Abstract
Background
Easily identifiable risk factors including: obesity and ethnicity at high risk of diabetes are commonly used to indicate which women should be offered the oral glucose tolerance test (OGTT) to diagnose gestational diabetes (GDM). Evidence regarding these risk factors is limited however. We conducted a systematic review (SR) and meta-analysis and individual participant data (IPD) analysis to evaluate the performance of risk factors in identifying women with GDM.
Methods
We searched MEDLINE, Medline in Process, Embase, Maternity and Infant Care and the Cochrane Central Register of Controlled Trials (CENTRAL) up to August 2016 and conducted additional reference checking. We included observational, cohort, case-control and cross-sectional studies reporting the performance characteristics of risk factors used to identify women at high risk of GDM. We had access to IPD from the Born in Bradford and Atlantic Diabetes in Pregnancy cohorts, all pregnant women in the two cohorts with data on risk factors and OGTT results were included.
Results
Twenty nine published studies with 211,698 women for the SR and a further 14,103 women from two birth cohorts (Born in Bradford and the Atlantic Diabetes in Pregnancy study) for the IPD analysis were included. Six studies assessed the screening performance of guidelines; six examined combinations of risk factors; eight evaluated the number of risk factors and nine examined prediction models or scores. Meta-analysis using data from published studies suggests that irrespective of the method used, risk factors do not identify women with GDM well.
Using IPD and combining risk factors to produce the highest sensitivities, results in low specificities (and so higher false positives). Strategies that use the risk factors of age (>25 or >30) and BMI (>25 or 30) perform as well as other strategies with additional risk factors included.
Conclusions
Risk factor screening methods are poor predictors of which pregnant women will be diagnosed with GDM. A simple approach of offering an OGTT to women 25 years or older and/or with a BMI of 25kg/m2 or more is as good as more complex risk prediction models. Research to identify more accurate (bio)markers is needed.
Systematic Review Registration: PROSPERO CRD42013004608
Introduction
Gestational diabetes mellitus (GDM) is hyperglycaemia of variable severity first identified in pregnancy. GDM is associated with an increased risk of a range of adverse perinatal outcomes,[1,2] including being born large (macrosomia) and there is growing evidence that the longer-term health of the mother and infant may be adversely affected.[3–5]
Treatment of GDM improves perinatal outcomes,[6–8] suggesting a role for identifying women with GDM. There is uncertainty about the effectiveness of different strategies for identifying these women, largely because of the lack of good quality evidence.[8,9] This has led to variation in clinical guidelines and practice for identifying GDM, both between and within countries. Strategies include selectively offering a 75g or 100g oral glucose tolerance test (OGTT) to high risk women only, identified using specific risk factors (usually easily identifiable maternal characteristics) or the administration of a 50g glucose challenge test. Alternatively all women can be offered an OGTT (universal offer of OGTT).[10,11] Restricting diagnostic testing to high risk women may be less costly than offering testing to all; the OGTT is relatively expensive and requires pregnant women to fast overnight and attend clinic for at least two hours. However, offering all women an OGTT may result in more women with GDM being identified and a reduction in adverse outcomes, as more affected women will receive treatment to reduce hyperglycaemia. GDM is also a risk factor for later development of type 2 diabetes,[3] if more women with GDM are identified, more could receive interventions aimed at reducing these risks and alongside lifelong screening to identify type 2 diabetes earlier, associated morbidities and costs may be reduced, however there is no robust evidence of longer-term benefit from the identification of GDM using a universal testing strategy.[8]
Risk factor screening involves the assessment of maternal characteristics, such as family history of diabetes; being of an ethnicity with a high prevalence of diabetes (i.e. non-white ethnicity: including Asian, black Caribbean or Middle Eastern); history of having GDM or a macrosomic infant; maternal obesity[12] and occasionally biochemical markers.[13,14]
Several healthcare agencies including the UK National Institute for Health and Care Excellence (NICE)[12], the American diabetes Association[15] and the Australasian Diabetes in Pregnancy Society (ADIPS)[16] recommend offering an OGTT to women with one or more risk factors (Table 1) in early pregnancy, some agencies then recommend repeat testing in this high risk group of women (those with risk factors), if GDM has not already been identified,[12] whilst others recommend all women (not previously identified as having GDM in early pregnancy) are offered an OGTT irrespective of risk factors.[16,17] For the majority of women the OGTT is conducted in mid-pregnancy (usually at 24–28 weeks gestation) so that the maximum number of women destined to develop hyperglycaemia will have a chance to be detected, while allowing enough time to provide treatment.
Table 1. Summary of selected screening strategies recommending the use of risk factors for the identification of gestational diabetes.
| Agency | Nature of screening strategy |
|---|---|
| National Institute for Health and Care Excellence (UK NICE)[12] 2015 | Offer women who have had GDM previously self-monitoring, blood glucose estimation or OGTT in early pregnancy.Offer OGTT at 24–28 weeks gestation only to women with at least one of:BMI >30kg/m2 |
| • Previous macrosomic baby (above 4.5kg) | |
| • Previous GDM | |
| • Family history of diabetes | |
| • Ethnic origin with a high prevalence of diabetes | |
| American Diabetes Association(ADA)[17] 2017 | Offer OGTT at first pregnancy visit to women who are overweight/obese (BMI≥25 kg/m2) or are Asian American and have at least one additional risk factor: |
| • A1C ≥5.7% (39 mmol/mol), IGT, or IFG on previous testing | |
| • first-degree relative with diabetes | |
| • High-risk race/ethnicity (e.g., African American, Latino, Native American, Asian American, Pacific Islander) | |
| • Women who were diagnosed with GDM | |
| • History of CVD | |
| • Hypertension (≥140/90 mmHg or on therapy for hypertension) | |
| • HDL cholesterol level, 35 mg/dL (0.90 mmol/L) and/or a triglyceride level .250 mg/dL(2.82 mmol/L) | |
| • Women with polycystic ovary syndrome | |
| • Physical inactivity | |
| • Other clinical conditions associated with insulin resistance (e.g., severe obesity, acanthosis nigricans | |
| Test all women at 24 to 28 weeks gestation not previously known to have diabetes | |
| Australasian Diabetes in Pregnancy Society (ADIPS)[16] 2014 | Offer OGTT early in pregnancy to women who have a BMI ≥25kg/m2 or are from an ethnicity at high risk of diabetes (e.g. