Abstract
Objectives
China has high burden on both of tuberculosis (TB) and tobacco use. This study aims to explore the potential link between smoking and TB infection using baseline survey data of a large-scale population-based prospective study in rural China
Methods
Between July 1 and Sept 30, 2013, based on the baseline survey of a population-based, prospective study in rural China, the relationship between smoking and TB infection, assessed by interferon-gamma release assays (IGRA), was investigated among the total study population and only among those smokers, respectively.
Results
A total of 21,008 eligible rural registered residents (≥ 5 years old) from 4 rural sites were included in the analysis. Ever-smokers were more likely to be QuantiFERON-TB Gold In-Tube (QFT) positive than never smokers with an adjusted odds ratio (OR) of 1.34 (95% confidence interval (CI): 1.21–1.49). Among ever smokers, a significant linear dose–response relation was observed between duration of smoking (by years) and QFT positivity (p < 0.001). Stratified analysis suggested that such an association was not influenced by gender and age. Evidence for interaction of smoking status with age was found.
Conclusions
Our results provide further evidence to support smoking might increase host susceptibility to TB infection. Populations under high risk of infection, such as elderly smokers, should be prior to TB infection controlling under a premise of community level intervention.
Introduction
The epidemics of tobacco smoking and tuberculosis (TB) are colliding, and increasing evidence showed smoking was associated with an increased risk of active TB [1–5]. However, the relation between smoking and TB infection has not been widely studied especially in China [6–7]. In spite of the accumulated evidence demonstrating a causal relationship between smoking and TB infection, whether smoking cessation in a community level could contribute to TB controlling is still being debated. Because most of the published studies were limited to special populations at high risk of TB infection, including prisoners [8–9], migrant workers [10–11], immigrants [12–13], and the homeless [14]. Furthermore, several studies even reported a negative association between cigarette smoking and TB infection [15–17]. Therefore, population-based data is urgent needed. Recently, we reported a causal link between smoking and TB infection in a population-based multicenter prospective study conducted in rural China. The annual risk of TB infection among ever-smokers has been found to be 1.53 fold higher as compared to never-smokers [18]. However, further more detailed analysis in the impact of smoking on TB infection was limited due to small sample size of the observed recently infected cases.
Therefore, this study aimed to investigate the association between smoking and TB infection in a community level based on the baseline survey of a large-scale population-based prospective study in rural China.
Materials and methods
Study participants
This cross-sectional study was based on the baseline survey of a multicenter prospective cohort study in rural China conducted between July 1st and September 30th, 2013, which has been reported in detail in elsewhere [19]. Registered rural residents at the four study sites were the target populations of the present and previous study. The study sites were selected based on a wide range of local TB epidemiology, economic conditions and geographic diversity (S1 Table) [19]. The inclusion criteria of the study participants were: resident population aged 5 years and above (birth prior to 1 June, 2008); resident population (more than 6 months’ residence in study site in the past year); provision of voluntary written informed consent. The exclusion criteria were suspected or current active tuberculosis (According to WHO guidelines, bacteriological confirmed TB cases (positive for sputum-smear or/and culture or/and X-pert MTB/RIF) or clinically diagnosed case of TB (cases diagnosed on the basis of chest digital radiography abnormalities without laboratory confirmation) were defined as active TB case), self-reported history of tuberculosis, and pregnancy.
Determination of TB infection
TB infection was tested using both tuberculin skin test (TST) and interferon-γ release assay (IGRA). IGRA result was used to determine TB infection status in the present study because our previous results proved TST results were affected by several factors including age, Bacillus Calmette-Guerin (BCG) vaccination and exposure to non-tuberculosis mycobacterium. QuantiFERON-TB Gold In-Tube (QFT, QIAGEN, USA), a commercial IGRA, was performed as recommended by the manufacturer using a cutoff value of ≥ 0.35 IU/ml.
Data collection
The study protocol was approved by the ethics committees of the Institute of Pathogen Biology, Chinese Academy of Medical Sciences (No: IPB-2013-5). Upon explanation of the study protocol, written informed consent was obtained from the participant or the legal guardian for all study participants. For each study participant, socio-demographic information was collected by a standardized questionnaire administered by trained interviewers. The questionnaire contained items on demographic characteristics (age, gender, educational level, and household per capita income (RMB)), alcohol consumption, cigarette smoking, history of reported tuberculosis disease, history of type 2 diabetes mellitus (T2DM) (With a self-reported history and/or fast blood glucose higher than 7.0 mmol/L at baseline examination) and history of close contact with a patient with tuberculosis. The following questions included items on smoking conditions: (1) Smoking status: never smoked (smoked less than 5 cigarettes per month or never smoked) and ever smoked (smoked 5 or more cigarettes per month). Ever smokers were further categorized as current smokers and former smokers. The information of duration of abstaining from smoking was collected. (2) Age of smoking onset. (3) Type of cigarette smoking. Two types of cigarette smoking are prevalent in rural China: filter tip cigarettes and sun-cured tobacco without filter tip. The latter was self-made by dried tobacco leaf rolling in a blank paper. (4) Duration of smoking by years and number of cigarettes per day: These exposure were converted into categorical variables for analysis (≤ 10 years, 10–20 years, 20–30 years, 30–40 years, 40–50 years, > 50 years) and (1–5, 6–10, 11–19, and ≥ 20 cigarettes per day), respectively. Body mass index (BMI) was calculated as weight over height squared (kg/m2), and was further categorized as underweight (< 18.5 kg/m2), normal weight (18.5 to 24.0 kg/m2), overweight (24.0 to 28.0 kg/m2), or obese (≥ 28.0 kg/m2) [20].
