ABSTRACT
With an increasing immunocompromised population which is linked to invasive fungal infections, it is clear that our present 3 classes of antifungal agents may not be sufficient to provide optimal management to these fragile patients. Furthermore, with widespread use of antifungal agents, drug-resistant fungal infections are on the rise. Therefore, there is some urgency to develop the antifungal pipeline with the goal of new antifungal agent discovery. In this review, a simple metabolic pathway, which forms the disaccharide, trehalose, will be characterized and its potential as a focus for antifungal target(s) explained. It possesses several important features for development of antifungal agents. First, it appears to have fungicidal characteristics and second, it is broad spectrum with importance across both ascomycete and basidiomycete species. Finally, this pathway is not found in mammals so theoretically specific inhibitors of the trehalose pathway and its enzymes in fungi should be relatively non-toxic for mammals. The trehalose pathway and its critical enzymes are now in a position to have directed antifungal discovery initiated in order to find a new class of antifungal drugs.
KEYWORDS: antifungal, drug development, trehalose
Introduction
The identification of new antifungal targets for the development of potential antifungal drugs has been rapidly accelerated and amplified because of both the clinical and economic climate.1,2 First, it is clear that there continues to be enlarging immunocompromised host populations such as transplant recipients, cancer victims, AIDS patients and those cared for in Intensive Care Units and it is in these fragile patients that invasive fungal diseases (IFD) arise and kill.3 Second, despite several classes of antifungal agents available for treatment, mortality rates in resource-available countries continue to hover in the 20–40%–range for IFDs depending on the clinical scenario and the infecting fungal species.4-7 The silent epidemic of IFDs has been generally managed by 3 antifungal classes: polyenes, azoles and echinocandins. Along with substantial clinical failures with these antifungal agents, there has been a pernicious acceleration in direct drug resistance to several antifungal classes.8-10 Although there has been remarkable progress in the management of fungal infections over the previous decades, improved antifungal drugs are urgently needed to possess rapid fungicidal potency, broad-spectrum antifungal activities and an excellent safety profile to reduce complications in this very sick patient population.
Fortunately, the antifungal pipeline has been rejuvenated by the slow but persistent ascent into clinical practice of fungal biomarkers such as β-D-glucan, mannan, galactomannan, lateral flow-detected antigens, PCR and T2 magnetic resonance technology so that diagnostic preciseness and earlier interventions with treatments have been improved. Furthermore, antifungal drug development has been pushed forward in a spectacular fashion by 2 economic factors. First, the GAIN (Generating Antibiotic Incentives Now) Act provides incentives to qualified infectious disease products like antifungal agents with fast track designations by the FDA. Second, the use of the Orphan Drug Act allows drugs for rare diseases such as fungal infections to qualify for 7-year orphan drug market exclusivity. Therefore, the perceived clinical needs for new antifungals with elevated mortality rates despite present treatments and the increasing drug resistance rates have met a favorable business climate to create the potential antifungal drug development tsunami occurring today.11,12
Over the last 2 decades our ability to identify and validate antifungal targets has significantly increased due to the development of sophisticated molecular biological techniques/manipulations of fungal pathogens13 combined with the establishment and integration of robust animal models of mycoses.13 This scientific progress has begun to allow rational drug designs in the antifungal space14 and recently, these carefully designed drugs such as the new azole VT-1161 have made it into clinical trials.15 In this investigative antifungal development milieu, there have been 2 major investigative goals to the antifungal drug discovery focus: (1) find a fungicidal target and its attendant inhibitor to efficiently eliminate pathogen(s) from the host; (2) because fungi and mammalian systems share basic eukaryotic cellular mechanisms, it is essential that the identified target and/or inhibitors have a favorable therapeutic-toxic ratio. In these fragile patients, the antifungal drug must be very safe. In the search for more antifungal drug(s), the fungal trehalose biosynthetic pathway has become a very interesting area to study as a potential target since it meets these 2 primary goals.
Trehalose α-D-glucopyranosyl-(1→1)-α-D-glucopyranoside is a simple non-reducing sugar containing 2 glucose subunits. This disaccharide has a substantial history in that it was first reported in 1832 in ergot of rye by Wiggers16 and in fact, there is even mention of it in 1681 as trehala in the cocoon of the Larinus butles.17 From these early beginnings, this sugar was extensively studied for its chemical properties and over time the entire simple pathway to trehalose formation and its degradation was deciphered in several biological systems. In Figure 1, there is a diagram of the proposed trehalose pathway for the basidiomycetous fungi, Cryptococcus neoformans and Cryptococcus gattii. The principle features of this pathway are its direct linkage to the glycolytic pathway18 and the presence of several critical enzymes including: Tps1,trehalose-6-phosphate synthase, and Tps2, trehalose-6-phosphate phosphatase, that participate in the formation of the disaccharide containing 2 glucose molecules. However, it is important to note that various fungal species may have different interactive and/or regulatory proteins within the trehalose biosynthetic complex but these 2 enzymes form the backbone of trehalose formation. Once trehalose is formed within a cell, it can then be degraded back to glucose by several described trehalases. A critical feature to this simple pathway as an antifungal target is that trehalose can be formed by bacteria, fungi, plants and invertebrates, but this sugar is not made by mammalian hosts and in fact, the enzyme machinery for the pathway has not been found in mammals. Importantly, in this respect is that targeting and blocking the pathway through its enzymes should have little consequences for mammal metabolism and biochemical networks. Thus, one can hypothesize that inhibitors and/or eventual drugs directed toward this pathway would have little toxicity for the host. In principle, this feature is extremely attractive for a target in the antifungal drug development space.
Figure 1.
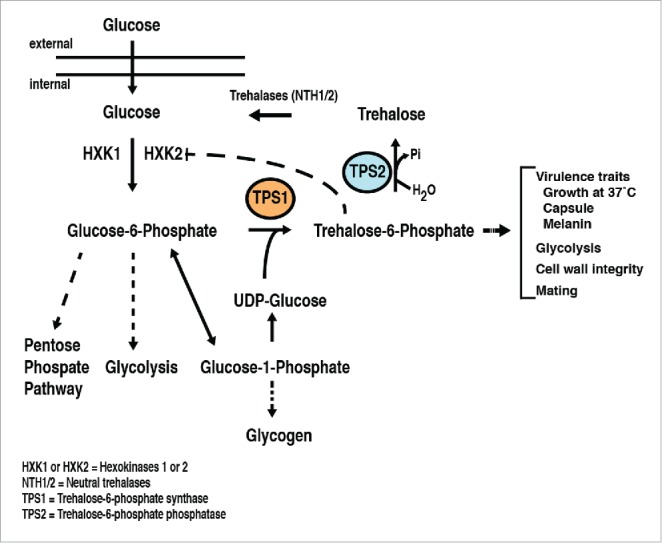
The putative trehalose pathway in cryptococcus.
What are the biological functions of trehalose in fungi?
Trehalose has been shown to be important for fungal conidia survival and germination secondary to its known function as a carbon source.19 It is an important component of the energy reserves for certain fungi as a carbohydrate source and with this stored energy, it might be primarily used during times of certain stresses. In fact, it appears that trehalose also has a critical role as a general stress protectant molecule for fungi in addition to its energy roles. Physically, it can interact with proteins and phospholipids to protect membrane structures and/or degradation or denaturation of intracellular proteins.20,21 It has been shown that trehalose and its pathway have been critical for survival of certain fungi at high environmental temperature stresses.22,23 However, there has been a shift in our understanding of the role of trehalose and the enzymes themselves as general stress protectants and more work is needed to clarify the roles.24 The importance of Tps1 in energy homeostasis and its impact on ATP levels during stress emphasizes the importance of this protein, outside of its role as a biosynthetic enzyme.24
This stress protectant function has been confirmed with the use of site-directed mutants in Candida albicans, Aspergillus nidulans and convincingly in both C. neoformans and C. gattii at mammalian body temperatures. Along with modulating temperature stresses, trehalose can act as a free radical scavenger under oxidative stress conditions and can protect against host cellular damages.25,26 Trehalose is important in the cell wall structure of pathogenic mycobacteria but the direct link in fungal cell wall structure is less certain, although it has been shown to have effects on cell wall and even capsule formation in cryptococcus.27
Trehalose and importantly, its intermediate trehalose-6-phosphate (T6P), are crucial to carbon metabolism and its regulation.28 It is likely that the intermediate molecule, T6P, is a major signaling molecule within the fungal cell.24,29 It is clear in Saccharomyces cerevisiae and Cryptococcus species that T6P inhibits the hexokinases and thus regulates glycolytic flux. In Magnaporthe oryzae, the rice blast fungus, T6P even controls carbon catabolite repression.30
What is the pathobiological impact of Tps1?
