Abstract
Local adaptation is an important mechanism underlying the adaptation of plants to environmental heterogeneity, and the toxicity of salt results in strong selection pressure on salt tolerance in plants and different ecotypes. Solidago canadensis, which is invasive in China, has spread widely and has recently colonized alkali sandy loams with a significant salt content. A common greenhouse experiment was conducted to test the role of local adaptation in the successful invasion of S. canadensis into salty habitats. Salt treatment significantly decreased the growth of S. canadensis, including rates of increase in the number of leaves and plant height; the root, shoot, and total biomass. Furthermore, salt stress significantly reduced the net photosynthetic rate, stomatal conductance, transpiration rate and relative chlorophyll content but significantly increased peroxidase activity and the proline content of S. canadensis and the root/shoot ratio. Two-way analysis of variance showed that salt treatment had a significant effect on the physiological traits of S. canadensis, except for the intercellular CO2 concentration, whereas the population and the salt × population interaction had no significant effect on any physiological traits. Most of the variation in plasticity existed within and not among populations, excep for the root/shoot ratio. S. canadensis populations from soil with moderate/high salt levels grew similarly to S. canadensis populations from soils with low salt levels. No significant correlation between salt tolerance indices and soil salinity levels was observed. The plasticity of the proline content, intercellular CO2 concentration and chlorophyll content had significant correlations with the salt tolerance index. These findings indicate a lack of evidence for local adaption in the existing populations of invasive S. canadensis in China; instead, plasticity might be more important than local adaptation in influencing the physiological traits and salt tolerance ability across the S. canadensis distribution.
Introduction
Invasive species are considered the second greatest threat to native biodiversity following habitat destruction and are a hotspot in the study of ecology and the environment [1]. When alien plants are introduced into a novel region, they confront novel abiotic and biotic factors that may determine invasion success [2, 3]. It has been well documented that invasive plants have stronger adaptation ability to heterogeneous environments than native species [4, 5], yet there is also evidence showing that invasive and native plants have equal ability to adapt to heterogeneous environments [6]. Nontheless, it remains unclear why and how invasive plants adapt to novel heterogeneous abiotic and biotic factors [7].
Because plants are sessile during a significant fraction of their life cycle and are subject to strong selection for optimal performance under local environmental conditions [8], local adaptation contributes to the successful occupation of different habitats and wide distribution [7, 9, 10]. Geographic variation can lead to the evolution of different adaptations to a local environment and thus generate ecotypic differentiation in important functional traits [11, 12]. Furthermore, local adaptation has been well verified as a major mechanism enhancing invasiveness [13], which helps invasive plants successfully adapt to novel heterogeneous environments [7, 9]. Alternatively, phenotypic plasticity, the ability of a single genotype to produce different phenotypes in response to environmental variation [14–16], also contributes to the success of an invasive plant species by allowing increased fitness across a range of habitats [3, 17–22] and plays an important role in successful establishment under novel conditions [19, 23]. A central issue in invasion ecology is determining the importance of phenotypic plasticity and local adaptation in the adaption of invasive plant to novel heterogeneous environments across its distribution. However, contradictory conclusions have been drawn, indicating that the importance of these factors might depend on the plant species (for phenotypic plasticity rather than local adaptation, see reference [3, 24]; for both phenotypic plasticity and local adaptation, see reference [13, 25]. Thus, more attention should be paid to invasive plants to explore the mechanisms underlying invasion success [24].
Soil salinity is a determining factor in species colonization because salt can be toxic [26]. It is well documented that salinity can inhibit the growth of plants through water deficit, ion toxicity, ion imbalance or a combination of these factors, and plants respond to salinity stress at physiological, biochemical, molecular and morphological levels [27, 28]. However, the effect of salinity on plant growth is dependent on the salt concentration. A high saline environment may reduce parameters, such as the photosynthetic rate, plant height, the number of leaves, root length and increase the root/shoot ratio [27], whereas low salinity may stimulate plant growth [28]. In addition, the effect of soil salinity on plant species varies among taxa [29], including invasive plants [26]. Both phenotypic plasticity and genetic adaptation may contribute to the salt tolerance of plants [7, 8], and soil salinity plays an important role in favoring or limiting the spread of alien species, especially in the colonization of saline environments [30]. For example, salinity was found to enhance the replacement of native Spartina alterniflora by invasive Phragmites australis [30] but to limit the replacement of native Salicornia subterminalis by invasive Polypogon monspeliensis [31]. Understanding the response of invasive plants to soil salinity when there is potential for colonizing saline environments is key to providing basic references for the management and control of invasive plants [30].
Solidago canadensis L. (Asteraceae), a long-lived rhizomatous perennial forb of North American origin, is one of the most widespread invasive alien plants [32]. This species was introduced to Shanghai in 1935 as an ornamental plant and then escaped into the wild [33] and has since become highly abundant in China [34]. Based on microsatellite and chloroplast locus data, Moran found that S. canadensis collected from colder locations in Switzerland tended to grow faster at a site of the highest elevation, whereas samples collected from warmer sites did not, indicating the possibility of local adaptation to cold weather [35]. Based on growth chamber and greenhouse experiments, we determined that the phenotypic plasticity of S. canadensis may have evolved rapidly in regions with different climatic conditions and might have contributed to the spread of this invasive species [22]. However, we also found that individual plasiticty, not local adaptation, plays an important role in the response of S. canadensis to shade [36]. Recently, S. canadensis was shown to be tolerant to salinity and to have invaded alkali sandy loams with a significant salt content, such as the Jiuduansha intertidal wetland shoals located at the junction of the Yangtze River and the East China Sea [37] and the polders on the wetland of Hangzhou Bay [38]. With regard to the toxic and selection pressure of salt, we hypothesized that local adaptation might play important role in the invasion success of S. canadensis into sodic soil. However, there is thus far no experimental evidence. Therefore, in this study, we conducted a greenhouse experiment with replicate cuttings of genets from different populations and addressed three questions: (1) Is the salt tolerance of S. canadensis populations related to soil salt levels? (2) Do S. canadensis populations from high salt-level soil grow better than those from low salt-level soil? If not, (3) does the variation in plasticity in response to salt exist within or among populations of S. canadensis? (4) Is the plasticity of physiological traits correlated with the salt tolerance of S. canadensis?