Statistical analysis
Data analyses were performed using SAS 9.2 (SAS Institute Inc., NC, USA). The Pearson’s χ2 test was used to compare the categorical variables. Variables with p < 0.05 in the univariate analysis were all included in the multivariable models based on our previous hypothesis Stepwise multiple logistic regression analysis was used to identify the variables independently associated with QFT positivity, the significance level for the variables stayed in the model was 0.05. The associations between TB infection and self-reported history of cigarette smoking were assessed by means of odds ratios (OR) and 95% confidence intervals (CI). Cochran–Armitage (chi-square) tests were used to explore TB infection trends with cigarette intensity. Evidence for interaction of smoking status with gender, age, BMI and TB contact history was assessed using the likelihood ratio test (LRT) by comparing logistic regression models with and without an interaction term. In additional sensitivity analyses, the association between TB infection and self-reported history of cigarette smoking was evaluated after excluding 5–19 years old people.
Results
A total of 21,022 eligible participants from four study sites completed baseline survey, 21,008 of them were included in this study after excluding 14 with missing data on smoking. Basic characteristics of the study population with respect to smoking status were shown in Table 1. In total, more than half (53.71%, 11,284/21,008) were females and the age ranged from 5 to 99 years with a median age 46 years (interquartile range [IQR]: 27–59 years). Among the study participants, 24.73% (5195/21008) were ever-smokers (4,835 (24.17%) were current-smokers, 360 (1.80%) were former-smokers), and 15,813 (75.27%) had never smoked. Ever-smokers reported higher educational levels and household incomes than never-smokers. BMI distribution showed that 2089 (40.21%) ever- and 5558 (35.15%) never smokers were overweight or obese. Ever smokers (53.38%, 2773/5195) were more likely to drink alcohol than never-smokers (7.70%, 1,217/15,813) (p < 0.001). 235 (4.53%) ever- and 579 (3.66%) never- smokers had a history of close contact with TB patients, respectively. 6.74% (350/5195) ever smokers and 4.22% (667/15813) never smokers had a self-reported history of T2DM or with a baseline fast blood glucose level ≥ 7.0 mmol/L. The QFT positivity for ever-smokers and never-smokers was 28.26% (1468/5195) and 15.72% (2,486/15,813), respectively (p < 0.001).
Table 1. Characteristics of the total study population by smoking status.
| Variables | Total† (N = 21008) |
% | Ever-smoker (n = 5195) |
% | Never-smoker (n = 15813) |
% | p for χ2 test‡ |
|---|---|---|---|---|---|---|---|
| Gender | <0.001 | ||||||
| Male | 9724 | 46.29 | 5061 | 97.42 | 4663 | 29.49 | |
| Female | 11284 | 53.71 | 134 | 2.58 | 11150 | 70.51 | |
| Age (years) | <0.001 | ||||||
| 5–19 | 3556 | 16.93 | 46 | 0.89 | 3510 | 22.20 | |
| 20–29 | 2053 | 9.77 | 514 | 9.89 | 1539 | 10.53 | |
| 30–39 | 2181 | 10.38 | 599 | 11.53 | 1582 | 12.06 | |
| 40–49 | 4648 | 22.12 | 1304 | 25.10 | 3344 | 21.15 | |
| 50–59 | 3612 | 17.19 | 1170 | 22.52 | 2442 | 15.44 | |
| 60–69 | 3136 | 14.93 | 1060 | 20.40 | 2076 | 13.13 | |
| ≥70 | 1822 | 8.67 | 502 | 9.66 | 1320 | 8.35 | |
| Median (inter quartile range) | 46 (27–59) | 50 (41–62) | 44 (23–58) | ||||
| Highest education level | <0.001 | ||||||
| Primary school or lower | 11172 | 53.18 | 2236 | 43.04 | 8936 | 56.51 | |
| Middle school | 6882 | 32.76 | 2198 | 42.31 | 4684 | 29.62 | |
| High school | 2301 | 10.95 | 650 | 12.51 | 1651 | 10.44 | |
| College or higher | 653 | 3.11 | 111 | 2.14 | 542 | 3.43 | |
| BMI (kg/m2) | <0.001 | ||||||
| <18.5 | 3278 | 15.60 | 278 | 5.35 | 3000 | 18.97 | |
| ≥18.5-<24.0 | 10082 | 47.99 | 2828 | 54.44 | 7254 | 45.88 | |
