Abstract
Background
The Neuberger review made a number of recommendations to improve end of life care, including research into the biology of dying. An important aspect of the biology of dying is the identification of biomarkers as indices of disease processes. Biomarkers have the potential to inform the current, limited understanding of the dying process and assist clinicians in recognising dying, in particular how to distinguish dying from reversible acute deterioration.
Objectives
To critically appraise the literature on biological factors that may be used as prognostic indicators in advanced cancer patients and to identify candidate biomarkers of the dying process that can be measured serially in cancer patients’ bodily fluids.
Methods
A systematically structured review was conducted using three electronic databases. A hand search of six peer-reviewed journals and conference abstracts was also conducted. Studies reporting prognostic biomarkers in cancer patients with a median survival of ≤90 days and post-mortem studies were included. Final levels of evidence and recommendations were made using the Evidence Based Medicine modified GRADE system.
Results
30 articles were included. Seven prognostic biological factors demonstrated Grade A evidence (lymphocyte count, white blood cell count, serum C-reactive protein, albumin, sodium, urea and alkaline phosphatase). An additional eleven prognostic factors were identified with Grade B evidence (platelet count, international normalised ratio, serum vitamin B12, prealbumin, bilirubin, cholesterol, aspartate aminotransferase, alanine transaminase, lactate dehydrogenase, pseudocholinesterase and urate). A number of biomarkers were specifically identified in the last two weeks of life but limitations exist. No post-mortem studies met the inclusion criteria.
Conclusion
The biology of dying is an important area for future research, with the evidence focused on signs, symptoms and prognostic factors. This review identifies a number of common themes shared amongst advanced cancer patients and highlights candidate biomarkers which may be indicative of a common biological process to dying.
Background
In a comprehensive evaluation of the challenges and actions required to provide the best care for dying patients, the Neuberger review identified the need for further research into the biology of dying as a priority [1]. The biology of dying is an umbrella topic that encompasses the physiological and biological changes attributed to the dying process, in addition to the aetiology of signs and symptoms commonly seen in the last days, weeks and months of life.
There is significant uncertainty in consistently and accurately identifying the dying phase (last days of life) and no definitive diagnostic criteria exist [1,2]. Little is known about the process of dying [1] and in the final days of life, new symptoms or exacerbation or recurrence of previously well-controlled symptoms can occur [3,4]. A prospective cohort study of 343 doctors found only 20% of prognostic estimates in hospice patients were accurate and that, overall doctors overestimated survival by a factor of five [5].
It has been extensively documented that cancer patients experience a sharp functional decline in the last months of life [6]. The prevalence of common terminal symptoms amongst patients with advanced cancer is suggestive of a common terminal trajectory; historically termed the “terminal cancer syndrome” [7]. Although the concept of the “terminal cancer syndrome” has been largely superseded, there is significant evidence for the prognostic importance of dyspnoea (Grade B), cancer anorexia-cachexia syndrome (CACS, Grade B), delirium (Grade B) and low performance status (Grade A) in advanced cancer [8]. A systematic review by Kehl et al. demonstrated that dyspnoea (56.7%), pain (52.4%), respiratory tract secretions (51.4%) and confusion (50.1%) were the most prevalent signs and symptoms that occur in the last two weeks of life [9]. Further, Hui et al. identified 13 signs highly predictive of death within three days [10,11].
An important aspect of the biology of dying is the identification of biomarkers as indices of disease processes. Biomarkers have the potential to inform the current, limited understanding of the process of dying and assist clinicians in recognising dying, in particular how to distinguish dying from reversible acute deterioration. In 2005, Maltoni et al. published evidence-based recommendations for a number of prognostic biological factors in advanced cancer patients [8]. Subsequently, a number of prognostic models have been developed that incorporate prognostic biomarkers [12]. No systematic reviews have been conducted that summarise the evidence for prognostic biomarkers in advanced cancer patients.
In this systematically structured review, the authors summarise the evidence for prognostic biomarkers in advanced cancer patients in the last months of life and extrapolate which biological processes are affected.
Objectives
This systematically structured review was conducted to collate and critically appraise the literature on prognostic biomarkers in advanced cancer. The following questions formed the basis of this review:
What biological factors are prognostic in advanced cancer patients in the last days, weeks or months of life?
Can serial measurement of identified biomarkers detect the last days to weeks of life in advanced cancer patients?
Methods
Given that no randomised controlled trials have been conducted on this research topic, a systematically structured review was conducted to ensure a replicable and systematic synthesis of the evidence. The review was structured according to the PRISMA standards for conducting a systematic review [13].
Literature search methods
On 5th February 2016, three electronic databases were searched (Medline, Scopus and Cochrane Database of Systematic Reviews) using combinations of the key words, described in Table 1. Limits were set to humans, adults (aged over 18 years of age) and publication between 1st January 2000 and 5th February 2016. Only published articles were sought. Two reviewers (VLR and SC) independently searched all designated databases for abstracts and titles. Agreement on inclusion was made by consensus. Additional articles were identified through a hand search of contents pages of the most recent issues (December 2014 to February 2016) of six relevant peer-reviewed Palliative Medicine journals: Cancer, BMJ Supportive and Palliative Care, Palliative Medicine, Journal of Palliative Care, Journal of Palliative Medicine, and Journal of Pain and Symptom Management. Grey literature was searched through citation tracking and conference abstracts from the European Association of Palliative Care World Congress 2016, European Association of Palliative Care World Congress 2015, the Marie Curie Research Conference 2015, the Arts and Science of Hospice Care Annual Conference 2015, the 5th International Conference on Advance Care Planning and End of Life Care 2015, and the International Congress of Palliative Care 2014. The last literature search was conducted on 16th June 2016. Institutional review board approval was not sought or required for this literature review.
Table 1. Search strategy for medline.
| Query Number | Query Content |
|---|---|
| #1 | death OR dying OR terminal care OR terminal illness OR “terminally ill” OR hospice* OR palliative care |
| #2 | biolog* OR physiolog* OR pathophysiolog* OR patholog* OR biomarker* OR biologic marker* OR biological marker* OR biological factor* OR “biology of dying” OR prognostic* OR prognosis OR predict OR mortality OR “terminal cancer syndrome” OR “common terminal pathway” OR “common terminal trajectory” OR “final common pathway” OR “terminal syndrome” |
| #3 | cancer OR tumor* OR tumour* OR neoplas* OR oncolog* OR carcinoma |
| #4 | #1 AND #2 AND #3 |
| #5 | Limit #4 to humans, all adults (19 years plus), publication year 2000—current |
*Truncation
Eligibility criteria
The primary objective was the identification of prognostic biomarkers in advanced cancer patients in the last days, weeks or months of life. Biomarkers were defined as objective, quantifiable characteristics of biological processes [14] quantifiable in bodily fluids and tissues. Given that most cancer patients seem to follow a common terminal trajectory, [6] only patients with cancer were included in this study.
Diagnosing imminent death is often difficult and imprecise [1] and terminal physiological changes are often seen many weeks before death. Further, the definition of “advanced cancer” is often lacking in the literature. In line with the study by Maltoni et al. this review included only study populations with a median survival of ≤90 days [8] that, for the purpose of this study, defines the term “advanced cancer.” This criterion enabled us to capture biological factors predictive of dying over days, weeks and months of life. Post-mortem studies of cancer patients specifically looking at biomarkers of the dying process were also included.
All types of peer-reviewed evidence were included and a 16-year timeframe was selected to ensure comprehensive yet current coverage of the literature, and build on the excellent review published in 2005 by Maltoni et al (last literature search conducted in 2003).
Articles were excluded if they described only signs, symptoms or physiological changes associated with imminent death. The following types of articles were also excluded: duplicates, non-English language, paediatric populations, editorials, commentaries, case reports, reviews and animal studies. The systematic review by Maltoni et al. was included as it provided an evidence-based summary of the literature up until 2005 [8]. Primary researchers were contacted by email, where necessary, to clarify information to ensure strict adherence to the inclusion criteria. Where survival data could not be confirmed, studies were excluded.
Study selection
A review protocol was developed by VLR in advance of the literature search (S2 File). VLR extracted the data from the studies and discussed the results with SC. Disagreements between reviewers were resolved by consensus. Reviewers were not blinded for authors, institutions, or journals of publication. The Liverpool Reviews and Implementation Group (LRiG) at University of Liverpool reviewed and agreed on the employed methodology.
Quality assessment
The primary authors (VLR and SC) used the UK National Institute for Health and Clinical Excellence (NICE) hierarchy of evidence to assign a quality rating to the potential articles for inclusion [15]. Given that a variety of research outputs were considered in this review, a critical appraisal tool described by Hawker et al. [16] was utilised to further evaluate the quality of studies. Briefly, a four-point scale from one (very poor) to four (good) was assigned to nine areas including: abstract and title, introduction and aims, methods and data, sampling, data analysis, ethics and bias, results, transferability/generalisability, and implications and usefulness with a total number between 9–36 assigned to each study [16]. The Maltoni et al. seven-point checklist of quality criteria for evaluation of studies on prognostic factors was also utilised, where one point is assigned to seven criteria including; prospective study design; well-defined cohort of patients assembled at a common point in the course of their disease; random patient selection, percentage of patients lost to follow-up ≤20%; ratio between the number of events (death) and the number of potential predictors ≥10; prognostic variables fully defined, accurately measured, and available for all or a high proportion of patients; and reliable measurement of outcome (date of death) [8]. A total score between 1–7 was assigned and high quality (or low probability of bias) was attributed to studies fulfilling at least five of the seven criteria [8]. VLR and SC assessed the quality of potential articles for inclusion independently and mean scores were assigned. Spearman’s rank correlation coefficient was used to measure rank correlations of scores between assessors. Data was analyzed using Statistical Software Package for the Social Sciences® (SPSS® version 22.0; IBM SPSS Inc., Chicago, IL). Final levels of evidence and recommendations were made using the Evidence Based Medicine modified Grading of Recommendations Assessments, Development and Evaluation (GRADE) system (Grade A = high quality evidence with consistent results, to Grade D = very low quality evidence such as expert opinion) [17].
Results
Study selection
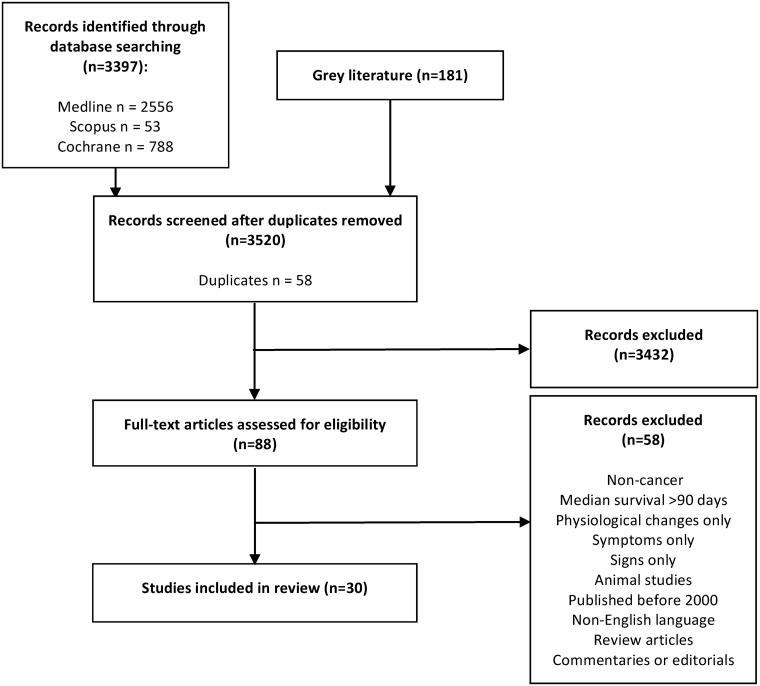
The results of the literature search are summarised in Fig 1. Based on firm application of the inclusion criteria, 30 articles were included in this systematically structured review (Table 2). A selected number of articles were excluded as patient populations had a median survival >90 days. An additional three articles were excluded due to lack of survival data.
Fig 1. PRISMA flow diagram of this systematically structured review.
Table 2. Summary of included studies.
| Citation (author, year, country) | Design & Objectives | Setting and Sample | Diagnosis | Days until Death | Weaknesses | Appraisal | Main Study Findings |
|---|---|---|---|---|---|---|---|
| Geissbűhler, P et al. 2000, Switzerland [18] |
|
|
|
|
|
|
|
| Pasanisi, F et al. 2001, Italy [19] |
|
|
|
|
|
|
|
| Glare, P et al. 2001, Australia [20] |
|
|
|
|
|
|
|
| McMillan, DC et al. 2001, UK [22] |
|
|
|
|
|
|
|
| Faris M. 2003, Oman [23] |
|
|
|
|
|
|
|
| Ho SY et al. 2003, Taiwan [24] |
|
|
|
|
|
|
|
| Iwase S, et al. 2004, Japan [25] |
|
|
|
|
|
|
|
| Maltoni M et al. 2005, Italy [8] |
|
|
|
|
|
|
|
| Shin, HS et al. 2006, Korea [26] |
|
|
|
|
|
|
|
| Lam PT et al. 2007, China [27] |
|
|
|
|
|
|
|
| Kelly, L et al. 2007, UK [28] |
|
|
|
|
|
|
|
| Suh, SY et al. 2007, South Korea [29] |
|
|
|
|
|
|
|
| Alsirafy SA et al. 2009, Saudi Arabia [30] |
|
|
|
|
|
|
|
| Hyodo, I et al. 2010, Japan [31] |
|
|
|
|
|
|
|
| Tarumi, Y et al. 2011, Canada [32] |
|
|
|
|
|
|
|
| Feliu, J et al. 2011, Spain [33] |
|
|
|
|
|
|
|
| Gwilliam, B et al. 2011, UK [34] |
|
|
|
|
|
|
|
| Cui, J et al. 2014, China [35] |
|
|
|
|
|
|
|
| Kim, ES et al. 2014, Korea [36] |
|
|
|
|
|
|
|
| Amano, K et al. 2015, Japan [37] |
|
|
|
|
|
|
|
| Baba, M et al. 2015, Japan [38] |
|
|
|
|
|
|
|
| Baba, M et al. 2015, Japan [39] |
|
|
|
|
|
|
|
| Malik S et al. 2015, Australia [40] |
|
|
|
|
|
|
|
| Taylor, P et al. 2015. UK [41] |
|
|
|
|
|
|
|
| Yoon, J et al. 2015, Korea [42] |
|
|
|
|
|
|
|
| Miura, T et al. 2015, Japan [43] |
|
|
|
|
|
|
|
| Coyle S et al. 2016, UK [44] |
|
|
|
|
|
|
|
| Niki K et al. 2016, Japan [45] |
|
|
|
|
|
|
|
| Paulsen O et al. 2016, Norway [46] |
|
|
|
|
|
|
|
| Wrafter S et al. 2016, Ireland [47] |
|
|
|
|
|
|
Only prognostic factors relevant to the objectives of this review are presented here.
AGP = α1-acid glycoprotein; ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate transaminase; AUC = area under the curve; BCI = B12 CRP index; BUN = blood urea nitrogen; B12 = vitamin B12; CACS = cancer anorexia-cachexia syndrome; CAR = CRP/albumin ratio; CPS = Clinicians’ Prediction of Survival; CRP = C-reactive protein; D-PAP = Delirium-Palliative Prognostic Score; ECOG PS = Eastern Cooperative Oncology Group Performance Status; ESR = erythrocyte sedimentation rate; GC-MS = Gas Chromatography Mass Spectrometry; GGT = gamma-glutamyl transpeptidase; GPS = Glasgow Prognostic Score; IFNγ = interferon gamma; IL-1ra = interleukin-1 receptor antagonist; IL-1β = interleurkin-1 beta; IL-2 = interleukin-2; IL-4 = interleukin-4; IL-6 = interleukin-6; IL-8 = interleukin-8; IL-10 = interleukin-10; IL-12(p70) = interleukin-12(p70); IL-18 = interleukin-18; INR = international normalised ratio; JPOS-PI = Japan Palliative Oncology Study-Prognostic Index; KPS = Karnofsky Performance Status; LDH = lactate dehydrogenase; LIF = leukaemia inhibiting factor; MCP-1 = monocyte chemoattractant protein-1; m-GPS = modified Glasgow Prognostic Score; MIF = macrophage migration inhibitory factor; MIP-1α = macrophage inflammatory protein-1α; MSSE = mini mental state examination; new-ChPS = new-Chinese Advanced Cancer Patients Scale; NLR = neutrophil-lymphocyte ratio; NMR = Nuclear Magnetic Resonance Spectrometry; NPV = negative predictive value; PaP Score = Palliative Prognostic Score; PINI = Prognostic Inflammatory and Nutritional Index; PiPS = Prognosis in Palliative Care Study; PiPS-A = modified Prognosis in Palliative Care Study-A; PiPS-B = modified Prognosis in Palliative Care Study-B; PPI = Palliative Performance Index; PPV = positive predictive value; PTH = parathyroid hormone; PTHrP = parathyroid hormone related protein; RNA = ribonucleic acid; sTNF- r1 = soluble tumour necrosis factor receptor 1; TGF-β1 = transforming growth factor β1; TNFα = tumour necrosis factor alpha; TTD = time from initial diagnosis to diagnosis of terminal disease; VOCs = volatile organic compounds; WBC = white blood cell count; WPBAL = WBC/platelet/BUN/AST/LDH prognostic score; WPCBAL = WBC/platelet/CRP/BUN/AST/LDH prognostic score.
Study characteristics
The characteristics of the included studies are summarised in Table 2. A formal meta-analysis was not conducted because of the heterogeneity of the published studies. A number of studies utilised univariate rather than multivariate analysis. Both types of studies were included to ensure a comprehensive summary of the literature. The use of the Evidence Based Medicine modified GRADE system has highlighted the limitations of univariate analysis for these types of studies [17].
Table 3 subdivides prognostic biomarkers by grades of evidence. Only five articles investigated changes in concentrations of identified biomarkers in the last days to weeks of life (Table 3).
Table 3. Prognostic biomarkers subdivided by evidence based medicine modified GRADE criteria.
| GRADE | Quality of Evidence | Biomarker |
|---|---|---|
| A | High | |
| B | Moderate | |
| C | Low | |
| D | Very Low |
|
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate transaminase; BUN = blood urea nitrogen; CRP = C-reactive protein; IL-1ra = interleukin-1 receptor antagonist; IL-1β = interleukin-1 beta; IL-6 = interleukin-6; IL-18 = interleukin-18; ESR = erythrocyte sedimentation rate; GGT = gamma-glutamyl transferase; INR = international normalised ratio; LDH = lactate dehydrogenase; MCP-1 = monocyte chemoattractant protein-1; MIF = macrophage migration inhibitory factor; sTNF- r1 = soluble tumour necrosis factor receptor 1; TGF-β1 = transforming growth factor β1; VOCs = volatile organic compounds; WBC = white blood cell count.
aBiomarkers detectable in the blood in the last two weeks of life
bPrognostic significance not assessed
The methodological quality of the included studies ranged between 26–36 using the Hawker et al. appraisal tool [16]. Using the seven-point checklist of quality criteria, the selected studies ranged between 3–6 [8]. Spearman’s rank correlation coefficient demonstrated a statistically significant rank correlation between assessors (rs 1⁄4 0.955 p = 0.000 and rs 1⁄4 0.921 p = 0.000, respectively). Frequently, sample size calculations were not conducted, and some studies lacked information about the study setting and patient characteristics. Further, the majority (97%) of studies had convenience rather than random sampling, a reliable method for identification of the date of death was often poor, and a large number of potential prognostic factors were analysed despite small sample sizes, which meant that the ratio between the number of deaths and the number of potential prognostic factors frequently scored less than ten.
Discussion
This is the first systematically structured review to evaluate prognostic biomarkers in a heterogeneous group of patients with advanced cancer. Seven prognostic biological factors had Grade A evidence: lymphocyte count, white blood cell (WBC) count, serum albumin, sodium, C-reactive protein (CRP), urea and alkaline phosphatase (ALP) (Table 3). Few studies have specifically investigated changes in biomarkers in the last days to weeks of life. In the last two weeks of life a number of biomarkers were elevated in the blood including: WBC count, platelet count, serum CRP, urea, urate, alanine transaminase (ALT), lactate dehydrogenase (LDH), sodium and plasma interleukin-6 (IL-6). However limitations exist as only five studies specifically investigated serial measurements of candidate biomarkers in the last weeks of life.
A number of common themes emerged: systemic inflammation, haematological changes, CACS, hepatic dysfunction, renal dysfunction, and electrolyte changes. A number of biomarkers, such as serum albumin could be explained by multiple themes.
Systemic inflammation & haematological changes
There is consistent evidence that the presence of a systemic inflammatory response is associated with reduced survival in patients with a variety of solid organ tumors [48–50]. Elevated serum CRP levels are associated with poor prognosis independent of tumour stage [37,51]. IL-6, interleukin-1 (IL-1) and tumour necrosis factor alpha (TNFα) induce the hepatic synthesis of CRP. Further, elevated CRP and IL-6 are associated with CACS in advanced cancer [51]. Measurement of serum CRP is readily available and is an ideal biomarker of systemic inflammation. There is Grade A evidence that elevated serum CRP is an independent prognostic factor in advanced cancer [18,22,29,34,37,42]. Amano et al. demonstrated a clear dose-related effect between elevated serum CRP and prognosis, and patients could be divided into four groups based on CRP concentrations [37] (Table 2).
Only two studies investigated the role of inflammatory cytokines at the end of life [25,46], this may be because most cytokines have a short plasma half-life and assays are expensive in relation to serum CRP. Iwase et al. measured changes in plasma levels of various cytokines in 28 terminally ill cancer patients with cachexia [25]. Only IL-6 was detected in all patients at a concentration greater than 10pg/mL [25]. The concentration of IL-6 was seen to gradually rise during the early stages of cachexia followed by a sharp rise in the week prior to death [25]. It was hypothesised that circulating macrophages and T lymphocytes produce IL-6 in response to tumour burden, or plasma IL-6 is produced by the tumour mass itself [25,52]. IL-6 is a pro-inflammatory cytokine that regulates immune reactions to tissue damage. There is a growing consensus that plasma IL-6 is a prognostic factor in solid organ malignancies [53,54], however changes in plasma IL-6 levels are affected by carcinoma type [55].
Paulsen et al. identified that a number of inflammatory cytokines were significantly elevated in the plasma in the last months of life [46] (Table 2). Interestingly, soluble tumour necrosis factor receptor-1 (sTNF-r1), IL-6, CRP and erythrocyte sedimentation rate (ESR) were highly correlated with quality of life, and interleukin-1β (IL-1β) was moderately correlated with breathlessness[46]. Further research is required to confirm these preliminary findings and assess their prognostic significance.