The first step in evaluation of an antifungal target is to identify its importance in disease through clinically-relevant animal models. There were 2 indirect observations in animal models that strongly suggest that Tps1 is important for survival of cryptococcus in a mammalian host. First, in studies on gene expression profiling of Cryptococcus neoformans cells taken directly from the subarachnoid space of rabbits, TPS1 was found to be highly expressed at this important site of infection. Second, mice brains infected with cryptococcus31 and its metabolites in the infected tissue were studied by NMR, substantial amounts of trehalose were found in and around murine brain cryptococcomas.32 Both these observations were only suggestive that the trehalose biosynthetic pathway played a part in the yeast stress reaction to the host. However, to be more certain of its impact, Δtps1 mutants in both C. neoformans and also C. gattii were created. In the murine disseminated model in which yeasts were inoculated through the lungs and the intracisternal rabbit model of meningoencephalitis, the Δtps1 mutants were severely attenuated in both models.27,33 While the Δtps1 mutant could not grow well at 37°C, it did survive in vitro but in the mammalian host it simply could not survive. It was a very rapid death of the yeasts from an initial large burden of infecting yeasts. The yeasts were consistently killed either in the lung or the central nervous system. Therefore, blockage of this target in cryptococcus was truly fungicidal in mammalian hosts and there was no apparent regrowth or survival of resistant colonies. These results in the host (fungicidal) vs the impact of high temperature on Δtps1 mutant survival in vitro (fungistatic) made us hypothesize that other stresses beside temperature also created the observed impact on yeast survival when this pathway was blocked in the host. This prediction was further validated when a cryptococcal Δtps1 mutant was found to be severely attenuated in both the nematode and zebrafish models34 in which host temperatures are substantially below mammalian body temperatures. In fact, their temperatures are in the range in which the mutant survives well and actually grows under simple in vitro nutritious conditions. We now appeared to have an in vivo fungicidal target (Tps1) in a variety of animal systems. However, it was interesting that even in highly-related species such as, C. neoformans and C. gattii there were some differences between the Δtps1 mutants in their specific impact on other virulence traits such as melanin and capsule biosynthesis.33 Despite these differences in network connections, the profound survival defect was consistent between the 2 species.
In contrast to the cryptococcal experience, the mold, Aspergillus fumigatus, has 2 TPS1 genes and when an aspergillus double Δtps1 mutant was made, there was not severe attenuation of virulence in a murine pulmonary aspergillosis model and in fact, it had a modest increase in virulence.35 The reasons for this difference in another fungal species could be multiple including the basic model system studied to the possible unique immunological responses induced by the mutant. In fact, there were some characteristics of the double Δtps1 mutant that suggested it would possess host survival defects. Furthermore, the C. albicans Δtps1 mutant has both decreased infectivity36 and macrophage survival.37 These observed differences between fungal species with respect to outcomes in Δtps1 mutants will need to be further understood.
Is Tps2 an antifungal target?
Phosphatases as druggable targets frequently have drawbacks because these enzymes commonly have “off target” activity or might have promiscuous phosphatase inhibitions. However, it is interesting and potentially helpful that our detailed biochemical studies have shown that the fungal phosphatase, Tps2, is very specific and has no characteristics that suggest it would be a promiscuous phosphatase enzyme target. For instance, it is very specific for only T6P and has no activity on other sugar phosphates or even the universal phosphatase substrate para-NitroPhenylPhosphate (pNPP).49 This substrate specificity has also been reported for the bacterial TPP (Tps2) from mycobacterium.38 As previously noted the cryptococcal Δtps2 mutant simply dies in vitro at 37°C and furthermore, cannot survive in the mammalian host. In addition, this target is truly broad spectrum in that Δtps2 mutants of C. albicans and A. fumigatus display attenuated virulence in mammalian hosts.39-42 It is likely that the variety of stresses in the mammalian host from high temperatures to host cell damage mechanisms creates high amounts of T6P inside the yeast cell with a specific phosphatase block and this important signaling molecule at high concentrations may actually become toxic to the fungus inside the cell and its machinery. Tps2 appears to be an ideal fungicidal, broad-spectrum antifungal target to identify inhibitors/drugs.
