The diagnosis of IPF was arrived at by standard accepted American Thoracic Society recommendations [2]. All experiments were approved by the Duke University Institutional Review Board and in accordance with the guidelines outlined by the board. Mouse primary fibroblasts were derived from mouse lungs as described [18]. The mouse fibroblasts were used from 3 to 6 passages.
4.2. RNA interference assay and cell proliferation
Transfection with siRNA duplexes against human HAS2 was performed as described previously [18]. Briefly, two siRNA duplexes designed to target different nucleotide sequences (HAS2-1 si, 1530–1550, CAGCTCGATCTAAGTGCCTTA; HAS2-4 si, 1777-1797, CCAGCTAGTAGGTCTCATAAA) of the human HAS2 gene [NM_005328], as well as a control siRNA (control si with sense sequence UUCUCCGAACGUGUCACGUdTdT) were obtained from Qiagen. Subconfluent fibroblasts (about 50–60% confluent) grown in complete medium were transfected separately with each siRNA duplexes or a control siRNA at 100 nM using HiPerFect transfection reagent (Qiagen) according to the manufacturer’s instructions. The suppression efficiency of each of the siRNA duplexes was confirmed by measuring the hyaluronan content in the conditioned culture media [18].
Fibroblasts with or without transfection were seeded in triplicates and cultured in complete medium (15% FBS-DMEM). Every 24 h, cell numbers were counted using hemocytometer and growth curves were generated.
For BrdU incorporation, cells in 4-well chamber slides (BD Bioscience) were transfected with HAS2 siRNA or control siRNA. At 48 h after transfection, the cells were labeled with 10 μg/ml of BrdU (Sigma) for 2 h. The cells were then fixed in 4% paraformadehyde for 10 min and followed by incubation with 2N HCl containing 0.1% Triton 100 in PBS for 10 min. The cells were washed and incubated with anti-BrdU antibody (Accurate Chemical) and biotin-HABP (Associates of Cape Cod Inc) overnight at 4°C. The cells were then incubated with donkey anti-rabbit second antibody, Alexa Fluor® 568 conjugate, and anti-streptavidin, Alexa Fluor® 488 conjugate (Invitrogen) to detect BrdU and HA. The processed cells were mounted in Fluoromount G (eBioscience) containing DAPI. Fluorescence microscopy images were taken from five random fields in each well using a Zeiss microscope with AxioCam MRc5 camera (AxioVision Rel 4.6 software).
4.3. Quantification of mRNA expression
Total RNA was purified using RNAqueous™-4PCR kit (Ambion) and was reversed to cDNA using SuperScriptTM II RNase H− Reverse Transcriptase Kit (Invitrogen) according to the manufacturer’s instructions. Gene expression in the resultant cDNAs were examined using ABI Prism 7500 Detection system (Applied Biosystems) with SYBR-green as fluorescent dye enabling real time detection of PCR products according to the manufacturer’s protocol (Power SYBR Green PCR Master Mix, Applied Biosystems). The relative expression levels of the gene were determined against GAPDH levels in the samples. The primers used were: human HAS2 (NM_005328) forward, 5′-TCG CAA CAC GTA ACG CAA T; human HAS2 reverse, 5′-ACT TCT CTT TTT CCA CCC CAT TT; human HAS3 (AF234839) forward, 5′-GGC ATT ATC AAG GCC ACC TA, human HAS3 reverse, 5′-GAC ACA GGA ATG AGG CCA AT; human GAPDH (NM_002046) forward, 5′-CCC ATG TTC GTC ATG GGT GT; human GAPDH reverse, 5′-TGG TCA TGA GTC CTT CCA CGA TA. The fold-change of the target genes was calculated by using the 2−ΔΔCT method. The expression level for gene of interest (GOI) was calculated as 2−Ct followed by normalization to GAPDH, using the formula 2−(Ct GOI - Ct GAPDH). Ultimately, the fold change in normalized gene expression was calculated by comparing values from treated fibroblasts (EXP) to control fibroblasts (CTL) according to the following formula: 2−ΔCt EXP/2−ΔCt CTL.