There is Grade A evidence that WBC count is an independent predictor of survival in advanced cancer [31,34,35]. In a UK multicentre cohort study, Gwilliam et al. demonstrated WBC count and platelet count independently predicted survival at two months and two weeks in a heterogeneous population of advanced cancer patients across a variety of palliative care settings [34].
There is Grade A evidence that lymphopenia is an independent predictor of survival in advanced cancer [23,31,33,34,41,43]. Gwilliam et al. confirmed that lymphocyte count was a significant predictor of two-month but not two-week survival [34]. The mechanisms driving lymphopenia in advanced cancer are unknown. Immunodeficiency in advanced cancer has been well documented and there is a significant trend of decreasing functional T cell populations (including CD4, CD8, CD4:CD8 ratio and naïve T cells) with cancer progression: this is associated with increased morbidity and mortality [56]. WBC differential rather than WBC counts may be a more useful prognostic marker for future studies.
The prognostic role of thrombocytopaenia (Grade B) [29,34] and haemoglobin count (Grade C) [33] has been indicated but further research is needed to confirm these findings.
Cancer anorexia-cachexia syndrome
CACS is a significant prognostic factor in advanced cancer [8] and the biological mechanisms have been extensively studied [57,58]. There is Grade A evidence for the independent prognostic role of hypoalbuminaemia in advanced cancer [19,27,33,34,41]. It is hypothesised that hypoalbuminaemia is caused by a combination of hepatic dysfunction and CACS. Interestingly, Gwilliam et al. demonstrated hypoalbuminaemia was a predictor of two-month but not two-week survival, suggesting serum albumin is a predictor of dying over a longer timeframe [34]. This is contrary to the findings by Taylor et al. who demonstrated a significant change over time and as death approached [41]. These findings highlight the need for further prospective studies that analyse serial measurements of serum albumin at the end of life.
One article demonstrated low serum prealbumin as a significant predictor of survival in terminally ill cancer patients by multivariate analysis (Grade B) [24]. Albumin and prealbumin levels reflect the visceral protein pool [24]. Given that prealbumin has a shorter half-life than albumin; prealbumin may be a more sensitive marker of nutritional status [24].
Hepatic dysfunction
A number of biomarkers of liver dysfunction have been indicated as prognostic in advanced cancer including: serum vitamin B12, albumin, bilirubin, aspartate aminotransferase (AST), ALT, ALP, gamma-glutamyl transferase (GGT), international normalised ratio (INR), LDH and cholesterol. The aetiology of hepatic dysfunction at the end of life is unclear. Certainly, studies have demonstrated mixed results on the prognostic significance of liver metastasis in advanced cancer [27,29,33,34,43].
There is Grade B evidence for the independent prognostic significance of elevated serum vitamin B12 in advanced cancer [18]. Geissbühler et al. demonstrated an inverse relationship between survival and serum vitamin B12 levels [18]. There was also a strong correlation between the presence of metastasis or hepatic dysfunction and an elevated serum vitamin B12 [18]. Importantly, there was no difference in serum vitamin B12 levels in patients with haematological malignancies (a potential confounding factor) compared to other cancers [18]. To our knowledge no additional studies have been published that investigate vitamin B12 as a prognostic factor.
Studies assessing serum bilirubin and other liver function tests have found mixed results. There is Grade B evidence that hyperbilirubinaemia is an independent prognostic factor [26,29,33,35]. Laboratory parameters ALP (Grade A), AST (Grade B) and ALT (Grade B) proved to be prognostically significant in at least one multivariate analysis [24,34,35]. Gwilliam et al. also found ALT predictive of two-week, but not two-month survival [34]. There are conflicting results whether GGT is prognostic (Grade C) [33,35]. These contrasting results may be explained by the geographical differences in patient groups, the type of patients included and differences in the methods of biochemical analysis of liver function tests. In contrast to serum AST, elevations in serum ALT are rarely seen in conditions other than liver parenchymal damage and may be a more sensitive marker for liver disease [24].
Prolonged INR was an independent prognostic factor by multivariate analysis in two prospective studies of terminally ill cancer patients (Grade B) [26,29]. To our knowledge, no studies have been published that measure serial changes in INR and liver function tests at the end of life.
Serum LDH is an independent prognostic factor in advanced cancer [26,29,33] (Grade B). Suh et al. demonstrated a significant increase in LDH concentrations in the last two weeks of life [29]. This retrospective study was limited however by sample size and was restricted to hospitalised patients [29]. Prospective multicentre studies across a variety of palliative care settings are required to confirm these preliminary findings. It has been extensively published that elevated serum LDH in cancer reflects tumour burden and aggressiveness [29,59]. Serum concentrations can be further elevated due to hepatic necrosis caused by hepatic dysfunction and/or metastasis in advanced cancer[29,60].
There is Grade B evidence for the prognostic role of hypocholesterolaemia in advanced cancer [26,29,33]. A number of risk factors for hypocholesterolaemia have been identified including: cancer, hepatic dysfunction, CACS, haematological disease and elderly populations [61].
Renal dysfunction
A number of potential prognostic biomarkers of renal dysfunction have been indicated in advanced cancer. Maltoni et al. demonstrated insufficient evidence for the prognostic role of proteinuria in advanced cancer [8]. Proteinuria is prognostic in a number of solid organ tumours [62,63] however, to our knowledge, there have been no recent published studies that investigate proteinuria as a predictor of survival in advanced cancer.
Elevated serum urea was demonstrated as a significant predictor of survival in three studies by multivariate analysis (Grade A) [24,34,41]. Gwilliam et al. identified serum urea as an independent predictor of two-week and two-month survival in advanced cancer [34]. Taylor et al. also found that serum urea and creatinine showed a statistically and clinically significant increase in the last two weeks of life [41]. There is Grade C evidence that serum creatinine is prognostic in advanced cancer [26,33].
There is Grade B evidence that serum urate is a significant prognostic factor in advanced cancer [26,29,33] and serum urate levels were significantly increased in the last two weeks of life [26]. A number of potential explanations for raised serum urate concentrations have been hypothesised including: renal dysfunction and cellular injury caused by hypoxia and/or inflammation [26].
The mechanisms for renal dysfunction at the end of life are unclear. Oliguria was identified as a significant prognostic factor in two univariate analyses [26,29]. It is hypothesised that glomerular filtration rate is reduced at the end of life. Although hypotension is a prognostic factor, there are conflicting results whether blood pressure falls during the dying phase [41,64].
Electrolyte changes
There is Grade A evidence that serum sodium is a significant predictor of survival in advanced cancer [30,35]. These studies involved hospitalised patients; and acute illness may be a confounding factor. One multivariate analysis identified hyponatraemia as an independent predictor of survival in advanced cancer [42]. The most common aetiology of hyponatraemia in cancer is syndrome of inappropriate secretion of antidiuretic hormone (SIADH) [42,65]. Conversely, hypernatraemia at the end life is commonly caused by dehydration [30] and is associated with shorter overall survival in hospitalised patients receiving palliative care [30]. Although, Taylor et al. demonstrated a statistically significant elevation in serum sodium in the last two weeks of life, results were not clinically significant [41]. Serum sodium levels rather than serum sodium may be a more useful prognostic factor for future studies.
The prognostic significance of hypercalcaemia has been described in a number of solid organ and haematological malignancies including: lung [66], prostate [67], renal [68], head and neck [69,70] and the aerodigestive tract [71]. There is conflicting evidence whether hypercalcaemia predicts survival in advanced cancer (Grade C). By univariate analysis, Alsirafy et al. found that hypercalcaemia was associated with a 69% inpatient death rate in hospitalised patients [30]. However, an additional three articles failed to demonstrate statistical significance [27,33,40]. Contrasting results may reflect differences in study settings, tumour type, and the presence of bony metastasis. One univariate analysis identified hypermagnesemia to be predictive of survival (Grade C) in patients referred to the hospital palliative care team [30].