What about the trehalases and the degradative trehalose pathway?
The completion of the trehalose pathway includes the cleavage of trehalose to glucose by trehalases that allow the resulting glucose molecules to be utilized by the glycolytic pathway.43 This part of the pathway can clearly be used by fungi in the utilization of trehalose as a carbon source for growth. The number of trehalase genes can vary between fungal species and include both neutral and acid trehalase proteins. Relevant to the importance of trehalases to virulence, the neutral trehalase genes in C. albicans (NTC1) and cryptococcus (NTH1) do not appear to have any impact on these yeasts' virulence composite or general microbial fitness. However, an acid trehalose (ACT1) mutant in C. albicans had an attenuated phenotype in mice44 and in Magnaporthe oryzae the neutral trehalase (NTH1) mutant showed some attenuation of its disease production by failure of the mutant to colonize plant tissue30 Validamycin A is a known trehalase inhibitor and it can possess anti-cryptococcal activity in vitro under certain growth conditions (unpublished data). In C. albicans, validamycin A had limited antifungal effects.45 In addition, its ability to work as an antifungal agent in animal models has been more challenging to show. While the trehalases participate in the degradation of trehalose in fungi, their potential as a target for antifungal drug development is hampered by the presence of multiple enzymes, variable impact on fungal fitness, and as yet no robust data that this target would be a consistent, broad-spectrum antifungal target(s). However, further work is encouraged to understand the impact of the trehalose degradation pathway on fungal diseases since it remains only partially understood.
Are there other targets within the trehalose pathway?
Future efforts are needed to understand fully the entire trehalose biosynthetic complex in pathogenic fungi. For instance, S. cerevisiae has 2 additional proteins in the complex (TPS3/TSL1) and work with pathogenic fungi have found that the trehalose biosynthetic complex can contain other interacting proteins.46 Furthermore, it is likely that there are multiple trehalose transporter(s), but these have not been characterized in the major pathogenic fungi. However, S. cerevisiae has an identified trehalose transporter (Agt1). The trehalose biosynthetic pathway is integrated with glycolytic regulation, cellular homeostasis, and stress responses. Thus, these network connections will provide multiple strategies and targets to block in this central pathway for potential synergy. For example, we observed that the cryptococcal Δtps1 mutant was particularly susceptible in vitro compared to the wild-type strain to the cell stress inhibitors such as fludioxonil or FK506, which target the HOG1-MAPK and the calcineurin pathways, respectively. These early observations underscore the potential that this pathway and its inhibitors may be able to synergize with or potentiate other signaling/stress pathway inhibitors and/or drugs. Another example has been described for C. albicans, where virulence was significantly reduced in a strain lacking both TPS2 and GPR1, which regulates the cAMP pathway.39
Is there concern for development of drug resistance to this pathway?
In molecular studies, it was not difficult to spontaneously isolate from both Δtps1 and Δtps2 mutants in C. neoformans and C. gattii, colonies that suppressed the temperature-sensitive phenotype. These suppressors are being analyzed for exactly how this pathway for temperature sensitivity can be circumvented. However, despite this observation, in the host stress environment we have not observed any development of resistance clones directly from the mammalian host. The killings of cryptococcal Δtps1 and Δtps2 mutants are simply efficient and complete within the host despite very high inocula (108 CFU). There have not been any resistant colonies appearing in tissue, which suggests that development of resistant colonies to this block, if occurring, is a very infrequent survival event under the stresses of the mammalian host.
How to search for inhibitors of TPS1 and TPS2?
The strategy for this search has taken 2 avenues. First, the Tps1 and Tps2 enzymes or their catalytically germane domains from C. neoformans, C. albicans and A. fumigatus have been produced in purified and large quantities for high throughput- inhibitor screening assays. With the recombinant cryptococcal Tps1 protein we have developed a Transcreener UDP fluorescence-polarization assay to screen a library of 1300 bioactive molecules of which we observed several inhibitor hits. One of these inhibitors, closantel, had potent in vitro activity against C. neoformans and C. gattii strains with minimum inhibitory concentrations under 1 mg/mL. However, the first murine cryptococcosis experiments with closantel treatment did not yield anti-cryptococcal activity. Regardless, this robust system is now ready for further screening of other novel trehalose inhibitors of Tps1.
A secondary strategy is to pursue the crystal structures of Tps1 and Tps2 to design “rationally derived” inhibitors for these proteins based on their active site interactions with substrates, substrate analogs or inhibitors. To date, only bacterial E. coli Tps147 and the substrate-free structure of the Tps2 protein from the parasite Brugia malayi have been solved.48 The best structure-guided inhibitor design typically requires medium to high-resolution structures (normally 2.5 Å or lower). Therefore, we recently have determined the first eukaryotic, substrate and inhibitor bound Tps1 and Tps2 structures from multiple pathogenic fungal species, including C. neoformans, C. albicans and A. fumigatus. The Tps 2 structures have recently been published to show specific mechanisms of substrate recognition and catalysis.49 These structures, which are all high resolution, reveal all key interactions between Tps1 or Tps2 and their substrates or substrate analogs. Importantly, these structures revealed the architecture of the active site of each enzyme thereby significantly aiding in silico inhibitor design and its subsequent optimization. With over a dozen structures of Tps1 and Tps2 from multiple fungal pathogens determined either in their substrate-free conformations or as their substrate-bound or substrate analog-bound complexes combined with biochemical analysis and functional mutational mapping studies of the fungal enzymes, this area is ready to be exploited for rational design50 to identify inhibitors of the trehalose biosynthetic pathway enzymes that may lead to treat fungal infections.
In summary, investigations into the molecular pathogenesis of cryptococcal disease have directed us to the trehalose biosynthetic pathway. Its pathobiological importance has been validated and its uniqueness is being explored. The tools are now available to make a concerted effort to explore the pathway as an entry point to discover potent, broad-spectrum antifungal agents. We anticipate that paradigms of the trehalose biosynthetic pathway will continue to shift with the increased effort toward uncovering its role in the cell and during pathogenesis and that the role of Tps1 and Tps2 in the cell will be expanded beyond what is currently described.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by U.S. Public Service grants P01 AI 04533 (to JRP and RGB), and R01s AI 73896 and AI 93257 (to JRP).
References
- [1].Butts A, Krysan DJ. Antifungal drug discovery: something old and something new. PLoS Pathog 2012; 8:e1002870; PMID:22969422; http://dx.doi.org/ 10.1371/journal.ppat.1002870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov 2010; 9:719-27; PMID:20725094; http://dx.doi.org/ 10.1038/nrd3074 [DOI] [PubMed] [Google Scholar]
- [3].Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med 2012; 4:165rv13; PMID:23253612; http://dx.doi.org/ 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- [4].Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 2005; 41:1232-9; PMID:16206095; http://dx.doi.org/ 10.1086/496922 [DOI] [PubMed] [Google Scholar]
- [5].Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis 2012; 73:45-8; PMID:22578938; http://dx.doi.org/ 10.1016/j.diagmicrobio.2012.02.001 [DOI] [PubMed] [Google Scholar]
- [6].Bratton E, EI Husseini N, Chastain C, Lee M, Poole C, Sturmer T, Juliano J, Weber D, Perfect J. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PloS One 2012; 7(8):e43582; PMID:22937064; http://dx.doi.org/ 10.1371/journal.pone.0043582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, Heinz WJ, Jagannatha S, Koh LP, Kontoyiannis DP, et al.. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med 2015; 162:81-9; PMID:25599346; http://dx.doi.org/ 10.7326/M13-2508 [DOI] [PubMed] [Google Scholar]