Materials and methods
Plant species
Solidago canadensis, a forb belonging to the Asteraceae family, can produce annual clonal aboveground shoots from persistent belowground rhizomes [34]. This clonal growth leads to dense stands of shoots that reduce native species diversity [34]. The seeds are small, numerous, and wind dispersed, which is necessary for long-distance dispersal [34]. In addition, S. canadensis is self-incompatible [39].
Sample collection and propagation of plant materials
In the winter of 2012, rhizome systems were collected from 11 populations of S. canadensis in China. The populations came from representative habitats; most of the field sites were located near roads and consisted largely of ruderal vegetation. The field sites did not involve any endangered or protected species, and none of the populations were privately owned or under nature protection. No specific permissions were required for these locations. The geographical information and the types of land uses of the populations are shown in Table 1. Soil salinity levels were extracted from the Harmonized World Soil Database at a 0.5-arc-minute spatial resolution from FAO (http://www.fao.org/; detailed information is shown in Table 1). Excess free salts, referred to as soil salinity, is measured as electrical conductivity (EC) or exchangeable sodium percentage (ESP). Among the populations, the salinity levels of 7 were low (EC <4 ds/m or ESP < 6%), whereas those of the other 4 were moderate (EC = 4–8 ds/m or ESP = 6–15%), high (EC = 8–16 ds/m or ESP = 15–25%) or very high (EC > 16 ds/m or ESP > 15%) [40]. Within each population, 3 randomly selected shoot bases with attached rhizomes were dug up and kept moist until replanting. The clonal sprouts of S. canadensis generate from belowground rhizome fragments; thus, the distances between the shoots were at least 10 m to avoid sampling the same genet twice. All rhizome systems were planted in pots in a common garden at Taizhou University (E 121°17´, N 28°87´) in Linhai City, Zhejiang Province, China. The pots were 30 cm in diameter and 30 cm deep and were filled with a mixed matrix composed of soil, sand and peat soil in a 6:3:1 ratio with a final pH of 6.80±0.10, an organic matter content of 27.66±1.19 g/kg, a total nitrogen content of 361.00±33.00 mg/kg, an available phosphorus content of 8.00±1.14 mg/kg, and an available potassium content of 12.00±1.00 mg/kg. Flow cytometry analysis using a tender leaf showed that individuals from all localities sampled in this study exhibited the same ploidy level (i.e., hexaploidy; 6n = 54).
Table 1. Geographical information, types of land use and soil salinity of Solidago canadensis populations.
| No. | Population abbreviation | Location | Longitude | Latitude | Altitude (m) | Types of land uses | Soil salinity |
|---|---|---|---|---|---|---|---|
| 1 | PD | Pudong District, Shanghai City | E121.804° | N31.354° | 3 | Abandoned farmland | Moderate salinity |
| 2 | TZ | Taizhou City, Zhejiang Province | E121.397° | N28.656° | 6 | Abandoned farmland | Low salinity |
| 3 | NT | Nantong City, Jiangsu Province | E120.843° | N32.070° | 5 | Abandoned farmland | Low salinity |
| 4 | WZ | Wenzhou City, Zhejiang Province | E120.607° | N28.126° | 4 | Abandoned farmland | Low salinity |
| 5 | HZ | Xiaoshan District, Hangzhou City, Zhejiang Province | E120.297° | N30.161° | 9 | Abandoned farmland | Low salinity |
| 6 | FZ | Fuzhou City, Fujian Province | E119.359° | N26.098° | 19 | Abandoned farmland | Low salinity |
| 7 | LYG | Lianyungang City, Jiangsu Province | E119.235° | N34.654° | 3 | Abandoned farmland | Very high salinity |
| 8 | WHu | Wuhu City, Anhui Province | E118.387° | N31.342° | 16 | Garbage dump | Moderate salinity |
| 9 | JDZ | Jingdezheng City, Jiangxi Province | E117.166° | N29.318° | 40 | Green belts | Low salinity |
| 10 | JJ | Jiujiang City, Jiangxi Province | E116.283° | N29.985° | 18 | Abandoned vegetable garden | High salinity |
| 11 | WH | Hankou District, Wuhan City, Hubei Province | E114.350° | N30.878° | 25 | Abandoned farmland | Low salinity |
Salt treatment experiment
The salt treatment experiment was conducted in a greenhouse at Taizhou University under identical light, humidity and temperature conditions in the summer of 2014. The mean daily temperature ranged from 25°C to 34°C. The photosynthetic active radiation is approximately 80% of the strength of natural sunlight. Rosettes with a similar height (mean = 15 cm) were selected and removed from the collected rhizome systems after sprouting and individually planted in pots (16 cm diameter, 14 cm height) containing the mixed matrix. For every population, three genotypes were used as replicates. For every genotype, two rosettes were used for the salt treatment and control. One rosette was planted in each pot. All plant materials used in the different treatments in this study were clonally propagated two years after being commonly planted so that the differences caused by different genotypes and the effect of the mother body were excluded in the experiment. Eight days after planting, 50 ml of 1/8 Hoagland solution was applied to each pot. Ten days after planting, the plant height (Ht1) and number of leaves (Nt1) were calculated as the initial data (t1 = 0 day). Plant height was recorded as the distance from the ground to the highest leaf position. To set up the periodic salt treatments, 50 ml of a 300 mmol/L NaCl solution was applied to each pot for two days, followed by 50 ml of tap water for one day, then 50 ml of Hoagland solution for one day. For the control, 50 ml of tap water was applied to each pot for three days, followed by 50 ml of Hoagland solution for one day. The positions of the pots were randomized within the experimental chamber every week to confirm that no position effect occurred.