Although Cui et al. demonstrated serum potassium as a prognostic factor by univariate analysis[35], an additional three articles were identified which failed to demonstrate statistical significance including two multivariate analyses (Grade D) [30,33,41]. In one univariate analysis, abnormal plasma glucose levels were predictive of survival (Grade C) [35].
Non-invasive research methodologies
Coyle et al. recently presented preliminary results of a statistically significant increase in the number of volatile organic compounds (VOCs) in the urine in the last weeks of life using Gas Chromatography Mass Spectrometry (GC-MS) [44]. Interestingly, the steepest rise in significant VOCs was seen in the last week of life [44].
Prognostic models
Based on the outcome of these studies a number of prognostic models have been developed to assist clinicians. Simmons et al. recently reviewed the role of prognostic models in advanced cancer [12]. They concluded that ‘various prognostic tools have been validated, but vary in their complexity, subjectivity and therefore clinical utility’ [12].
What makes this study unique?
The Neuberger review made a number of recommendations to improve end of life care, including research into the biology of dying [1]. This is the first study to specifically investigate biomarkers in advanced cancer patients in the last months of life and attempts to extrapolate which biological processes are affected. A number of common themes emerged including: systemic inflammation, organ dysfunction and CACS.
What is the significance of the findings of this analysis?
This review demonstrates that there are many biological prognostic factors that have an association with the dying process. Although this review is unable to provide evidence of causation it is important for healthcare professionals to be aware of these prognostic factors and their incorporation into existing prognostic models [12], which may be useful in clinical practice. However, further research is needed to understand the application of biomarkers in prognostication at the end of life.
Identification of biomarkers of dying is an important area for future research that will lead to both improved clinical tools for managing patients, and shed light on fundamental processes such as the answer to the question: why do patients die from cancer?
The “terminal cancer syndrome” theory is characterised by common terminal symptoms including dry mouth, dyspnoea, malnutrition and susceptibility to infection, which are shared amongst heterogeneous groups patients with advanced cancer [7]. In the last three days of life, Bruera et al. demonstrated that blood pressure and oxygen saturations decrease significantly in cancer patients [64]. Heart rate variability [72] and autonomic dysfunction [73] have also been described. Thus, changes in vital signs and biomarkers suggest end organ dysfunction.
In non-cancer animal models, McDonald et al. demonstrated a rapid loss of body weight and disintegration of the circadian rhythmicity in deep body temperature several days before death in senescent but not presenescent rats [74]. Tankersley et al. also demonstrated a predictable sequence of pathophysiological events associated with dying including a fall in daily mean heart rate and a loss of circadian pattern in deep body temperature 3–4 weeks before death [75]. Further, increased lung permeability, reduced lung volume and compliance were seen during a period of terminal senescence [76].
At post-mortem, Kadhim et al. demonstrated the over-expression of interleukin-2 (IL-2) in situ in brainstem neuronal centres implicated in autonomic control of vital homeostatic functions in adults and children who died from severe illness [77,78]. It was hypothesised that biological stressors trigger over-expression of IL-2 in brainstem neuronal centres, inducing a neurochemical cascade that results in disturbed homeostatic control of cardiorespiratory responses, and eventual death; however, populations were confined to non-cancer diagnoses [78]. Further, Perry et al. hypothesised that a neurochemicals including glutamate decarboxylase, tissue pH and tryptophan could reflect novel biomarkers of agonal status [79] and hence the dying phase.
Prognostic factors and post-mortem therefore studies suggest a common biological process to dying. Measurable parameters in the blood suggest a systemic inflammatory response including: elevated CRP, IL-6 and other pro-inflammatory cytokines, hypoalbuminaemia, leucocytosis and neutrophilia. Interestingly, post-mortem studies demonstrated evidence of silent pneumonia in advanced cancer [80,81]. Although it is not possible to assume causation, these findings are of clinical and research interest. For example, the presence of silent pneumonia, may to some extent explain why 24% of patients with terminal cancer have breathlessness despite a lack of risk factors [7]. Further, Kontoyiannis et al. demonstrated pulmonary candidiasis in 21% of patients with evidence of pneumonia at post-mortem and 42% of these patients had disseminated candidiasis [82]. What is the role of immunodeficiency towards the end of life? Is there an overarching aetiology for common terminal symptoms? Does over-expression of IL-2 in brainstem neuronal centres together with silent pneumonia, contribute to breathing changes seen during the dying phase? When is the “point of no return” when the administration of antibiotics is futile? The current lack of research in these areas, together with our desire to improve patient care, highlights the importance for further research into the biology of dying. Biomarkers are important adjuncts in prognostication and the recognition of dying, but equally increase our understanding of the dying process.
Key areas for future research include: serial blood measurements of candidate biomarkers including inflammatory cytokines during the dying phase, intracerebral measurement of cytokines; and correlation with physiological parameters observed during the dying phase. Interestingly, Coyle et al. recently presented a feasibility study for taking serial urine samples from hospice inpatients towards the end of life[83] and demonstrated elevated levels of a number of volatile VOCs in the urine during the dying phase[44]. Non-invasive research methodologies are an important area for future research.
Limitations
There are several limitations with this review. This review only included published studies from year 2000, in order to conduct an in-depth analysis of current studies since the Maltoni et al. paper. The authors recognise that a systematic review of randomised controlled trials is the gold standard in research synthesis, however within the constraints of current available evidence, the PRISMA standards for reporting evidence were applied to ensure a systematic structure to the search, selection and review of the literature [13]. However, reviewers were not blinded to the authors, institutions, or journals of publication, which could have introduced selection bias.
This review excluded non-cancer conditions and was limited to patient populations with a median survival of ≤90 days, which meant that a selected number of articles were excluded. This was important given that “advanced cancer” is poorly defined in the literature; exclusion of these articles did not change the overall findings of this review.
Many of the studies included in Table 2 were heterogeneous in nature, small and underpowered, and the quality of studies varied considerably. Few studies have specifically investigated changes in the last weeks of life. We screened some studies that may have contained information specific to the objectives of this review, but data was not presented in a way that was specific to advanced cancer. There are considerable ethical implications of conducting research in patients in the last few weeks of life, in particular when this involves invasive procedures. We recognise that this may have limited the number of studies for inclusion in this review. Certainly, a number of included studies were limited by sample size and lack of protocols for collection of blood samples.
It was not possible to provide a synthesis of the evidence due to the heterogeneity of the sample. Despite this, the findings of this review are of particular research interest. This review makes an important contribution to the evidence base on the biology of dying and highlights potential areas for research funding and analysis. Attention has been given to the ethical and methodological implications of research into the biology of dying and future strategies are described.
Conclusion
The biology of dying is an important area for future research interest. The evidence to date is largely focused on signs, symptoms and prognostic factors. Despite appearances, the extent to which cancer patients follow a common terminal trajectory is uncertain and high quality research should be conducted to explore this concept further. This review identifies a number common themes shared amongst advanced cancer patients and highlights candidate biomarkers which may be indicative of a common biological process to dying. Attention should be placed on understanding the physiological process of dying and identification of candidate biomarkers of imminent death. This will increase clinicians’ confidence in identifying the dying process, inform decision making surrounding end of life care, and ensure the best possible care for patients and their families.
Supporting information
(PDF)
(PDF)
Acknowledgments
VLR took a lead role in writing the manuscript, appraising the evidence and rewriting the manuscript in response to feedback. RM, ACN, SRM, CP, JJE provided support with the review process and critique of manuscripts. SC devised the concept of the study, appraised the data, oversaw the project, and supported the writing of the manuscript.