- [8].Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Bruggemann RJ, Chowdhary A, Cornely OA, Denning DW, Groll AH, Izumikawa K, et al.. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updates 2015; 21-22:30-40; PMID:26282594; http://dx.doi.org/ 10.1016/j.drup.2015.08.001 [DOI] [PubMed] [Google Scholar]
- [9].Alexander BD JM, Pfeiffer CD, Jiménez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 2013; 56:1724-32; PMID:23487382; http://dx.doi.org/ 10.1093/cid/cit136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shor E, Perlin DS. Coping with stress and the emergence of multidrug resistance in fungi. PLoS Pathog 2015; 11:e1004668; PMID:25790300; http://dx.doi.org/ 10.1371/journal.ppat.1004668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Perfect JR. “Is there an emerging need for new antifungals?.” Expert Opin Emerg Drugs 2016:1-3; PMID:2666760926667609 [Google Scholar]
- [12].Denning DW, Bromley MJ. Infectious Disease. How to bolster the antifungal pipeline. Science 2015; 347:1414-6; PMID:25814567; http://dx.doi.org/ 10.1126/science.aaa6097 [DOI] [PubMed] [Google Scholar]
- [13].Casadevall A, Mitchell A, Berman J. CSH Monograph on Human Fungal Pathogens. Cold Spring Harbor Laboratory Press; 2014; pp.1-563. [Google Scholar]
- [14].Medina Marrero R, Marrero-Ponce Y, Barigye SJ, Echeverria Diaz Y, Acevedo-Barrios R, Casanola-Martin GM, Garcia Bernal M, Torrens F, Perez-Gimenez F. QuBiLs-MAS method in early drug discovery and rational drug identification of antifungal agents. SAR QSAR Environ Res 2015; 26:943-58; PMID:26567876; http://dx.doi.org/ 10.1080/1062936X.2015.1104517 [DOI] [PubMed] [Google Scholar]
- [15].Warrilow AG, Hull CM, Parker JE, Garvey EP, Hoekstra WJ, Moore WR, Schotzinger RJ, Kelly DE, Kelly SL. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother 2014; 58:7121-7; PMID:25224009; http://dx.doi.org/ 10.1128/AAC.03707-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Harding T. History of trehalose. Its discovery and methods of preparation 1923; 25:476-8. [Google Scholar]
- [17].Hanbury D. Note on Two Insect-products from Persia. J Proc Lin Soc London 1859; 3:178-83; http://dx.doi.org/ 10.1111/j.1096-3642.1859.tb00078.x [DOI] [Google Scholar]
- [18].Thevelein JM, Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem Sci 1995; 20:3-10; http://dx.doi.org/ 10.1016/S0968-0004(00)88938-0 [DOI] [PubMed] [Google Scholar]
- [19].Lillie SH, Pringle JR. Reserve carbohydrate metabolism in Saccharomyces cerevisiae : responses to nutrient limitation. J Bacteriol 1980; 143:1384-94; PMID:6997270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Crowe JH, Crowe LM, Chapman D. Preservation of membranes in anhydrobiotic organisms; the role of trehalose. Science 2006; 233 701-3. [DOI] [PubMed] [Google Scholar]
- [21].Gadd GM, Chalmers K, Reed RH. The role of trehalose in dehydration resistance of Saccharomyces cerevisiae. FEMS Microbiol Lett 1987; 48 249-54; http://dx.doi.org/ 10.1111/j.1574-6968.1987.tb02551.x [DOI] [Google Scholar]
- [22].Elliott B, Haltiwanger RS, Futcher B. Synergy between trehalose and Hsp104 for thermotolerance in Saccharomyces cerevisiae. Genetics 1996; 144:923-33; PMID:8913738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Singer MA, Lindquist S. Thermotolerance in Saccharomyces cerevisiae : the Yin and Yang of trehalose. Trends Biotechnol 1998; 16:460-8; PMID:9830154; http://dx.doi.org/ 10.1016/S0167-7799(98)01251-7 [DOI] [PubMed] [Google Scholar]
- [24].Petitjean M, Teste MA, Francois JM, Parrou JL. Yeast Tolerance to Various Stresses Relies on the Trehalose-6P Synthase (Tps1) Protein, Not on Trehalose. J Biol Chem 2015; 290:16177-90; PMID:25934390; http://dx.doi.org/ 10.1074/jbc.M115.653899 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [25].Wiemken A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Van Leeuwenhoek 1990; 58:209-17; PMID:2256682; http://dx.doi.org/ 10.1007/BF00548935 [DOI] [PubMed] [Google Scholar]