| ≥24.0-<28.0 | 5596 | 26.64 | 1542 | 29.68 | 4054 | 25.64 | |
| ≥28.0 | 2051 | 9.76 | 547 | 10.53 | 1504 | 9.51 | |
| Household per capita income (RMB) | <0.001 | ||||||
| <6000 | 13411 | 63.84 | 3178 | 61.17 | 10233 | 64.72 | |
| ≥6000 | 7596 | 36.16 | 2017 | 38.83 | 5579 | 35.28 | |
| Alcohol drinking | <0.001 | ||||||
| No | 17017 | 81.01 | 2422 | 46.62 | 14595 | 92.30 | |
| Yes | 3990 | 18.99 | 2773 | 53.38 | 1217 | 7.70 | |
| TB contact history | 0.005 | ||||||
| No | 20185 | 96.12 | 4958 | 95.47 | 15227 | 96.34 | |
| Yes | 814 | 3.88 | 235 | 4.53 | 579 | 3.66 | |
| History of T2DM | <0.001 | ||||||
| Yes | 1017 | 4.84 | 350 | 6.74 | 667 | 4.22 | |
| No | 19911 | 95.16 | 4845 | 93.26 | 15146 | 95.78 | |
| QFT test | <0.001 | ||||||
| Positive | 3954 | 18.82 | 1468 | 28.26 | 2486 | 15.72 | |
| Negative | 16454 | 78.32 | 3624 | 69.76 | 12830 | 81.14 | |
| Indeterminate | 600 | 2.86 | 103 | 1.98 | 497 | 3.14 |
Abbreviations: BMI = body mass index; IQR = interquartile range; QFT = QuantiFERON-TB Gold In-Tube; T2DM = type 2 diabetes mellitus; TB = tuberculosis.
†Sum might not always be in total because of missing data. Frequency of missing data did not differ significantly between smoking statuses.
‡ P values refer to the comparison of different variables between ever- and never-smokers.
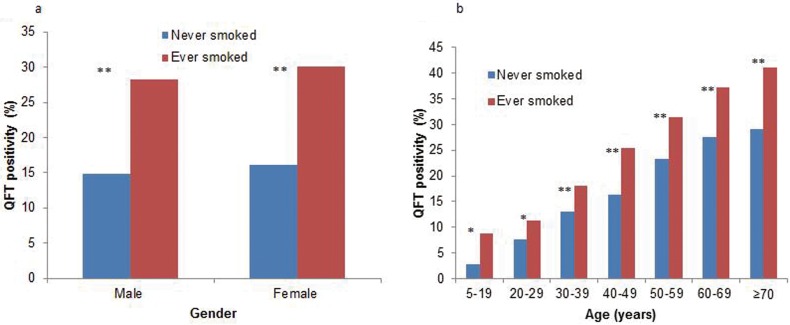
Univariate and multivariate analysis of QFT positivity were conducted firstly among total study population (Table 2). Factors significantly associated with QFT positivity were male sex, increasing age, a BMI of 28.0 kg/m2 or more, ever smoked and history of close contact with TB patients. The risk of QFT positivity among ever-smokers was 1.34 times higher than never-smokers (95% CI: 1.21–1.49) in multivariate analysis. After classified by gender and age groups, ever-smokers were still more likely to be QFT positive than never smokers as depicted in Fig 1.
Table 2. Identification of the factors independently associated with QFT positivity in the total study population.
| Variables | QFT positivity(n/N†) | % | p for χ2 test | Adjusted OR# (95% CI) |
|---|---|---|---|---|
| Gender | ||||
| Female | 1832/10863 | 16.86 | <0.001 | Reference |
| Male | 2122/9545 | 22.23 | 1.26 (1.14, 1.39) | |
| Age (years) | ||||
| 5–19 | 100/3485 | 2.87 | <0.001 | Reference |
| 20–29 | 175/2009 | 8.71 | 2.72 (2.07, 3.56) | |
| 30–39 | 313/2114 | 14.81 | 4.83 (3.73, 6.25) | |
| 40–49 | 876/4510 | 19.42 | 6.64 (5.22, 8.45) | |
| 50–59 | 934/3478 | 26.85 | 9.97 (7.83, 12.69) | |
| 60–69 | 966/3047 | 31.70 | 12.65 (9.95, 16.07) | |
| ≥70 | 590/1765 | 33.43 | 14.27 (11.19, 18.21) | |
| Education level | ||||
| Primary school or lower | 2236/10796 | 20.71 | <0.001 | |
| Middle school | 1275/6714 | 18.99 | ||
| High school | 375/2259 | 16.60 | ||
| College or higher | 68/639 | 10.64 | ||
| Household per capita income (RMB) | ||||
| <6000 | 2512/12978 | 19.36 | 0.924 | |
| ≥6000 | 1442/7429 | 19.41 | ||
| BMI (kg/m2) | ||||
| <18.5 | 230/3203 | 7.18 | <0.001 | 0.86 (0.73, 1.02) |
| ≥18.5-<24.0 | 2002/9762 | 20.51 | Reference | |
| ≥24.0-<28.0 | 1257/5444 | 23.09 | 1.06 (0.98, 1.16) | |
| ≥28.0 | 465/1998 | 23.27 | 1.15 (1.02, 1.29) | |
| Smoking status | ||||
| Never smoked | 2486/15316 | 16.23 | <0.001 | Reference |
| Ever smoked | 1468/5092 | 28.83 | 1.34 (1.21, 1.49) | |
| Alcohol drinking | ||||
| No | 2957/16486 | 17.94 | <0.001 | |
| Yes | 997/3921 | 25.21 | ||
| TB contact history | ||||
| No | 3722/19618 | 18.97 | <0.001 | Reference |
| Yes | 231/781 | 29.58 | 1.62 (1.37, 1.90) | |
| History of T2DM | ||||
| No | 3689/19427 | 18.99 | <0.001 | |
| Yes | 265/981 | 27.01 |
Abbreviations: BMI = body mass index; CI = confidence interval; OR = odds ratio; QFT = QuantiFERON-TB Gold In-Tube; T2DM = type 2 diabetes mellitus; TB = tuberculosis.