Abbreviations
- AGP
α1-acid glycoprotein
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate transaminase
- AUC
area under the curve
- B12
vitamin B12
- BCI
B12 CRP index
- BUN
blood urea nitrogen
- CACS
cancer anorexia-cachexia syndrome
- CAR
CRP/albumin ratio
- CPS
Clinicians’ Prediction of Survival
- CRP
C-reactive protein
- D-PAP
Delirium-Palliative Prognostic Score
- ECOG PS
Eastern Cooperative Oncology Group Performance Status
- ESR
erythrocyte sedimentation rate
- GC-MS
Gas Chromatography Mass Spectrometry
- GGT
gamma-glutamyl transpeptidase
- GPS
Glasgow Prognostic Score
- IFNγ
interferon gamma
- IL-10
interleukin-10
- IL-12(p70)
interleukin-12(p70)
- IL-18
interleukin-18
- IL-1ra
interleukin-1 receptor antagonist
- IL-1β
interleurkin-1 beta
- IL-2
interleukin-2
- IL-4
interleukin-4
- IL-6
interleukin-6
- IL-8
interleukin-8
- INR
international normalised ratio
- JPOS-PI
Japan Palliative Oncology Study-Prognostic Index
- KPS
Karnofsky Performance Status
- LDH
lactate dehydrogenase
- LIF
leukaemia inhibiting factor
- MCP-1
monocyte chemoattractant protein-1
- m-GPS
modified Glasgow Prognostic Score
- MIF
macrophage migration inhibitory factor
- MIP-1α
macrophage inflammatory protein-1α
- MSSE
mini mental state examination
- new-ChPS
new-Chinese Advanced Cancer Patients Scale
- NLR
neutrophil-lymphocyte ratio
- NMR
Nuclear Magnetic Resonance Spectrometry
- NPV
negative predictive value
- PaP Score
Palliative Prognostic Score
- PINI
Prognostic Inflammatory and Nutritional Index
- PiPS
Prognosis in Palliative Care Study
- PiPS-A
modified Prognosis in Palliative Care Study-A
- PiPS-B
modified Prognosis in Palliative Care Study-B
- PPI
Palliative Performance Index
- PPV
positive predictive value
- PTH
parathyroid hormone
- PTHrP
parathyroid hormone related protein
- RNA
ribonucleic acid
- sTNF- r1
soluble tumour necrosis factor receptor 1
- TGF-β1
transforming growth factor β1
- TNFα
tumour necrosis factor alpha
- TTD
time from initial diagnosis to diagnosis of terminal disease
- VOCs
volatile organic compounds
- WBC
white blood cell count
- WPBAL
WBC/platelet/BUN/AST/LDH prognostic score
- WPCBAL
WBC/platelet/CRP/BUN/AST/LDH prognostic score
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Neuberger J, Guthrie C, Aaronovitch D, Hameed K, Bonser T, Harries R, et al. More care, less pathway: a review of the Liverpool Care Pathway. 2013: 1–61. [Google Scholar]
- 2.Kennedy C, Brooks-Young P, Brunton Gray C, Larkin P, Connolly M, Wilde-Larsson B, et al. Diagnosing dying: an integrative literature review. BMJ Support Palliat Care. 2014;4: 263–270. 10.1136/bmjspcare-2013-000621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichter I, Hunt E. The last 48 hours of life. J Palliat Care. 1990;6: 7–15. [PubMed] [Google Scholar]
- 4.Vigano A, Bruera E, Suarez-Almazor M. Terminal cancer syndrome: myth or reaity?. 1999;15: 32–39. [PubMed] [Google Scholar]
- 5.Christakis NA, Lamont EB. Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320: 469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teno JM, Weitzen S, Fennell ML, Mor V. Dying trajectory in the last year of life: does cancer trajectory fit other diseases? J Palliat Med. 2001;4: 457–464. 10.1089/109662101753381593 [DOI] [PubMed] [Google Scholar]
- 7.Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med. 1988;148: 1586–1591. [PubMed] [Google Scholar]
- 8.Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23: 6240–6248. 10.1200/JCO.2005.06.866 [DOI] [PubMed] [Google Scholar]
- 9.Kehl KA, Kowalkowski JA. A systematic review of the prevalence of signs of impending death and symptoms in the last 2 weeks of life. Am J Hosp Palliat Care. 2013;30: 601–616. 10.1177/1049909112468222 [DOI] [PubMed] [Google Scholar]
- 10.Hui D, dos Santos R, Chisholm G, Bansal S, Silva TB, Kilgore K, et al. Clinical signs of impending death in cancer patients. Oncologist. 2014;19: 681–687. 10.1634/theoncologist.2013-0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui D, Dos Santos R, Chisholm G, Bansal S, Souza Crovador C, Bruera E. Bedside clinical signs associated with impending death in patients with advanced cancer: preliminary findings of a prospective, longitudinal cohort study. Cancer. 2015;121: 960–967. 10.1002/cncr.29048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons CPL, McMillan DC, McWilliams K, Sande TA, Fearon KC, Tuck S, et al. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5: 463–466. 10.1097/COH.0b013e32833ed177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Methods for development of NICE public health guidance. 2006: 1–131. [PubMed]
- 16.Hawker S, Payne S, Kerr C, Hardey M, Powell J. Appraising the evidence: reviewing disparate data systematically. Qual Health Res. 2002;12: 1284–1299. 10.1177/1049732302238251 [DOI] [PubMed] [Google Scholar]
- 17.Barry H, Ebell M, Shaughnessy A, Slawson D. Essential Evidence Plus. 2016. https://www.essentialevidenceplus.com/product/ebm_loe.cfm?show=grade.