- [26].Lewis JG, Learmonth RP, Watson K. Induction of heat, freezing and salt tolerance by heat and salt shock in Saccharomyces cerevisiae. Microbiology 1995; 141 (Pt 3):687-94; PMID:7711907; http://dx.doi.org/ 10.1099/13500872-141-3-687 [DOI] [PubMed] [Google Scholar]
- [27].Ngamskulrungroj P, Himmelreich V, Breger JA, Wilson C, Chayakulkeeree M, Krockenberger MB, Malik R, Daniel HM, Toffaletti D, Djordjevic JT, et al.. The trehalose pathway: an integral part of virulence composite for Cryptococcus gattii. Infect Immun 2009; 77:4584-96; PMID:19651856; http://dx.doi.org/ 10.1128/IAI.00565-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wilson RA, Jenkinson JM, Gibson RP, Littlechild JA, Wang ZY, Talbot NJ. Tps1 regulates the pentose phosphate pathway, nitrogen metabolism and fungal virulence. EMBO J 2007; 26:3673-85; PMID:17641690; http://dx.doi.org/ 10.1038/sj.emboj.7601795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].O'Hara LE, Paul MJ, Wingler A. How do sugars regulate plant growth and development? New insight into the role of trehalose-6-phosphate. Mol Plant 2013; 6:261-74; PMID:23100484; http://dx.doi.org/ 10.1093/mp/sss120 [DOI] [PubMed] [Google Scholar]
- [30].Foster AJ, Jenkinson JM, Talbot NJ. Trehalose synthesis and metabolism are required at different stages of plant infection by Magnaporthe grisea. EMBO J 2003; 22:225-35; PMID:12514128; http://dx.doi.org/ 10.1093/emboj/cdg018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Steen BR, Zuyderduyn S, Toffaletti DL, Marra M, Jones SJ, Perfect JR, Kronstad J. Cryptococcus neoformans gene expression during experimental cryptococcal meningitis. Eukaryot Cell 2003; 2:1336-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Himmelreich U, Dzendrowskyj TE, Allen C, Dowd S, Malik R, Shehan BP, Russell P, Mountford CE, Sorrell TC. Cryptococcomas distinguished from gliomas with MR spectroscopy: an experimental rat and cell culture study. Radiology 2001; 220:122-8; PMID:11425983; http://dx.doi.org/ 10.1148/radiology.220.1.r01jl25122 [DOI] [PubMed] [Google Scholar]
- [33].Petzold EW, Himmelreich U, Mylonakis E, Rude T, Toffaletti D, Cox GM, Miller JL, Perfect JR. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect Immun 2006; 74:5877-87; PMID:16988267; http://dx.doi.org/ 10.1128/IAI.00624-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tenor JL, Oehlers SH, Yang JL, Tobin DM, Perfect JR. Live Imaging of Host-Parasite Interactions in a Zebrafish Infection Model Reveals Cryptococcal Determinants of Virulence and Central Nervous System Invasion. MBio 2015; 6:e01425-15; PMID:26419880; http://dx.doi.org/ 10.1128/mBio.01425-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Al-Bader N, Vanier G, Liu H, Gravelat FN, Urb M, Hoareau CM, Campoli P, Chabot J, Filler SG, Sheppard DC. Role of trehalose biosynthesis in Aspergillus fumigatus development, stress response, and virulence. Infect Immun 2010; 78:3007-18; PMID:20439478; http://dx.doi.org/ 10.1128/IAI.00813-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zaragoza O, Blazquez MA, Gancedo C. Disruption of the Candida albicans TPS1 gene encoding trehalose-6-phosphate synthase impairs formation of hyphae and decreases infectivity. J Bacteriol 1998; 180:3809-15; PMID:9683476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Martinez-Esparza M, Aguinaga A, Gonzalez-Parraga P, Garcia-Penarrubia P, Jouault T, Arguelles JC. Role of trehalose in resistance to macrophage killing: study with a tps1/tps1 trehalose-deficient mutant of Candida albicans. Clin Microbiol Infect 2007; 13:384-94; PMID:17359322; http://dx.doi.org/ 10.1111/j.1469-0691.2007.01663.x [DOI] [PubMed] [Google Scholar]
- [38].Klutts S, Pastuszak I, Edavana VK, Thampi P, Pan YT, Abraham EC, Carroll JD, Elbein AD. Purification, cloning, expression, and properties of mycobacterial trehalose- phosphate phosphatase. J Biol Chem 2003; 278:2093-100; PMID:12417583; http://dx.doi.org/ 10.1074/jbc.M209937200 [DOI] [PubMed] [Google Scholar]