† Participants with indeterminate results were not included in this analysis. Sum might not always be in total because of missing data.
# Adjusted for variables with p < 0.05 in univariate analysis by stepwise selection. Sex and age kept in the model.
Fig 1.
Distribution of QFT positivity among ever-smokers and never-smokers by smoking status, gender (1A) and age (1B). After classified by gender and age groups, ever-smokers were still more likely to be QFT positivity than never-smokers (*: p < 0.05, **: p < 0.01). Abbreviations: QFT = QuantiFERON-TB Gold In-Tube.
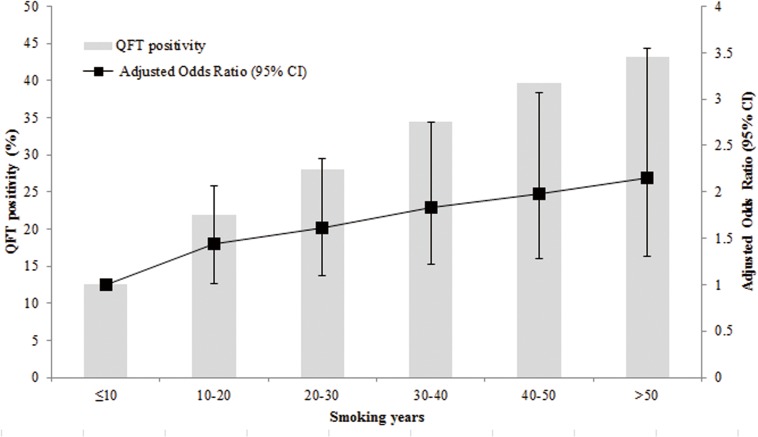
Factors associated with QFT positivity among smokers were shown in Table 3. An increasing TB infection risk was observed to be positively related to ages of 60 years or older, history of close contact with TB patients and duration of smoking years. In addition, as shown in Fig 2, a significant dose–response relation between QFT positivity and duration of smoking years was observed among smokers (p trend = 0.001).
Table 3. Association analysis for QFT positivity among smokers.
| Variables | N† | % | p for χ2 test | Adjusted OR#(95% CI) |
|---|---|---|---|---|
| Gender | ||||
| Female | 41/131 | 31.30 | 0.528 | Reference |
| Male | 1427/4961 | 28.76 | 0.91 (0.62.1.35) | |
| Age (years) | ||||
| 5–19 | 4/46 | 8.70 | <0.001 | Reference |
| 20–29 | 58/506 | 11.46 | 1.30 (0.45,3.75) | |
| 30–39 | 108/590 | 18.31 | 1.72 (0.58,5.09) | |
| 40–49 | 331/1285 | 25.76 | 2.27 (0.76,6.72) | |
| 50–59 | 367/1139 | 32.22 | 2.74 (0.91,8.19) | |
| 60–69 | 394/1033 | 38.14 | 3.25 (1.08,9.79) | |
| ≥70 | 206/493 | 41.78 | 3.53 (1.15, 10.85) | |
| Education level | ||||
| Primary school or lower | 731/2179 | 33.55 | <0.001 | |
| Middle school | 560/2162 | 25.90 | ||
| High school | 155/643 | 24.11 | ||
| College or higher | 22/108 | 20.37 | ||
| Household per capita income (RMB) | ||||
| <6000 | 924/3108 | 29.74 | 0.076 | |
| ≥6000 | 544/1984 | 27.42 | ||
| BMI (kg/m2) | ||||
| 18.5–24.0 | 814/2766 | 29.43 | 0.520 | |
| <18.5 | 71/276 | 25.72 | ||
| 24.0–28.0 | 436/1515 | 28.78 | ||
| ≥28.0 | 147/535 | 27.48 | ||
| Alcohol drinking | ||||
| No | 732/2358 | 31.04 | 0.001 | |
| Yes | 736/2734 | 26.92 | ||
| TB contact history | ||||
| No | 1381/4862 | 28.40 | 0.002 | Reference |
| Yes | 87/228 | 38.16 | 1.48 (1.12,1.97) | |
| History of T2DM | ||||
| No | 1352/4751 | 28.46 | 0.029 | |
| Yes | 116/341 | 34.02 | ||
| Smoking status | ||||
| Former smoker | 101/352 | 28.69 | 0.953 | |
| Current smoker | 1367/4740 | 28.84 | ||
| Cigarette type | ||||
| Without filter | 108/324 | 33.33 | 0.064 | |
| With filter | 1360/4768 | 28.52 | ||
| Onset of cigarette smoking (years) | ||||
| <18 | 279/927 | 30.10 | 0.346 | |
| ≥18 | 1189/4165 | 28.55 | ||
| Duration of smoking (years) | ||||
| ≤10 | 96/753 | 12.75 | <0.001 | Reference |
| 10–20 | 199/891 | 22.33 | p for trend<0.001 | 1.45 (1.02, 2.06) |
| 20–30 | 366/1305 | 28.05 | 1.65 (1.14, 2.40) | |
| 30–40 | 392/1138 | 34.45 | 1.90(1.28, 2.82) | |
| 40–50 | 294/726 | 40.50 | 2.09 (1.36, 3.19) | |
| >50 | 121/279 | 43.37 | 2.26 (1.38, 3.69) | |
| Number of cigarettes per day | ||||
| 1–5 | 126/507 | 24.85 | 0.009 | |
| 5–10 | 182/683 | 26.65 | p for trend<0.001 | |
| 10–19 | 525/1912 | 27.46 | ||
| ≥20 | 635/1990 | 31.91 |
Abbreviations: BMI = body mass index; CI = confidence interval; OR = odds ratio; QFT = QuantiFERON-TB Gold In-Tube; T2DM = type 2 diabetes mellitus; TB = tuberculosis.