Measurements
Twenty-eight days after salt treament, plant height (Ht2) was measured via ruler with an accuracy of 0.1 cm. The number of leaves (Nt2) was also recorded. The increase rate in plant height was calculated as (Ht2-Ht1)/(t2-t1) and the rate of increase of the number of leaves was calculated as (Nt2-Nt1)/(t2-t1).
In situ photosynthesis measurements were made on the third fully expanded leaf, using a portable photosynthesis-measurement system (LI-6400 XT, Li-COR Inc., Lincoln, NE, USA). Measurements were obtained between 9:00 AM and 11:00 AM under a photosynthetically active radiation of 1,400 μmol m-2 s-1 (i.e. at light saturation) at a leaf temperature of 25°C, a CO2 concentration of 400 ppm, and relative humidity of 70%. Net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) were measured.
Three tender leaves per plant were collected and transferred to the lab immediately upon collection. The content of proline was determined via acid ninhydrin colorimetry using L-proline as the standard [41]. The activity of peroxidase was measured by ultraviolet spectrometry [42]. The relative chlorophyll content was measured using a chlorophyll content meter (CCM-200 plus, Opti-Science Inc. Hudson, NH, USA).
Following measurements, plants were harvested and divided into leaves, stems, and roots. Plant material was over-dried (at 105°C for 1 h and then at 80°C until a constant weight was reached). The leaf, stem, and root biomasses were weighed using a balance with a precision of 0.0001 g (Shanghai Jingtian Electronic Instrument Co., Ltd, Shanghai, China). Total biomass and root/shoot ratios were calculated.
Statistical analyses
The tolerance index (TI) in terms of the root, shoot, total biomass and plant height was calculated for each population, i.e., the index of the plants under salinity stress divided by the index of the plants in the control [43]. The phenotypic plasticity index was calculated based on the difference between the maximum mean and minimum mean divided by the maximum mean [44].
All data are shown as the mean ± standard error. A t-test was applied to test the effect of the salt treatment on the plants. A two-way analysis of variance (ANOVA) was applied to test the effects of salt, population and their interaction on physiological traits, with the treatment as a fixed factor and the population and genotype (nested to population) as random factors. One-way ANOVA was applied to test the differences in plasticity among different populations. A variance component analysis was conducted to calculate the differentiation coefficient of the phenotypic plasticity index within or between different populations. The relationships between phenotypic plasticity and the salt tolerance index were evaluated separately by Pearson’s correlation analysis. The relationships between the salt tolerance index and soil salinity level were evaluated using Spearman’s rho value through a nonparametric test (binomial test). All statistical analyses were conducted using SPSS 16.0 software.
Results
Effects of salinity on growth and salt tolerance
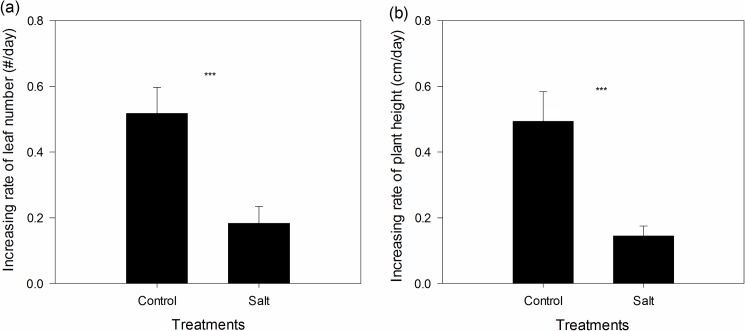
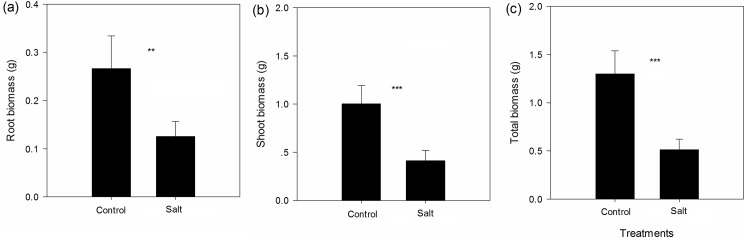
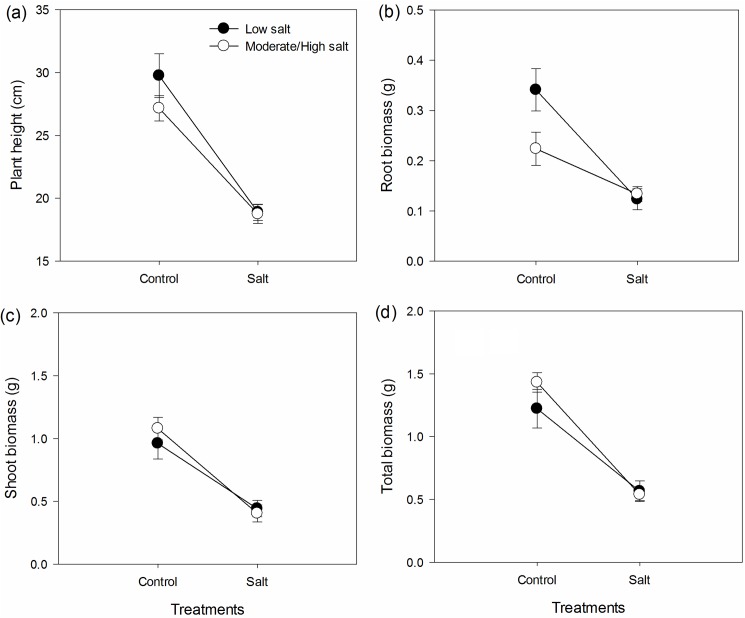
The salt treatment significantly inhibited the rate of increase in the number of leaves (Fig 1A, paired t value = 8.987, p<0.001) and the rate of increase in plant height (Fig 1B, paired t value = 7.245, p<0.001). Salt treatment also significantly reduced the root biomass (Fig 2A, paired t value = 4.306 p = 0.002), shoot biomass (Fig 2B, paired t value = 6.597, p<0.001) and total biomass (Fig 2C, paired t value = 6.733, p<0.001). Under salt treatment, the root, shoot, and total biomass and plant height of S. canadensis populations from moderate/severe salt-level soil were similar to those of S. canadensis populations from low salt-level soil (Fig 3).