4.4. Cellular senescence (SA-β-gal staining) assay
For cellular senescence, fibrotic fibroblasts or fibroblasts transfected with HAS2 siRNA or control siRNA were fixed in phosphate-buffered 2% paraformadehyde-2% glutalaldehyde for 7 min, β-galactosidase activity was detected by using the senescence histochemical staining kit from Sigma-Aldrich according to the manufacturer’s instruction [10]. For detecting senescence in vivo, lung tissues were fixed in phosphate-buffered 2% paraformadehyde-0.5% glutalaldehyde for 30 min, washed with PBS, rinsed in SA-β-gal staining buffer for 30 min, and then stained in SA-β-gal staining solution at 37°C for 6 h. After fixing in 10% formalin for additional 2 h, the tissues were embedded in OPC compound (Tissue-Tek) and kept at −80°C until section. The frozen tissue sections (6 μm) were washed with PBS and counterstained with or without Fast Red.
4.5. Cell cycle analysis
For cell cycle distribution analysis, fibroblasts were harvested, washed with PBS and fixed in 70% ethanol. After centrifugation, the cells were suspended in staining solution (200 μg/ml propidium iodide, 0.1% Triton X-100 and 2 mg/ml RNase A) and incubated for 20 min at room temperature. The distribution of cells was determined using FACS Canto II (BD Biosciences). Flow data were analyzed using FlowJo software (Tree Star).
4.6. Western blotting analysis
Western blotting analysis was performed as described previously [61]. Total cell lysates were prepared in RIPA buffer (150 mM NaCl, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris) (Sigma) supplemented with protease inhibitor cocktail (Sigma). 20 μg total proteins were used for Western. The proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using gradient gel (4–20%, Bio-Rad) and electroblotted onto nitrocellulose membrane (Bio-Rad). The membranes were probed with antibodies against p21, p27, p53, phospho-elF2α, Perk, Bip, and SKP2, CDK2, and CDK4. SKP2 was from Santa Cruz, the rest of antibodies were from Cell Signaling. All antibodies were used at 1:1,000 dilution, except SKP2, which was at 1:200 dilution for Western-actin was used as a loading control. Quantitative densitometric analysis relative to β-actin was used to aid clarity
4.7. Mice and bleomycin administration
ASMA-human HAS2 transgenic mice (ASMA-HAS2+) [62] and HAS2 mesenchyme conditional knock out mice (FSP-1-Cre+/Has2flox/flox, termed Has2FKO/FKO) were described previously [18]. All mice were housed in a pathogen-free facility at Duke University, and all animal experiments were approved by the Institutional Animal Care and Use Committee at Duke University. Bleomycin was injected intratracheally at 1.75 U/kg body weight as previously described [18]. At designated time points after bleomycin injection, mouse lungs were harvested for fibroblast isolation.
4.8. HA quantification
Conditioned media were collected from fibroblast culture. The HA content in conditioned media was measured using a microtiter-based assay taking advantage of the formation of a complex between hyaluronan and the hyaluronan binding protein domain of aggrecan, as described previously [18].
4.9. Statistical analysis
We assessed differences in measured variables using the Student t-test. Differences between multiple groups were calculated using one-way Anova with Tukey-Kramer or two-way ANOVA with bonferroni multiple comparisons Data are expressed as the mean ± SEM where applicable. Statistical difference was accepted at p < 0.05.
Highlights.
IPF fibroblasts show senescence in vitro
HAS2 expression correlates fibroblast senescence
Deletion of HAS2 in fibroblasts increases fibroblast senescence in vitro and in vivo
HAS2 regulates fibroblast senescence through a p27-CDK2-SKP2 pathway
Acknowledgments
This study was supported by National Institutes of Health grants AI052201, HL06539, and P01 HL108793 (to P.W.N.), and HL122068 (to D.J.), and a grant from CIRM (RB5-07302 to P.W.N.).