- 18.Geissbuhler P, Mermillod B, Rapin CH. Elevated serum vitamin B12 levels associated with CRP as a predictive factor of mortality in palliative care cancer patients: a prospective study over five years. J Pain Symptom Manage. 2000;20: 93–103. [DOI] [PubMed] [Google Scholar]
- 19.Pasanisi F, Orban A, Scalfi L, Alfonsi L, Santarpia L, Zurlo E, et al. Predictors of survival in terminal-cancer patients with irreversible bowel obstruction receiving home parenteral nutrition. Nutrition. 2001;17: 581–584. [DOI] [PubMed] [Google Scholar]
- 20.Glare P, Virik K. Independent prospective validation of the PaP score in terminally ill patients referred to a hospital-based palliative medicine consultation service. J Pain Symptom Manage. 2001;22: 891–898. [DOI] [PubMed] [Google Scholar]
- 21.Pirovano M, Maltoni M, Nanni O, Marinari M, Indelli M, Zaninetta G, et al. A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage. 1999;17: 231–239. [DOI] [PubMed] [Google Scholar]
- 22.McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, McArdle CS. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41: 64–69. 10.1080/01635581.2001.9680613 [DOI] [PubMed] [Google Scholar]
- 23.Faris M. Clinical estimation of survival and impact of other prognostic factors on terminally ill cancer patients in Oman. Support Care Cancer. 2003;11: 30–34. [DOI] [PubMed] [Google Scholar]
- 24.Ho SY, Guo HR, Chen HH, Peng CJ. Nutritional predictors of survival in terminally ill cancer patients. J Formos Med Assoc. 2003;102: 544–550. [PubMed] [Google Scholar]
- 25.Iwase S, Murakami T, Saito Y, Nakagawa K. Steep elevation of blood interleukin-6 (IL-6) associated only with late stages of cachexia in cancer patients. Eur Cytokine Netw. 2004;15: 312–316. [PubMed] [Google Scholar]
- 26.Shin HS, Lee HR, Lee DC, Shim JY, Cho KH, Suh SY. Uric acid as a prognostic factor for survival time: a prospective cohort study of terminally ill cancer patients. J Pain Symptom Manage. 2006;31: 493–501. 10.1016/j.jpainsymman.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 27.Lam PT, Leung MW, Tse CY. Identifying prognostic factors for survival in advanced cancer patients: a prospective study. Hong Kong Med J. 2007;13: 453–459. [PubMed] [Google Scholar]
- 28.Kelly L, White S, Stone PC. The B12/CRP index as a simple prognostic indicator in patients with advanced cancer: a confirmatory study. Ann Oncol. 2007;18: 1395–1399. 10.1093/annonc/mdm138 [DOI] [PubMed] [Google Scholar]
- 29.Suh SY, Ahn HY. Lactate dehydrogenase as a prognostic factor for survival time of terminally ill cancer patients: a preliminary study. Eur J Cancer. 2007;43: 1051–1059. 10.1016/j.ejca.2007.01.031 [DOI] [PubMed] [Google Scholar]
- 30.Alsirafy SA, Sroor MY, Al-Shahri MZ. Predictive impact of electrolyte abnormalities on the admission outcome and survival of palliative care cancer referrals. J Palliat Med. 2009;12: 177–180. 10.1089/jpm.2008.0200 [DOI] [PubMed] [Google Scholar]
- 31.Hyodo I, Morita T, Adachi I, Shima Y, Yoshizawa A, Hiraga K. Development of a predicting tool for survival of terminally ill cancer patients. Jpn J Clin Oncol. 2010;40: 442–448. 10.1093/jjco/hyp182 [DOI] [PubMed] [Google Scholar]
- 32.Tarumi Y, Watanabe SM, Lau F, Yang J, Quan H, Sawchuk L, et al. Evaluation of the Palliative Prognostic Score (PaP) and routinely collected clinical data in prognostication of survival for patients referred to a palliative care consultation service in an acute care hospital. J Pain Symptom Manage. 2011;42: 419–431. 10.1016/j.jpainsymman.2010.12.013 [DOI] [PubMed] [Google Scholar]
- 33.Feliu J, Jimenez-Gordo AM, Madero R, Rodriguez-Aizcorbe JR, Espinosa E, Castro J, et al. Development and validation of a prognostic nomogram for terminally ill cancer patients. J Natl Cancer Inst. 2011;103: 1613–1620. 10.1093/jnci/djr388 [DOI] [PubMed] [Google Scholar]
- 34.Gwilliam B, Keeley V, Todd C, Gittins M, Roberts C, Kelly L, et al. Development of prognosis in palliative care study (PiPS) predictor models to improve prognostication in advanced cancer: prospective cohort study. BMJ. 2011;343: d4920 10.1136/bmj.d4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui J, Zhou L, Wee B, Shen F, Ma X, Zhao J. Predicting survival time in noncurative patients with advanced cancer: a prospective study in China. J Palliat Med. 2014;17: 545–552. 10.1089/jpm.2013.0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DH, Kim JA, Choi YS, Kim SH, Lee JY, Kim YE. Heart rate variability and length of survival in hospice cancer patients. J Korean Med Sci. 2010;25: 1140–1145. 10.3346/jkms.2010.25.8.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amano K, Maeda I, Morita T, Miura T, Inoue S, Ikenaga M, et al. Clinical Implications of C-Reactive Protein as a Prognostic Marker in Advanced Cancer Patients in Palliative Care Settings. J Pain Symptom Manage. 2016;51: 860–867. 10.1016/j.jpainsymman.2015.11.025 [DOI] [PubMed] [Google Scholar]
- 38.Baba M, Maeda I, Morita T, Hisanaga T, Ishihara T, Iwashita T, et al. Independent validation of the modified prognosis palliative care study predictor models in three palliative care settings. J Pain Symptom Manage. 2015;49: 853–860. 10.1016/j.jpainsymman.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 39.Baba M, Maeda I, Morita T, Inoue S, Ikenaga M, Matsumoto Y, et al. Survival prediction for advanced cancer patients in the real world: A comparison of the Palliative Prognostic Score, Delirium-Palliative Prognostic Score, Palliative Prognostic Index and modified Prognosis in Palliative Care Study predictor model. Eur J Cancer. 2015;51: 1618–1629. 10.1016/j.ejca.2015.04.025 [DOI] [PubMed] [Google Scholar]
- 40.Mallik S, Mallik G, Macabulos ST, Dorigo A. Malignancy associated hypercalcaemia-responsiveness to IV bisphosphonates and prognosis in a palliative population. Support Care Cancer. 2016;24: 1771–1777. 10.1007/s00520-015-2962-8 [DOI] [PubMed] [Google Scholar]
- 41.Taylor P, Crouch S, Howell DA, Dowding DW, Johnson MJ. Change in physiological variables in the last 2 weeks of life: an observational study of hospital in-patients with cancer. Palliat Med. 2015;29: 120–127. 10.1177/0269216314554967 [DOI] [PubMed] [Google Scholar]
- 42.Yoon J, Ahn SH, Lee YJ, Kim CM. Hyponatremia as an independent prognostic factor in patients with terminal cancer. Support Care Cancer. 2015;23: 1735–1740. 10.1007/s00520-014-2522-7 [DOI] [PubMed] [Google Scholar]
- 43.Miura T, Matsumoto Y, Hama T, Amano K, Tei Y, Kikuchi A, et al. Glasgow prognostic score predicts prognosis for cancer patients in palliative settings: a subanalysis of the Japan-prognostic assessment tools validation (J-ProVal) study. Support Care Cancer. 2015;23: 3149–3156. 10.1007/s00520-015-2693-x [DOI] [PubMed] [Google Scholar]
- 44.Coyle S, Scott A, Nwosu A, Latten R, Phelan M, Mason S, et al. A process to dying? Metabolomic changes in urine towards the end of life. Palliat Med. 2016;30: NP1–NP401. [Google Scholar]
- 45.Niki K, Okamoto Y, Tabata Y, Murata T, Matsumura Y, Takagi T, et al. A new approach of determining short-term prognostic predictive methods in terminal cancer patients based on the change-point in laboratory test values. Palliat Med. 2016;30: NP1–NP401. [DOI] [PubMed] [Google Scholar]
- 46.Paulsen O, Kaasa S, Aass N, Lea T, Klepstad P. Are serum concentrations of cytokines associated with symptom intensities or response to corticosteroids in advanced cancer patients receiving opioids? Palliat Med. 2016;30: NP1–NP401. [Google Scholar]
- 47.Wrafter S, Lorton C, Brady B, Dhuibhir Ui P, Joyce D, O’Leary N, et al. The prognostic role of C-reactive protein and albumin in advanced cancer palliative care inpatients: A retrospective study. Palliat Med. 2016;30: NP1–NP401. [Google Scholar]
- 48.Falconer JS, Fearon KC, Ross JA, Elton R, Wigmore SJ, Garden OJ, et al. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75: 2077–2082. [DOI] [PubMed] [Google Scholar]
- 49.O'Gorman P, McMillan DC, McArdle CS. Prognostic factors in advanced gastrointestinal cancer patients with weight loss. Nutr Cancer. 2000;37: 36–40. 10.1207/S15327914NC3701_4 [DOI] [PubMed] [Google Scholar]
- 50.Scott H, McMillan D, Forest L, Brown D, McArdle C, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87: 264–267. 10.1038/sj.bjc.6600466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep. 2002;4: 250–255. [DOI] [PubMed] [Google Scholar]
- 52.Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101: 2727–2736. 10.1002/cncr.20672 [DOI] [PubMed] [Google Scholar]
- 53.Wu CW, Wang SR, Chao MF, Wu TC, Lui WY, P'eng FK, et al. Serum interleukin-6 levels reflect disease status of gastric cancer. Am J Gastroenterol. 1996;91: 1417–1422. [PubMed] [Google Scholar]
- 54.Martin F, Santolaria F, Batista N, Milena A, Gonzalez-Reimers E, Brito MJ, et al. Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine. 1999;11: 80–86. [DOI] [PubMed] [Google Scholar]
- 55.Nakano T, Chahinian AP, Shinjo M, Tonomura A, Miyake M, Togawa N, et al. Interleukin 6 and its relationship to clinical parameters in patients with malignant pleural mesothelioma. Br J Cancer. 1998;77: 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang C, Wu C, Lu Y. Cancer-associated immune deficiency: a form of accelerated immunosenescence? 2012.