Fig 1.
Effect of salt treatment on the rate of increase in the number of leaves (a) and plant height (b). Values are shown in mean ± standard error (SE). ***, indicates that the difference between the control and the salt treatment is significant at p<0.001.
Fig 2.
Effect of salt treatment on root biomass (a) shoot biomass (b) and total biomass (c). Values are shown in mean ± standard error (SE). *, **, ***, indicate that the difference between the control and salt treatment is significant at p<0.05, p<0.01 and p<0.001, respectively.
Fig 3.
Effect of salt treatment on the plant height (a) and root (b), shoot (c) and total biomass (d) of S. canadensis populations from moderate/severe salt-level soil and low salt-level soil. Values are shown in mean ± standard error (SE).
Based on the root, shoot and total biomass, the salt tolerance of S. canadensis of the NT population was highest, whereas the salt tolerance of the LYG population was lowest. Based on plant height, the salt tolerance of the HZ and TZ populations was highest and lowest, respectively (Table 2). However, no significant differences were found among the populations (p>0.05). Nonparametric correlations analysis showed no significant correlation between salt tolerance indices and soil salinity levels (Table 2).
Table 2. Mean ± standard error (SE) of the salt tolerance for each trait of Solidago canadensis and nonparametric correlations with soil salinity levels.
| Population | Plant height | Shoot biomass | Root biomass | Total biomass |
|---|---|---|---|---|
| PD | 0.71±0.14 | 0.33±0.03 | 0.59±0.20 | 0.38±0.17 |
| TZ | 0.43±0.03 | 0.43±0.21 | 0.50±0.29 | 0.42±0.26 |
| NT | 0.85±0.10 | 0.94±0.06 | 0.77±0.09 | 0.88±0.08 |
| WZ | 0.50±0.07 | 0.36±0.08 | 0.43±0.06 | 0.34±0.04 |
| HZ | 0.90±0.18 | 0.35±0.14 | 0.39±0.08 | 0.36±0.12 |
| FZ | 0.42±0.01 | 0.47±0.03 | 0.62±0.25 | 0.49±0.08 |
| LYG | 0.54±0.03 | 0.14±0.03 | 0.24±0.07 | 0.18±0.07 |
| WHu | 0.62±0.03 | 0.52±0.18 | 0.47±0.17 | 0.62±0.31 |
| JDZ | 0.58±0.10 | 0.39±0.21 | 0.35±0.16 | 0.39±0.0.21 |
| JJ | 0.72±0.15 | 0.50±0.27 | 0.52±0.30 | 0.50±0.28 |
| WH | 0.57±0.13 | 0.36±0.14 | 0.47±0.19 | 0.37±0.15 |
| Spearman’s rho (p value) | 0.171 (0.425) | -0.329 (0.116) | -0.207 (0.331) | -0.286 (0.175) |
Effects of salinity on physiological traits, root/shoot ratio and their plasticities
The salt treatment significantly increased peroxidase activity, proline content and root/shoot ratio, and significantly decreased the net photosynthetic rate, stomatal conductance, and transpiration rate; however, the salt treatment had no significant effect on the intercellular CO2 concentration (Table 3). Two-way ANOVA showed that the salt treatment had a significant effect on the root/shoot ratio and the physiological traits of S. canadensis, except for the intercellular CO2 concentration; In contrast, the population, genotype and the interaction salt × population had no significant effect on all traits. The proline content showed the strongest plasticity, whereas the intercellular CO2 concentration and root/shoot ratio showed the weakest plasticity (Table 3). Most of the variation in plasticity existed within populations and not among populations, except for the root/shoot ratio (Table 3).
Table 3. Mean ± standard error (SE) of each physiological trait under the control and salt treatment and the mean ± SD and differentiation coefficient (Vst) of the phenotypic plasticity index (PPI) for each physiological trait of Solidago canadensis.
Different small letters on the same line indicate that the difference between the control and salt treatment was significant. The results of two-way ANOVA and the population differentiation coefficient are listed.
| Effect of salinity | Phenotypic plasticity | |||||||
|---|---|---|---|---|---|---|---|---|
| Trait | Control (mean±SE) | Salt (mean±SE) | Fsalt | Fpop | Fsalt×pop | Fgeno | PPI (mean±SD) | Vst |
| Proline content | 18.10±15.81a | 342.45±174.94b | 80.61*** | 1.30 | 1.05 | 0.69 | 0.93±0.07 | 0.44 |
| Peroxidase activity | 2.10±0.81a | 3.13±1.94b | 7.78* | 0.82 | 1.34 | 1.36 | 0.37±0.23 | 0.48 |
| Net photosynthetic rate | 11.35±1.96a | 6.21±2.57b | 64.16*** | 1.83 | 1.09 | 0.71 | 0.47±0.21 | 0.30 |
| Stomatal conductance | 0.70±0.17a | 0.31±0.16b | 89.89*** | 0.60 | 0.60 | 0.29 | 0.56±0.22 | 0.28 |
| Intercellular CO2 concentration | 344.66±7.87a | 341.42±13.79a | 2.53 | 10.11 | 0.58 | 0.53 | 0.03±0.03 | 0.56 |
| Transpiration rate | 7.68±1.41a | 4.97±2.01b | 36.93*** | 11.10 | 0.78 | 0.33 | 0.37±0.27 | 0.39 |
| Chlorophyll content | 1.04±0.22a | 0.57±0.18b | 71.26*** | 5.57 | 1.31 | 0.34 | 0.46±0.19 | 0.48 |
| Root/shoot ratio | 0.28±0.05b | 0.34±0.06a | 3.75* | 0.99 | 1.01 | 0.78 | 0.28±0.03 | 0.97 |
Note:
*, *** indicate significance at the 0.05 and 0.001 levels, respectively.
Correlation between the salt tolerance index and the plasticity of physiological traits and the root/shoot ratio
Pearson’s correlation analysis revealed significant correlations between the plasticity of proline content and the salt tolerance index based on plant height; the plasticity of the intercellular CO2 concentration and salt tolerance indices based on total biomass, shoot biomass and plant height; and the plasticity of the chlorophyll content and the salt tolerance index based on plant height (Table 4).
Table 4. Pearson’s correlation between the plasticity index of physiological traits and the salt tolerance index.
The p values are listed in parentheses. The figures in bold indicate significant correlations.
| Salt tolerance index | Proline content | Peroxidase activity | Net photosynthetic rate | Stomatal conductance | Intercellular CO2 concentration | Transpiration rate | Chlorophyll content | Root/shoot ratio |
|---|---|---|---|---|---|---|---|---|
| Total biomass | -0.003(0.987) | -0.125(0.550) | 0.071(0.737) | 0.231(0.266) | 0.417(0.038) | 0.196(0.347) | -0.245(0.248) | -0.074(0.724) |
| Root biomass | -0.099(0.639) | -0.032(0.880) | 0.261(0.208) | 0.325(0.113) | 0.166(0.427) | 0.214(0.304) | -0.192(0.369) | 0.065(0.758) |
| Shoot biomass | -0.020(0.926) | -0.119(0.571) | 0.027(0.899) | 0.197(0.346) | 0.414(0.039) | 0.151(0.471) | -0.242(0.254) | -0.014(0.947) |
| Plant height | -0.570(0.003) | 0.163(0.435) | -0.215(0.302) | -0.103(0.625) | 0.500(0.011) | -0.135(0.519) | -0.431(0.036) | -0.060(0.775) |
Discussion
Salinity is a limiting environmental factor that impairs plant growth and development [45]. The toxicity of salt results in strong selection pressure on the salt tolerance of plants and different ecotypes, and genetic mutants with salt tolerance have evolved during the adaption to salt-affected soil [46]. In our greenhouse experiment, we did not find that S. canadensis from higher saline-level soil performed better than plants from low saline-level soil; furthermore, the salt tolerance indices in terms of root, shoot, total biomass and height did not show any significant correlations with the soil salinity levels. Our results suggest the absence of local adaption in the existing populations of S. canadensis collected from soils with medium, high and extremely high salinity, which was contrary to our original hypothesis. Similar phenomena have been observed in the perennial clonal plant Leymus chinensis in response to water [7] and the invasive plant Fallopia japonica in response to salt [3], but not in invasive Ipomoea cairica in response to salt [47] and invasive S. canadensis in response to cold [35]. Richards et al. hypothesized that local adaptation would occur in F. japonica established in salt marsh habitats because of increased salt tolerance compared to those established in the more common roadside habitat. However, these authors found that F. japonica from the salt marsh habitats did not perform better in the salt treatment, suggesting that selection by the salt content of the marsh habitat did not generate genotypes adapted to high salinity [3]. Having conducted a meta-analysis of local adaptation in plants, Leimu and Fischer suggested that local adaption is less common in plant populations than is generally assumed [48]. The occurrence and strength of local adaptation by plants in response to environmental variation may be dependent on species as well as on the life history, population size, and study characteristics [49].