Abbreviations used
- IPF
Idiopathic pulmonary fibrosis
- HA
hyaluronan
- HAS2
hyaluronan synthase 2
- ECM
extracellular matrix
- NHF
normal human lung fibroblasts
- ER
endoplasmic reticulum
- SKP2
S-phase kinase associated protein-2
- SA-β-gal
senescence-associated β-galactosidase
- ASMA
α-smooth muscle actin
Footnotes
Disclosure
The authors have no conflicting financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. Journal of hepatology. 2012;56:1171–1180. doi: 10.1016/j.jhep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 2.ATS/ERS. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 3.Moodley YP, Caterina P, Scaffidi AK, Misso NL, Papadimitriou JM, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA. Comparison of the morphological and biochemical changes in normal human lung fibroblasts and fibroblasts derived from lungs of patients with idiopathic pulmonary fibrosis during FasL-induced apoptosis. J Pathol. 2004;202:486–495. doi: 10.1002/path.1531. [DOI] [PubMed] [Google Scholar]
- 4.Henke C, Bitterman P, Roongta U, Ingbar D, Polunovsky V. Induction of fibroblast apoptosis by anti-CD44 antibody: implications for the treatment of fibroproliferative lung disease. Am J Pathol. 1996;149:1639–1650. [PMC free article] [PubMed] [Google Scholar]
- 5.Hadden HL, Henke CA. Induction of lung fibroblast apoptosis by soluble fibronectin peptides. Am J Respir Crit Care Med. 2000;162:1553–1560. doi: 10.1164/ajrccm.162.4.2001015. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz JC, Rogers DS, Simon RH, Sisson TH, Thannickal VJ. Plasminogen activation induced pericellular fibronectin proteolysis promotes fibroblast apoptosis. Am J Respir Cell Mol Biol. 2008;38:78–87. doi: 10.1165/rcmb.2007-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 8.Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Campisi J. Aging, cellular senescence, and cancer. Annual review of physiology. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L391–401. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi M, Kamiya N, Hirano J, Odaka M, Morikawa T, Nishimura SL, Kawabata Y, Hano H, Nakayama K, Kuwano K. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L56–69. doi: 10.1152/ajplung.00213.2012. [DOI] [PubMed] [Google Scholar]
- 14.Aoshiba K, Tsuji T, Nagai A. Bleomycin induces cellular senescence in alveolar epithelial cells. Eur Respir J. 2003;22:436–443. doi: 10.1183/09031936.03.00011903. [DOI] [PubMed] [Google Scholar]
- 15.Jiang D, Liang J, Noble PW. Regulation of non-infectious lung injury, inflammation, and repair by the extracellular matrix glycosaminoglycan hyaluronan. Anat Rec (Hoboken) 2010;293:982–985. doi: 10.1002/ar.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang D, Liang J, Noble P. Hyaluronan as an Immune Regulator in Human Diseases. Physiol Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauer ME, Dweik RA, Garantziotis S, Aronica MA. The Rise and Fall of Hyaluronan in Respiratory Diseases. Int J Cell Biol. 2015;2015:712507. doi: 10.1155/2015/712507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjermer L, Lundgren R, Hallgren R. Hyaluronan and type III procollagen peptide concentrations in bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. Thorax. 1989;44:126–131. doi: 10.1136/thx.44.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evanko SP, Potter-Perigo S, Petty LJ, Workman GA, Wight TN. Hyaluronan Controls the Deposition of Fibronectin and Collagen and Modulates TGF-beta1 Induction of Lung Myofibroblasts. Matrix Biol. 2015;42:74–92. doi: 10.1016/j.matbio.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Udabage L, Brownlee GR, Waltham M, Blick T, Walker EC, Heldin P, Nilsson SK, Thompson EW, Brown TJ. Antisense-mediated suppression of hyaluronan synthase 2 inhibits the tumorigenesis and progression of breast cancer. Cancer Res. 2005;65:6139–6150. doi: 10.1158/0008-5472.CAN-04-1622. [DOI] [PubMed] [Google Scholar]