- [39].Maidan MM, De Rop L, Relloso M, Diez-Orejas R, Thevelein JM, Van Dijck P. Combined inactivation of the Candida albicans GPR1 and TPS2 genes results in avirulence in a mouse model for systemic infection. Infect Immun 2008; 76:1686-94; PMID:18268028; http://dx.doi.org/ 10.1128/IAI.01497-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van Dijck P, De Rop L, Szlufcik K, Van Ael E, Thevelein JM. Disruption of the Candida albicans TPS2 gene encoding trehalose-6-phosphate phosphatase decreases infectivity without affecting hypha formation. Infect Immun 2002; 70:1772-82; PMID:11895938; http://dx.doi.org/ 10.1128/IAI.70.4.1772-1782.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zaragoza O, de Virgilio C, Ponton J, Gancedo C. Disruption in Candida albicans of the TPS2 gene encoding trehalose-6-phosphate phosphatase affects cell integrity and decreases infectivity. Microbiology 2002; 148:1281-90; http://dx.doi.org/ 10.1099/00221287-148-5-1281 [DOI] [PubMed] [Google Scholar]
- [42].Puttikamonkul S, Willger SD, Grahl N, Perfect JR, Movahed N, Bothner B, Park S, Paderu P, Perlin DS, Cramer RA Jr. Trehalose 6-phosphate phosphatase is required for cell wall integrity and fungal virulence but not trehalose biosynthesis in the human fungal pathogen Aspergillus fumigatus. Mol Microbiol 2010; 77:891-911; PMID:20545865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sanchez-Fresneda R, Gonzalez-Parraga P, Esteban O, Laforet L, Valentin E, Arguelles JC. On the biochemical classification of yeast trehalases: Candida albicans contains two enzymes with mixed features of neutral and acid trehalase activities. Biochem Biophys Res Commun 2009; 383:98-102; PMID:19336219; http://dx.doi.org/ 10.1016/j.bbrc.2009.03.134 [DOI] [PubMed] [Google Scholar]
- [44].Pedreno Y, Gonzalez-Parraga P, Martinez-Esparza M, Sentandreu R, Valentin E, Arguelles JC. Disruption of the Candida albicans ATC1 gene encoding a cell-linked acid trehalase decreases hypha formation and infectivity without affecting resistance to oxidative stress. Microbiology 2007; 153:1372-81; PMID:17464051; http://dx.doi.org/ 10.1099/mic.0.2006/003921-0 [DOI] [PubMed] [Google Scholar]
- [45].Guirao-Abad JP, Sanchez-Fresneda R, Valentin E, Martinez-Esparza M, Arguelles JC. Analysis of validamycin as a potential antifungal compound against Candida albicans. Int Microbiol 2013; 16:217-25; PMID:25102722 [DOI] [PubMed] [Google Scholar]
- [46].Bell W, Sun W, Hohmann S, Wera S, Reinders A, De Virgilio C, Wiemken A, Thevelein JM. Composition and functional analysis of the Saccharomyces cerevisiae trehalose synthase complex. J Biol Chem 1998; 273:33311-9; PMID:9837904; http://dx.doi.org/ 10.1074/jbc.273.50.33311 [DOI] [PubMed] [Google Scholar]
- [47].Gibson RP, Turkenburg JP, Charnock SJ, Lloyd R, Davies GJ. Insights into trehalose synthesis provided by the structure of the retaining glucosyltransferase OtsA. Chem Biol 2002; 9:1337-46; PMID:12498887; http://dx.doi.org/ 10.1016/S1074-5521(02)00292-2 [DOI] [PubMed] [Google Scholar]
- [48].Farelli JD, Galvin BD, Li Z, Liu C, Aono M, Garland M, Hallett OE, Causey TB, Ali-Reynolds A, Saltzberg DJ, et al.. Structure of the trehalose-6-phosphate phosphatase from Brugia malayi reveals key design principles for anthelmintic drugs. PLoS Pathog 2014; 10:e1004245; PMID:24992307; http://dx.doi.org/ 10.1371/journal.ppat.1004245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Miao Y, JL T, Toffaletti D, Perfect J, Brennan R. Structures of trehalose-6-phosphate phosphatase from pathogenic fungi reveal the mechanisms of substrate recognition and catalysis. Proc Nat Acad Sci 2016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xue Y, Shui G, Wenk MR. TPS1 drug design for rice blast disease in magnaporthe oryzae. SpringerPlus 2014; 3:18; PMID:24478940; http://dx.doi.org/ 10.1186/2193-1801-3-18 [DOI] [PMC free article] [PubMed] [Google Scholar]