The absence of local adaptation of S. canadensis in sodic soils might be due to three reasons. The first is the extremely high plasticity of S. canadensis, which might cover the effect of genotype variation. The second is the relatively short invasion history in colonization of sodic soil. Although differences in soil salinity levels existed in the different population, the short invasion history and weak selection pressure might not have contributed to the local adaptation of S. canadensis populations. The third is the population origin and non-quantitative measurment of soil salinity, which might also be the main limitation of the experiment design. The populations used in the study were collected from normal habitats and, not from coastal areas, where the difference in soil salinity levels might be greater. In addition, we extracted the soil salinity level data from the Harmonized World Soil Database at a 0.5-arc-minute spatial resolution from FAO, which might not be consistent with the local habitat conditions from which the S. canadensis individuals were collected. The relatively small difference in soil salinity levels and the relatively coarse broad-scale information of soil salinity levels might have affected the corrlation between salt tolerance indices and soil salinity levels. The existence of local adaption to cold temperature has been reported [35]. Thus, although no clear evidence for local adaptation was found in this study, we should still be careful in drawing a general conclusion about the absence of local adaption of S. canadensis in colonization of sodic soils. Further study should involve collection of plants from sodic soils with clear and relatively greater differences in salinity levels, direct measurement of the soil salinity content and calculation of the relationship between salt tolerance indices and the quantitative sat content.
Plasticity for ecologically important traits may enhance the ability to withstand adverse environmental conditions or to respond positively to favorable conditions, thus promoting invasiveness [49]. It has been well documented that phenotypic plasticity may be a common trait of plant invaders (reviewed by Richards et al. [19]. Richards et al. demonstrated that plasticity in salt tolerance traits might allow invasive Fallopia japonica and F. × bohemica to live in saline habitats without any specific adaptation to tolerate salt [3]. Our previous study showed that the phenotypic plasticity of S. canadensis is high in response to temperature and water availability [22]. In the present study, we observed strong plasticity of the proline content, intermediate plasticity of the net photosynthetic rate, stomatal conductance and relative chlorophyll content, and relatively weaker plasticity of the transpiration rate and the activity of peroxidase. Pearson’s correlation analysis revealed a significantly negative correlation between the plasticity of proline content or chlorophyll content and the salt tolerance index based on plant height and a significant positive correlation between the plasticity of the intercellular CO2 concentration and salt tolerance indices based on total biomass, shoot biomass and plant height. Our experimental evidence suggests that phenotypic plasticity may be the primary adaptive strategy for invasive S. canadensis with regard to salinity tolerance. These results show that the ability of S. canadensis to maintain higher chlorophyll content, lower proline content and higher intercellular CO2 concentration enables greater growth at higher levels of salinity.
Although the variation of phenotypic plasticity among invading populations has rarely been documented [50], some studies have indicated that variation of plasticity among introduced populations may allow an invader to evolve greater plasticity, resulting in the colonization of more diverse habitats and plant communities [17, 49, 50]. A previous study showed that individual plasticity, not local adaptation, played a more prominent role in the shade response of invasive S. canadensis populations under similar light conditions [36]. In the present study, we found that most of the variation in the plasticity of the physiological traits of S. canadensis occurred among individuals within populations and not among populations, indicating that the potential of S. canadensis to evolve greater tolerance or adaptation to saline soil was small. Similar results were obtained for the clonal perennial plant Leymus chinensis [7] but not the invasive plant Microstegium vimineum [49] and Ipomoea cairica [47]. In addition, we also found taht most of the variation in the plasticity of the root/shoot ratio of S. canadensis occurred among populations, indicating that the evolutionary potential of the variation in root/shoot ratio was large. However, we did not find a significant correlation between salt tolerance indices and the plasticity of the root/shoot ratio, which might also be due to the short colonization history of S. canadensis in saline habitats or the small difference in soil salinity levels.
Taken together, these findings indicate that individual plasticity might be more important than local adaptation in affecting physiological traits and salt tolerance across the distribution of the species. We predict that S. canadensis, which is dominant on broadsides [34], might have the potential to become a serious problem in sodic soils, including coastal areas, in the future. More attention should focus on monitoring the occurrence of S. canadensis on sodic soils, and timely prevention should be implemented to control new invaders.