- 23.Reed MJ, Damodarasamy M, Chan CK, Johnson MN, Wight TN, Vernon RB. Cleavage of hyaluronan is impaired in aged dermal wounds. Matrix Biol. 2013;32:45–51. doi: 10.1016/j.matbio.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes & development. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 27.d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 28.Noble PW. Idiopathic pulmonary fibrosis: natural history and prognosis. Clin Chest Med. 2006;27:S11–16. doi: 10.1016/j.ccm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Hammel P, Couvelard A, O’Toole D, Ratouis A, Sauvanet A, Flejou JF, Degott C, Belghiti J, Bernades P, Valla D, Ruszniewski P, Levy P. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. The New England journal of medicine. 2001;344:418–423. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- 30.Wynes MW, Edelman BL, Kostyk AG, Edwards MG, Coldren C, Groshong SD, Cosgrove GP, Redente EF, Bamberg A, Brown KK, Reisdorph N, Keith RC, Frankel SK, Riches DW. Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis: implications for fibroblast accumulation in idiopathic pulmonary fibrosis. J Immunol. 2011;187:527–537. doi: 10.4049/jimmunol.1100447. [DOI] [PubMed] [Google Scholar]
- 31.Huang SK, White ES, Wettlaufer SH, Grifka H, Hogaboam CM, Thannickal VJ, Horowitz JC, Peters-Golden M. Prostaglandin E(2) induces fibroblast apoptosis by modulating multiple survival pathways. FASEB J. 2009;23:4317–4326. doi: 10.1096/fj.08-128801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frankel SK, Cosgrove GP, Cha SI, Cool CD, Wynes MW, Edelman BL, Brown KK, Riches DW. TNF-alpha sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis. Am J Respir Cell Mol Biol. 2006;34:293–304. doi: 10.1165/rcmb.2005-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nature cell biology. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra247. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson RM, Meran S, Thomas D, Stephens P, Bowen T, Steadman R, Phillips A. Age-related changes in pericellular hyaluronan organization leads to impaired dermal fibroblast to myofibroblast differentiation. Am J Pathol. 2009;175:1915–1928. doi: 10.2353/ajpath.2009.090045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- 37.Suwan K, Choocheep K, Hatano S, Kongtawelert P, Kimata K, Watanabe H. Versican/PG-M Assembles Hyaluronan into Extracellular Matrix and Inhibits CD44-mediated Signaling toward Premature Senescence in Embryonic Fibroblasts. J Biol Chem. 2009;284:8596–8604. doi: 10.1074/jbc.M806927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rock K, Tigges J, Sass S, Schutze A, Florea AM, Fender AC, Theis FJ, Krutmann J, Boege F, Fritsche E, Reifenberger G, Fischer JW. miR-23a-3p causes cellular senescence by targeting hyaluronan synthase 2: possible implication for skin aging. The Journal of investigative dermatology. 2015;135:369–377. doi: 10.1038/jid.2014.422. [DOI] [PubMed] [Google Scholar]
- 39.Lompardia SL, Papademetrio DL, Mascaro M, Alvarez EM, Hajos SE. Human leukemic cell lines synthesize hyaluronan to avoid senescence and resist chemotherapy. Glycobiology. 2013;23:1463–1476. doi: 10.1093/glycob/cwt074. [DOI] [PubMed] [Google Scholar]
- 40.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 41.Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR, Pointer JN, Gruber SB, Su LD, Nikiforov MA, Kaufman RJ, Bastian BC, Soengas MS. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nature cell biology. 2006;8:1053–1063. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- 42.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. The New England journal of medicine. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 43.Toussaint O, Royer V, Salmon M, Remacle J. Stress-induced premature senescence and tissue ageing. Biochem Pharmacol. 2002;64:1007–1009. doi: 10.1016/s0006-2952(02)01170-x. [DOI] [PubMed] [Google Scholar]
- 44.Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, Cordon-Cardo C, Teruya-Feldstein J, Pandolfi PP. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campaner S, Doni M, Hydbring P, Verrecchia A, Bianchi L, Sardella D, Schleker T, Perna D, Tronnersjo S, Murga M, Fernandez-Capetillo O, Barbacid M, Larsson LG, Amati B. Cdk2 suppresses cellular senescence induced by the c-myc oncogene. Nature cell biology. 2010;12:54–59. 51–14. doi: 10.1038/ncb2004. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe K, Yamaguchi Y. Molecular identification of a putative human hyaluronan synthase. J Biol Chem. 1996;271:22945–22948. doi: 10.1074/jbc.271.38.22945. [DOI] [PubMed] [Google Scholar]