† Indeterminate results had been excluded from the analysis. Sum might not always be in total because of missing data.
# Adjusted for variables with p < 0.05 in univariate analysis by stepwise selection. Sex and age kept in the model.
Fig 2. Association of QFT positivity with smoking years among smokers.
Multiple logistic regression analysis was conducted to assess the association of smoking years with QFT positivity. Variables with p < 0.05 in univariate analysis were included in the model. Gender, age, education level, history of close contact with tuberculosis patients, alcohol drinking, number of cigarettes smoked per day were adjusted. Adjusted odds ratio (ORs) and the 95% confidence intervals (CIs) were given in the figure. Abbreviations: CI = confidence interval; QFT = QuantiFERON-TB Gold In-Tube.
As shown in Table 4, we found evidence of modification effect by age (p for interaction < 0.001) with an OR ranged from 3.44 (1.16, 10.16) for those aged less than 19 to an OR of 1.31 (1.10, 1.55) for those older than 60. We did not observe a modification effect of gender (p for interaction, 0.842), BMI (p for interaction, 0.838) or history of TB contacts (p for interaction, 0.931). In additional sensitivity analyses, excluding 3556 subjects who were 5–19 years old did not materially alter the results (S2 and S3 Tables).
Table 4. The association of QFT positivity and smoking status by age, gender and TB contact history among total study populations.
| Variables& | QFT positivity (n/N) | % | Adjusted OR (95%CI) | |
|---|---|---|---|---|
| Gender and smoking status† | ||||
| Female | Never-smoker | 1791/10732 | 16.69 | Reference |
| Ever-smoker | 41/131 | 31.30 | 1.70 (1.16, 2.50) | |
| Male | Never-smoker | 695/4584 | 15.16 | Reference |
| Ever-smoker | 1427/4961 | 28.76 | 1.45 (1.30, 1.61) | |
| p for likelihood ratio test | 0.842 | |||
| Age and smoking status‡ | ||||
| 5–19 years | Never-smoker | 96/3439 | 2.79 | Reference |
| Ever-smoker | 4/46 | 8.70 | 3.44 (1.16, 10.16) | |
| 20–39 years | Never-smoker | 322/3027 | 10.64 | Reference |
| Ever-smoker | 166/1096 | 15.15 | 1.40 (1.07, 1.85) | |
| 40–59 years | Never-smoker | 1112/5564 | 19.99 | Reference |
| Ever-smoker | 698/2424 | 28.80 | 1.32 (1.13, 1.55) | |
| ≥ 60 years | Never-smoker | 956/3286 | 29.09 | Reference |
| Ever-smoker | 600/1526 | 39.32 | 1.31 (1.10,1.55) | |
| p for likelihood ratio test | 0.002 | |||
| TB contact history and smoking status# | ||||
| No | Never-smoker | 2341/14756 | 15.86 | Reference |
| Ever-smoker | 1381/4862 | 28.40 | 1.49 (1.34, 1.66) | |
| Yes | Never-smoker | 144/553 | 26.04 | Reference |
| Ever-smoker | 87/228 | 38.16 | 1.32 (0.84, 2.06) | |
| p for likelihood ratio test | 0.931 | |||
| BMI and smoking status* | ||||
| <28.0(kg/m2) | Never-smoker | 2168/13853 | 15.65 | Reference |
| Ever-smoker | 1321/4557 | 28.99 | 1.47 (1.32, 1.97) | |
| ≥28.0 (kg/m2) | Never-smoker | 318/1463 | 21.74 | Reference |
| Ever-smoker | 147/535 | 27.48 | 1.55 (1.11, 2.15) | |
| p for likelihood ratio test | 0.838 | |||
Abbreviations: BMI = Body mass index; CI = confidence interval; OR = odds ratio; QFT = QuantiFERON-TB Gold In-Tube; TB = tuberculosis. Participants with indeterminate results were not included in this analysis. Sum might not always be in total because of missing data.