Acknowledgments
We thank Jianqing Ding and Zhengsheng He for their kind help with plant collection. We would also like to thank the two anonymous reviewers for helpful comments.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was financially support by the National Key Research and Development Program (2016YFC1201102; 2016YFC1201100), the National Natural Science Foundation of China (No. 31270461), and the Qianjiang Talents Project of Zhejiang Province (type D) (No. QJD1302021). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vitousek PM, D’Antonio CM, Loope LL, Rejmánek M, Westbrooks R. Introduced species: a significant component of human-caused global change. New Zealand Journal of Ecology. 1997; 21: 1–16. [Google Scholar]
- 2.Minchinton TE, Simpson JC, Bertness MD. Mechanisms of exclusion of native coastal marsh plants by an invasive grass. Journal of Ecology. 2006; 94: 342–354. [Google Scholar]
- 3.Richards CL, Walls RL, Bailey JP, Parameswaran R, George T, Pigliucci M. Plasticity in salty tolerance traits allows for invasion of novel habitat by Japanese knotweed S.L. (Fallopia japonica and F. × bohemica, Polygonaceae). American Journal of Botany. 2008; 95: 931–942. doi: 10.3732/ajb.2007364 [DOI] [PubMed] [Google Scholar]
- 4.Xu GF, Shen SC, Zhang FD. Adaptability and reproductive characteristics of Mikania micrantha H.B.K. under different habitats. Ecology and Environment Sciences. 2014; 8: 1258–1264. [Google Scholar]
- 5.Keser LH, Visser EJW, Dawson W, Song YB, Yu FH, Fisher M, Dong M. van Kleunen M. Herbaceous plant species invading natural areas tend to have stronger adaptive root foraging than other naturalized species. Frontiers in Plant Science. 2015; 6: 273–283. doi: 10.3389/fpls.2015.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drenovsky RE, Khasanova A, James JJ. Trait convergence and plasticity among native and invasive species in resource-poor environments. American Journal of Botany. 2012; 99(4): 629–639. doi: 10.3732/ajb.1100417 [DOI] [PubMed] [Google Scholar]
- 7.Liu YJ, Zhang LR, Xu XL, Niu HS. Understanding the wide geographic range of a clonal perennial grass: plasticity versus local adaptation. AoB Plant. 2015; 7: plv141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu JK. Genetic analysis of plant salt tolearnce using Arabidopsis. Plant Physiology. 2010; 124(3): 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siebenkäs A, Schumacher J, Roscher C. Phenotypic plasticity to light and nutrient availability alters functional trait ranking across eight perennial grassland species. AoB Plant. 2015; 7: plv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grassein F, Lavorel S, Till-Bottraud I. The importance of biotic interactions and local adaptation for plant response to environmental changes: field evidence along an elevational gradient. Global Change Biology. 2014; 20:1452–1460. doi: 10.1111/gcb.12445 [DOI] [PubMed] [Google Scholar]
- 11.Kawecki TJ, Ebert D. Conceptual issues in local adaptation. Ecology Letters. 7: 1225–1241. [Google Scholar]
- 12.Savolainen O, Pyhajarvi T, Knurr T. Gene flow and local adaptation in trees. Annual Review in Ecology and Evolutionary Systematics. 2007; 38: 595–619. [Google Scholar]
- 13.Godoy O, Saldaña A, Fuentes N, Valladares F, Gianoli E. Forests are not immune to plant invasions: phenotypic plasticity and local adaptation allow Prunella vulgaris to colonize a temperate evergreen rainforest. Biological Invasions. 2011; 13(7):1615–1625. [Google Scholar]
- 14.Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics. 1965; 13:115–155. [Google Scholar]
- 15.Sultan SE. Evolutionary implications of phenotypic plasticity in plants. Evolutionary Biology. 1987; 21:127–178. [Google Scholar]
- 16.Lortie CJ, Aarssen LW. The specialization hypothesis for phenotypic plasticity in plants. International Journal of Plant Sciences. 1996; 157(4): 484–487. [Google Scholar]
- 17.Cheplick GP. A modular approach to biomass allocation in an invasive annual (Microstegium vimineum; Poaceae). American Journal of Botany. 2006; 93: 539–545. doi: 10.3732/ajb.93.4.539 [DOI] [PubMed] [Google Scholar]
- 18.Pan XY, Geng YP, Zhang WJ, Li B, Chen JK. The influence of abiotic stress and phenotypic plasticity on the distribution of invasive Alternanthera philoxeroides along a riparian zone. Acta Oecologica. 2006; 30: 333–341. [Google Scholar]
- 19.Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M. Jack of all trades, master of some? On the role of phenotypic in plant invasions. Ecology Letters. 2006; 9: 981–993. doi: 10.1111/j.1461-0248.2006.00950.x [DOI] [PubMed] [Google Scholar]
- 20.Muth NZ, Pigliucci M. Implementation of a novel framework for assessing species plasticity in biological invasions: responses of centaurea and crepis to phosphorus and water availability. Journal of Ecology. 2007; 95: 1001–1013. [Google Scholar]
- 21.Du LS, Yang BF, Guan WB, Li JM. Phenotypic variation and water selection potential in the stem struture of invasive alligator weed. Acta Oecologica. 2016; 71: 22–30. [Google Scholar]
- 22.Li JM, Du LS, Guan WB, Yu FH, van Kleunen M. Latitudinal and longitudinal clines of phenotypic plasticity in the invasive herb Solidago canadensis in China. Oecologia. 2016; 182(3): 755–764. doi: 10.1007/s00442-016-3699-x [DOI] [PubMed] [Google Scholar]
- 23.Huang QQ, Pan XY, Fan ZW, Peng SL. Stress relief may promote the evolution of greater phenotypic plasticity in exotic invasive species: a hypothesis. Ecology & Evolution. 2015; 5(6):1169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riis T, Lambertini C, Olesen B, Clayton JS, Brix H, Sorrell BK. Invasion strategies in clonal aquatic plants: are phenotypic differences caused by phenotypic plasticity or local adaptation?. Annals of Botany. 2010; 106(5): 813–822. doi: 10.1093/aob/mcq176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Si C, Dai Z, Lin Y, Qi S, Huang P, Miao S, Du D. Local adaptation and phenotypic plasticity both occurred in Wedelia trilobata invasion across a tropical island. Biological Invasions. 2014; 16: 2323–2337. [Google Scholar]