- 47.Brinck J, Heldin P. Expression of recombinant hyaluronan synthase (HAS) isoforms in CHO cells reduces cell migration and cell surface CD44. Experimental cell research. 1999;252:342–351. doi: 10.1006/excr.1999.4645. [DOI] [PubMed] [Google Scholar]
- 48.Kuroda Y, Kasai K, Nanashima N, Nozaka H, Nakano M, Chiba M, Yoneda M, Nakamura T. 4-Methylumbelliferone inhibits the phosphorylation of hyaluronan synthase 2 induced by 12-O-tetradecanoyl-phorbol-13-acetate. Biomed Res. 2013;34:97–103. doi: 10.2220/biomedres.34.97. [DOI] [PubMed] [Google Scholar]
- 49.Simpson RM, Wells A, Thomas D, Stephens P, Steadman R, Phillips A. Aging fibroblasts resist phenotypic maturation because of impaired hyaluronan-dependent CD44/epidermal growth factor receptor signaling. Am J Pathol. 2010;176:1215–1228. doi: 10.2353/ajpath.2010.090802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowe D, Raj K. Premature aging induced by radiation exhibits pro-atherosclerotic effects mediated by epigenetic activation of CD44 expression. Aging Cell. 2014;13:900–910. doi: 10.1111/acel.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mun GI, Boo YC. Identification of CD44 as a senescence-induced cell adhesion gene responsible for the enhanced monocyte recruitment to senescent endothelial cells. Am J Physiol Heart Circ Physiol. 2010;298:H2102–2111. doi: 10.1152/ajpheart.00835.2009. [DOI] [PubMed] [Google Scholar]
- 52.Bourguignon LY. Matrix hyaluronan-activated CD44 signaling promotes keratinocyte activities and improves abnormal epidermal functions. Am J Pathol. 2014;184:1912–1919. doi: 10.1016/j.ajpath.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Debidda M, Williams DA, Zheng Y. Rac1 GTPase regulates cell genomic stability and senescence. J Biol Chem. 2006;281:38519–38528. doi: 10.1074/jbc.M604607200. [DOI] [PubMed] [Google Scholar]
- 54.Costa ET, Forti FL, Matos TG, Dermargos A, Nakano F, Salotti J, Rocha KM, Asprino PF, Yoshihara CK, Koga MM, Armelin HA. Fibroblast growth factor 2 restrains Ras-driven proliferation of malignant cells by triggering RhoA-mediated senescence. Cancer Res. 2008;68:6215–6223. doi: 10.1158/0008-5472.CAN-08-0342. [DOI] [PubMed] [Google Scholar]
- 55.Meran S, Thomas D, Stephens P, Martin J, Bowen T, Phillips A, Steadman R. Involvement of hyaluronan in regulation of fibroblast phenotype. J Biol Chem. 2007;282:25687–25697. doi: 10.1074/jbc.M700773200. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Rahmanian M, Widstrom C, Lepperdinger G, Frost GI, Heldin P. Irradiation-induced expression of hyaluronan (HA) synthase 2 and hyaluronidase 2 genes in rat lung tissue accompanies active turnover of HA and induction of types I and III collagen gene expression. Am J Respir Cell Mol Biol. 2000;23:411–418. doi: 10.1165/ajrcmb.23.3.4102. [DOI] [PubMed] [Google Scholar]
- 57.Li L, Asteriou T, Bernert B, Heldin CH, Heldin P. Growth factor regulation of hyaluronan synthesis and degradation in human dermal fibroblasts: importance of hyaluronan for the mitogenic response of PDGF-BB. Biochem J. 2007;404:327–336. doi: 10.1042/BJ20061757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007;35:661–664. doi: 10.1042/BST0350661. [DOI] [PubMed] [Google Scholar]
- 59.Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1) Am J Respir Cell Mol Biol. 1999;21:658–665. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 60.Yamasaki M, Kang HR, Homer RJ, Chapoval SP, Cho SJ, Lee BJ, Elias JA, Lee CG. P21 regulates TGF-beta1-induced pulmonary responses via a TNF-alpha-signaling pathway. Am J Respir Cell Mol Biol. 2008;38:346–353. doi: 10.1165/rcmb.2007-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Li L, Brown TJ, Heldin P. Silencing of hyaluronan synthase 2 suppresses the malignant phenotype of invasive breast cancer cells. Int J Cancer. 2007;120:2557–2567. doi: 10.1002/ijc.22550. [DOI] [PubMed] [Google Scholar]
- 62.Chai S, Chai Q, Danielsen CC, Hjorth P, Nyengaard JR, Ledet T, Yamaguchi Y, Rasmussen LM, Wogensen L. Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circ Res. 2005;96:583–591. doi: 10.1161/01.RES.0000158963.37132.8b. [DOI] [PubMed] [Google Scholar]