& Variables independently associated with QFT positivity identified in Table 2 were considered in this analysis.
†Adjusted for age, BMI and TB contact history.
‡ Adjusted for gender, BMI and TB contact history.
# Adjusted for age, BMI and gender.
* Adjusted for age, TB contact history and gender.
Discussion
In this large cross-sectional analysis among 21,008 participants in rural China, ever smoking was found to be significantly associated with TB infection. Among smokers, smoking years was identified as an independent risk factor which was strongly related to TB infection in a dose-response manner. Our results suggested that the development of smoking bans in China might benefit TB control as well. High risk populations for TB infection, such as smoking elderly found in this study, might be potential target populations for TB infection monitoring and management in China.
The World Health Organization published a monograph to announce the integration of tobacco control into TB programs in 2007 [21]. However, the impact of smoking on TB infection has not been clearly estimated. China not only has the greatest number of smokers but also carry some of the highest smoking rates. The geographic overlap between rural areas of China with a higher prevalence of cigarette smoking and regions with higher prevalence of latent and active TB is striking and complex, but the potential for significant public health impact is unparalleled. Also, based on the results of our previous study, 13.5%-19.8% of participants were QFT positive in rural China. This may represent an enormous reservoir for tuberculosis transmission and rural areas are a logical and strategic focus for intervention. The results in our investigation were consistent with several studies in population survey settings such as South Africa [22], America [23] which found a positive link between smoking and TST positivity. More importantly, our results might be more robust after excluding the potential interferences from BCG vaccination and non-tuberculosis mycobacteria (NTM) by using IGRA. However, by contrast with the increasing risk of TB infection among smokers in our study, such a relation was not found in the study performed in Zambia and South Africa [15, 17]. The different characteristics of the study populations might account for the disparity. Both of the studies examined the influence of smoking on QFT results in human immunodeficiency virus (HIV) infections. The decreased sensitivity of QFT test in immune-suppressed individuals underestimated the prevalence of TB infection and might finally lead to bias [24]. In addition, smoking habit might be different among populations at higher risk of HIV infection and might be changed after HIV infection acquisition. Therefore, we should take care to generalize the findings from the specific population to the general population.
This study showed that there was a positive dose-response relationship between smoking years and TB infection. The adverse effects of cigarette smoking on pulmonary immunity might explain the susceptibility of smoking population to TB infection [25]. Cigarette smoking may attenuate host defense mechanisms by preventing expansion and activation of pathogen-specific CD4+ T-cells and reducing the numbers of IFN-γ-producing adenoid-specific CD4+ and CD8+ T-cells [26]. Cumulative stimulation of cigarette smoking might selectively down-regulates the production of IL-12 and TNF-α. Simultaneously, nicotine could turn off production of TNF-α by macrophages while leaving the secretion of IL-10 intact [27]. However, it is worth concern that the aged population probably had longer duration and/or higher intensity in cigarette smoking and the lung structure impairment in aged patients may also increase their susceptibilities to TB infection. A study from Taiwan found evidence of effect modification by age because the effect of current smoking on active TB disappeared among those aged 65 or older [28]. Evidence of effect modification between age and smoking were also found in our study. Unlike the study from Taiwan, the prevalence of TB infection increased from 2.79% among < 20 years’ never-smokers to 39.32% among ≥ 60 years’ ever-smokers (classified by 20 years). The effect of smoking on TB infection was always statistically significant although it decreased with age, which hinted the necessity of advocating smoking cessation among older smokers. Otherwise, in contrast to several studies [8, 11], our study failed to find a correlation between numbers of cigarettes smoked per day and TB infection among smoking populations. There are two explanations for this discrepancy. Firstly, our large sample size gives us the opportunity to stratify this analysis among smokers, but the former studies mainly used never-smokers as control. Second, co-linearity might exist between years of smoking and numbers of cigarettes smoked due to the potential tendency for smokers to smoke more and more as times went on. We verified our hypotheses by analyzing the effect of numbers of cigarettes smoked per day in whole population. Comparing with never-smokers, a dose-effect association was observed with OR ranged from 1.19 to 1.44 after controlling for age, gender, BMI and close contact history (S4 Table). However, the associations were non-significant when adding smoking years into the model. But the effect of smoking years was still significant in such a model. Therefore, smoking years might be a better indicator to reflect the impact of cigarette intensity on TB infection.