- 26.Rouifed S, Byczek C, Laffray D, Piola F. Invasive knotweeds are highly tolerant to salt stress. Environmental Management. 2012; 50: 1027–1034. doi: 10.1007/s00267-012-9934-2 [DOI] [PubMed] [Google Scholar]
- 27.Parida AK, Das AB. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety. 2005; 60: 324–349. doi: 10.1016/j.ecoenv.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 28.Li JM, Liao JJ, Guan M, Wang EF, Zhang J. Salt tolerance of Hibiscus hamabo seedlings: a candidate halophyte for reclamation areas. Acta Physiologiae Plantarum. 2012; 34: 1747–1755. [Google Scholar]
- 29.Nasim M, Qureshi RH, Aziz T, Saqib M, Nawaz S, Akhtar J, et al. Different eucalyptus species show different mechanisms of tolerance to salinity and salinity × hypoxia. Journal of Plant Nutrition 2009; 32: 1427–1439. [Google Scholar]
- 30.Vasquez EA, Glenn EP, Guntenspergen GR, Brown JJ. Salt toterance and osmotic adjustment of Spartina alterniflora (Poaceae) and the invasive M haplotype of Phragmites australis (Poaceae) along a salinity gradient. American Journal of Botany. 2006; 93: 1784–1790. doi: 10.3732/ajb.93.12.1784 [DOI] [PubMed] [Google Scholar]
- 31.Kuhn NL, Zedler JB. Differential effects of salinity and soil saturation on native and exotic plants of a coastal salt marsh. Estuaries and Coasts. 1997; 20(2): 391–403. [Google Scholar]
- 32.Schittko C, Wurst S. Above- and belowground effects of plant-soil feedback from exotic Solidago canadensis on native Tanacetum vulgare. Biological Invasions. 2014; 16: 1465–1479. [Google Scholar]
- 33.Lu JZ, Weng ES, Wu XW, Weber E, Zhao B, Li B. Potential distribution of Solidago canadensis in China. Acta Phytotaxonomica Sinica. 2007; 45: 670–674. [Google Scholar]
- 34.Dong M, Lu JZ, Zhang WJ, Chen JK, Li B. Canada goldernrod (Solidago canadensis): an invasive alien weed rapidly spreading in China. Acta Phytotaxonomica Sinica. 2006; 44: 72–85. [Google Scholar]
- 35.Moran EV. Gene flow and local adaptation along elevation gradients in an invasive plant (Solidago canadensis). 99th ESA Annual Convention 2014.
- 36.Du LS, Liu HY, Yan M, Li JM, Li JS. Individual plasiticty of the shade response of the invasive Solidago canadensis in China. PLoS ONE. 2017; 12(1): e0170049 doi: 10.1371/journal.pone.0170049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu LL. Distribution and ecological adaptation characteristics of Solidago canadensis to Jiuduansha, Shanghai. Master degree thesis of Shanghai Normal University. 2012.
- 38.Xu XQ. Wang YY, Lu Q, Lin ZH, Chen HL. Effect of Solidago canadensis invasions on soil nematode communities in Hangzhou Bay. Biodiversity Science. 2011; 19: 519–527. [Google Scholar]
- 39.Mulligan GA, Findlay JN. Reproductive systems and colonization in Canadian weeds. Canadian Journal of Botany. 1970; 48: 859–860. [Google Scholar]
- 40.Fischer G, Nachtergaele F, Prieler S, van Velthuizen HT, Verelst L, Wiberg D. Global Agro-ecological Zones Assessment for Agriculture (GAEZ 2008). IIASA, Laxenburg, Austria and FAO, Rome, Italy; 2008.
- 41.Lindsay RWB, Hyland L, Cook FC, Hou S. Quantitative determination of total free-amino acid in Nervilia fordii (Hance) Schltr. by ninhydrin colorimetric method. Chinese Journal of Information on Traditional Chinese Medicine. 2010; 53: 1849–1859. [Google Scholar]
- 42.Sun WC, Liang YC, Yang YF. Influences of silicon and inoculation with Collectotrichum lagenarium on peroxidase activity in leaves of cucumber and their relation to resistance to anthracnose. Scientia Agricultura Sinica. 2002; 35: 1560–1564. [Google Scholar]
- 43.Ates E, Tekeli AS. Salinity tolerance of Persian clover (Trifolium resupinatum Var. Majus Boiss.) lines at germination and seedling stage. World Journal of Agricultural Sciences. 2007; 3: 71–81. [Google Scholar]
- 44.Valladares F, Sanchez-Gomez D, Zavala MA. Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. Journal of Ecology. 2006; 94:1103–1116. [Google Scholar]
- 45.Gondim EA, Miranda RS, Gomes-Filho E, Prisco JT. Enhanced salt tolerance in maize plants induced by H2O2 leaf spraying is associated with improved gas exchange rather than with non-enzymatic antioxidant system. Theoretical and Experimental Plant Physiology. 2013; 25: 251–260. [Google Scholar]
- 46.Wang Y, Yang L, Zheng Z, Grumet R, Loescher W, Zhu JK, Yang P, Hu Y, Chan Z. Transcriptomic and physiological variation of three Arabidopsis ecotypes in response to salt stress. PLoS ONE. 2013; 8(7): e6903647. doi: 10.1371/journal.pone.0069036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu G, Gao Y, Huang FF, Yuan MY, Peng SL. The invasion of coastal areas in south China by Ipomoea cairica may be accelerated by the ecotype being more locally adapted to salt stress. PloS ONE. 2016; 11(2): e0149262 doi: 10.1371/journal.pone.0149262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leimu R, Fischer M. A meta-analysis of local adaptation in plants. PLoS ONE. 2008; 3(12): e4010 doi: 10.1371/journal.pone.0004010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Droste T, Flory S L, Clay K. Variation for phenotypic plasticity among populations of an invasive exotic grass. Plant Ecology. 2010; 207(2): 297–306. [Google Scholar]
- 50.Lavergne S, Molofsky J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proceedings of the National Academy Sciences of the United States of America. 2007; 104: 3883–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.