- 57.Tisdale MJ. Biology of cachexia. J Natl Cancer Inst. 1997;89: 1763–1773. [DOI] [PubMed] [Google Scholar]
- 58.Strasser F, Bruera ED. Update on anorexia and cachexia. Hematol Oncol Clin North Am. 2002;16: 589–617. [DOI] [PubMed] [Google Scholar]
- 59.Miao P, Sheng S, Sun X, Liu J, Huang G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life. 2013;65: 904–910. 10.1002/iub.1216 [DOI] [PubMed] [Google Scholar]
- 60.Baur M, Schlappack O, Havelec L, Wrba F, Dittrich C. Prognostic significance of liver metastases as first site of generalisation in patients with breast cancer—a retrospective analysis. Acta Med Austriaca. 2001;28: 135–140. [DOI] [PubMed] [Google Scholar]
- 61.Elmehdawi R. Hypolipidemia: a word of caution. Libyan J Med. 2008;3: 84–90. 10.4176/071221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pedersen LM, Milman N. Prevalence and prognostic significance of proteinuria in patients with lung cancer. Acta Oncol. 1996;35: 691–695. [DOI] [PubMed] [Google Scholar]
- 63.Liu J, Wang X. Incidence and prognostic significance of proteinuria in patients with gastric carcinoma. 2005;2: 511–515. [Google Scholar]
- 64.Bruera S, Chisholm G, Dos Santos R, Crovador C, Bruera E, Hui D. Variations in vital signs in the last days of life in patients with advanced cancer. J Pain Symptom Manage. 2014;48: 510–517. 10.1016/j.jpainsymman.2013.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berghmans T, Paesmans M, Body JJ. A prospective study on hyponatraemia in medical cancer patients: epidemiology, aetiology and differential diagnosis. Support Care Cancer. 2000;8: 192–197. [DOI] [PubMed] [Google Scholar]
- 66.Sculier JP, Chansky K, Crowley JJ, Van Meerbeeck J, Goldstraw P, International Staging Committee and Participating Institutions. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol. 2008;3: 457–466. 10.1097/JTO.0b013e31816de2b8 [DOI] [PubMed] [Google Scholar]
- 67.Tucci M, Mosca A, Lamanna G, Porpiglia F, Terzolo M, Vana F, et al. Prognostic significance of disordered calcium metabolism in hormone-refractory prostate cancer patients with metastatic bone disease. Prostate Cancer Prostatic Dis. 2009;12: 94–99. 10.1038/pcan.2008.10 [DOI] [PubMed] [Google Scholar]
- 68.Fahn HJ, Lee YH, Chen MT, Huang JK, Chen KK, Chang LS. The incidence and prognostic significance of humoral hypercalcemia in renal cell carcinoma. J Urol. 1991;145: 248–250. [DOI] [PubMed] [Google Scholar]
- 69.Won C, Decker DA, Drelichman A, Al-Sarraf M, Reed ML. Hypercalcemia in head and neck carcinoma. Incidence and prognosis. Cancer. 1983;52: 2261–2263. [DOI] [PubMed] [Google Scholar]
- 70.Alsirafy SA, Sroor MY, Al-Shahri MZ. Hypercalcemia in advanced head and neck squamous cell carcinoma: prevalence and potential impact on palliative care. J Support Oncol. 2009;7: 154–157. [PubMed] [Google Scholar]
- 71.Penel N, Berthon C, Everard F, Neu JC, Clisant S, N'guyen M, et al. Prognosis of hypercalcemia in aerodigestive tract cancers: study of 136 recent cases. Oral Oncol. 2005;41: 884–889. 10.1016/j.oraloncology.2005.04.013 [DOI] [PubMed] [Google Scholar]
- 72.Kim ES, Lee JK, Kim MH, Noh HM, Jin YH. Validation of the prognosis in palliative care study predictor models in terminal cancer patients. Korean J Fam Med. 2014;35: 283–294. 10.4082/kjfm.2014.35.6.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walsh D, Nelson KA. Autonomic nervous system dysfunction in advanced cancer. Support Care Cancer. 2002;10: 523–528. 10.1007/s00520-002-0376-x [DOI] [PubMed] [Google Scholar]
- 74.McDonald RB, Horwitz BA. Brown adipose tissue thermogenesis during aging and senescence. J Bioenerg Biomembr. 1999;31: 507–516. [DOI] [PubMed] [Google Scholar]
- 75.Tankersley CG, Irizarry R, Flanders SE, Rabold R, Frank R. Unstable heart rate and temperature regulation predict mortality in AKR/J mice. Am J Physiol Regul Integr Comp Physiol. 2003;284: R742–50. 10.1152/ajpregu.00416.2002 [DOI] [PubMed] [Google Scholar]
- 76.Tankersley CG, Shank JA, Flanders SE, Soutiere SE, Rabold R, Mitzner W, et al. Changes in lung permeability and lung mechanics accompany homeostatic instability in senescent mice. J Appl Physiol (1985). 2003;95: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 77.Kadhim H, Deltenre P, De Prez C, Sebire G. Interleukin-2 as a neuromodulator possibly implicated in the physiopathology of sudden infant death syndrome. Neurosci Lett. 2010;480: 122–126. 10.1016/j.neulet.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 78.Kadhim H, Deltenre P, Segers V, Sebire G. Selective expression of a neuromodulatory cytokine (IL-2) in specific brainstem neurovegetative centers: a possible final common neuro-molecular pathway in dying patients. Med Hypotheses. 2012;78: 793–795. 10.1016/j.mehy.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 79.Perry EK, Perry RH, Tomlinson BE. The influence of agonal status on some neurochemical activities of postmortem human brain tissue. Neurosci Lett. 1982;29: 303–307. [DOI] [PubMed] [Google Scholar]
- 80.Abdel-Karim IA, Sammel RB, Prange MA. Causes of death at autopsy in an inpatient hospice program. J Palliat Med. 2007;10: 894–898. 10.1089/jpm.2006.0240 [DOI] [PubMed] [Google Scholar]
- 81.Pautex S, Vayne-Bossert P, Jamme S, Herrmann F, Vilarino R, Weber C, et al. Anatomopathological causes of death in patients with advanced cancer: association with the use of anticoagulation and antibiotics at the end of life. J Palliat Med. 2013;16: 669–674. 10.1089/jpm.2012.0369 [DOI] [PubMed] [Google Scholar]
- 82.Kontoyiannis DP, Reddy BT, Torres HA, Luna M, Lewis RE, Tarrand J, et al. Pulmonary candidiasis in patients with cancer: an autopsy study. Clin Infect Dis. 2002;34: 400–403. 10.1086/338404 [DOI] [PubMed] [Google Scholar]
- 83.Coyle S, Scott A, Nwosu A, Aggio R, Latten R, Wilson J, et al. Biological changes towards the end of life: a feasibility study. European Association of Palliative Care 2015. 2015: 206 Available: http://www.eapc-2015.org/tl_files/eapc15/Downloads/EAPC_2015_Abstract_Book.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.