Our findings should be interpreted with consideration of the following limitations. First, the cross-sectional analysis could not absolutely determine the causal link between cigarette smoking and TB infection. During the follow-up period of the study, the relationship between smoking and the risk of TB infection acquisition will be further studied to clarify this important issue. Second, smoking status was self-reported rather than determined by biochemical methods. A previous systematic review showed trends of underestimation when smoking prevalence was based on self-report compared to cotinine-assessed [29]. Although a serious of related questions on smoking were collected which might reduce the possibility of such information bias, but we couldn’t exclude it completely. Thirdly, Second Hand Smoke (SHS) exposure or passive smoking [30] and air pollution [6] had been reported to be associated with elevated risk for TB infection. While we failed to clarify this effect as we didn’t collect related information.
Conclusions
Despite of the limitations, our findings support that cigarette smoking was independently associated with increased risk of TB infection. More attention should be attached to smokers, especially elderly smokers, as one potential target population for TB infection controlling in rural China. Reinforced programs to reduce cigarette use or development of regimen on tobacco cessation might contribute to lighten the burden of TB infection. In addition, active case finding among populations with specific risks such as smokers [31], close contacts [32], diabetes [33] should be strengthened as well.
Supporting information
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
We appreciate Prof. Lixia Wang and Prof. Shuigao Jin from Chinese Center for Disease Prevention and Control, and Prof. Yude Chen from Peking University for their help on study implement and data analysis. We thank Dr. Shiming Cheng and Dr. Liya Wan from Chinese Anti-tuberculosis Association for their support on study design. We thank all the investigators from the study sites for their contribution to the site work for baseline survey.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Science and Technology Major Project of China (grant number: 2013ZX10003004-002, 2014ZX10003001-001), and the Program for Changjiang Scholars and Innovative Research Team in University of China (grant number: IRT13007) and the Sanming Project of Medicine in Shenzhen (GCZX2015043015340574). The content is solely the responsibility of the authors and the funding sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
References
- 1.Aryanpur M, Masjedi MR, Mortaz E, Hosseini M, Jamaati H, Tabarsi P, et al. Intention to Quit Smoking and Associated Factors in Smokers Newly Diagnosed with Pulmonary Tuberculosis. Tanaffos. 2016; 15(1): 17–24. [PMC free article] [PubMed] [Google Scholar]
- 2.Bai KJ, Lee JJ, Chien ST, Suk CW, Chiang CY. The Influence of Smoking on Pulmonary Tuberculosis in Diabetic and Non-Diabetic Patients. PLoS One. 2016; 11(6): e0156677 doi: 10.1371/journal.pone.0156677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aryanpur M, Masjedi MR, Hosseini M, Mortaz E, Tabarsi P, Soori H, et al. Cigarette smoking in patients newly diagnosed with pulmonary tuberculosis in Iran. Int J Tuberc Lung Dis. 2016; 20(5): 679–684. doi: 10.5588/ijtld.15.0662 [DOI] [PubMed] [Google Scholar]
- 4.Loddenkemper R, Brönnecke M, Castell S, Diel R. Tuberculosis and Tobacco Smoking. Pneumologie. 2016; 70(1): 17–22. doi: 10.1055/s-0041-109601 [DOI] [PubMed] [Google Scholar]
- 5.Pednekar MS, Gupta PC. Prospective study of smoking and tuberculosis in India. Prev Med. 2007; 44(6): 496–498. doi: 10.1016/j.ypmed.2007.02.017 [DOI] [PubMed] [Google Scholar]
- 6.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PloS Med. 2007; 4(1): e20 doi: 10.1371/journal.pmed.0040020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Archives Int Med. 2007; 167 (4): 335–342. [DOI] [PubMed] [Google Scholar]
- 8.Hussain H, Akhtar S, Nanan D. Prevalence of and risk factors associated with Mycobacterium tuberculosis infection in prisoners, North West Frontier Province, Pakistan. Int J Epidemiol. 2003; 32 (5): 794–799. [DOI] [PubMed] [Google Scholar]
- 9.Anderson RH, Sy FS, Thompson S, Addy C. Cigarette smoking and tuberculin skin test conversion among incarcerated adults. Am J Prev Med. 1997; 13(3): 175–181. [PubMed] [Google Scholar]
- 10.McCurdy SA, Arretz DS, Bates RO. Tuberculin reactivity among California Hispanic migrant farm workers. Am J Ind Med. 1997; 32 (6): 600–605. [DOI] [PubMed] [Google Scholar]
- 11.Plant AJ, Watkins RE, Gushulak B, O'Rourke T, Jones W, Streeton J, et al. Predictors of tuberculin reactivity among prospective Vietnamese migrants: the effect of smoking. Epidemiol Infect. 2002; 128 (1): 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh M, Mynak ML, Kumar L, Mathew JL, Jindal SK. Prevalence and risk factors for transmission of infection among children in household contact with adults having pulmonary tuberculosis. Arch Dis Child. 2005; 90 (6): 624–628. doi: 10.1136/adc.2003.044255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solsona J, Cayla JA, Nadal J, Bedia M, Mata C, Brau J, et al. Screening for tuberculosis upon admission to shelters and free-meal services. Eur J Epidemiol. 2001; 17 (2): 123–128. [DOI] [PubMed] [Google Scholar]
- 14.Nisar M, Williams CS, Ashby D, Davies PD. Tuberculin testing in residential homes for the elderly. Thorax. 1993; 48(12): 1257–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shanaube K, Hargreaves J, Fielding K, Schaap A, Lawrence KA, Hensen B, et al. Risk factors associated with positive QuantiFERON-TB Gold In-Tube and tuberculin skin tests results in Zambia and South Africa. PloS One. 2011; 6 (4): e18206 doi: 10.1371/journal.pone.0018206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He G, Li Y, Zhao F, Wang L, Cheng S, Guo H, et al. The Prevalence and Incidence of Latent Tuberculosis Infection and Its Associated Factors among Village Doctors in China. PloS One. 2015; 10(5): e0124097 doi: 10.1371/journal.pone.0124097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oni T, Gideon HP, Bangani N, Tsekela R, Seldon R, Wood K, et al. Smoking, BCG and employment and the risk of tuberculosis infection in HIV-infected persons in South Africa. PloS One. 2012; 7 (10): e47072 doi: 10.1371/journal.pone.0047072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao L, Bai L, Liu J, Lu W, Wang X, Li X, et al. Annual risk of tuberculosis infection in rural China: a population-based prospective study. Eur Respir J. 2016; 48(1): 168–178. doi: 10.1183/13993003.00235-2016 [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Lu W, Bai L, Wang X, Xu J, Catanzaro A, et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis. 2015; 15(3): 310–319. doi: 10.1016/S1473-3099(14)71085-0 [DOI] [PubMed] [Google Scholar]
- 20.Ministry of Health of the People's Republic of China. Criteria of Weight for Adults (WS/T428-2013); 2013.
- 21.World Health Organization. International Union against Tuberculosis and Lung Disease. A WHO/the Union monograph on TB and tobacco control: joining efforts to control two related global epidemics. 2007.
- 22.den Boon S, van Lill SW, Borgdorff MW, Verver S, Bateman ED, Lombard CJ, et al. Association between smoking and tuberculosis infection: a population survey in a high tuberculosis incidence area. Thorax. 2005; 60(7): 555–557. doi: 10.1136/thx.2004.030924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horne DJ, Campo M, Ortiz JR, Oren E, Arentz M, Crothers K, et al. Association between smoking and latent tuberculosis in the U.S. population: an analysis of the National Health and Nutrition Examination Survey. PloS One. 2012; 7(11): e49050 doi: 10.1371/journal.pone.0049050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santin M, Munoz L, Rigau D. Interferon-gamma release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PloS One. 2012; 7 (3): e32482 doi: 10.1371/journal.pone.0032482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Y, Kong Y, Barnes PF, Huang FF, Klucar P, Wang X, et al. Exposure to cigarette smoke inhibits the pulmonary T-cell response to influenza virus and Mycobacterium tuberculosis. Infect Immun. 2011; 79 (1): 229–237. doi: 10.1128/IAI.00709-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins CS, Dawe DE, Goncharova SI, Pouladi MA, Drannik AG, Swirski FK, et al. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Am J Resp Cell Mol. 2004; 30 (2): 202–211. [DOI] [PubMed] [Google Scholar]
- 27.Matsunaga K, Klein TW, Friedman H, Yamamoto Y. In vitro therapeutic effect of epigallocatechin gallate on nicotine-induced impairment of resistance to Legionella pneumophila infection of established MH-S alveolar macrophages. J Infect Dis. 2002; 185 (2): 229–236. doi: 10.1086/338449 [DOI] [PubMed] [Google Scholar]
- 28.Lin HH, Ezzati M, Chang HY, Murray M. Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Resp Crit Care. 2009; 180 (5): 475–480. [DOI] [PubMed] [Google Scholar]
- 29.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009; 11 (1): 12–24. doi: 10.1093/ntr/ntn010 [DOI] [PubMed] [Google Scholar]
- 30.Patra J, Bhatia M, Suraweera W, Morris SK, Patra C, Gupta PC, et al. Exposure to Second-Hand Smoke and the Risk of Tuberculosis in Children and Adults: A Systematic Review and Meta-Analysis of 18 Observational Studies. PLoS Med. 2015; 12 (6):e1001835 doi: 10.1371/journal.pmed.1001835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei X, Zou G, Chong MK, Xu L. An intervention of active TB case finding among smokers attending routine primary care facilities in China: an exploratory study. Trans R Soc Trop Med Hyg. 2015; 109 (9): 545–552. doi: 10.1093/trstmh/trv063 [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Wei X, Zou G, Walley J, Zhang H, Guo X, et al. Evaluation of active tuberculosis case finding through symptom screening and sputum microscopy of close contacts in Shandong, China. Trop Med Int Health. 2011; 16 (12): 1511–1517. doi: 10.1111/j.1365-3156.2011.02869.x [DOI] [PubMed] [Google Scholar]
- 33.Lin Y, Li L, Mi F, Du J, Dong Y, Li Z, et al. Screening patients with diabetes mellitus for tuberculosis in China. Trop Med Int Health. 2012; 17 (10):1302–1308. doi: 10.1111/j.1365-3156.2012.03069.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.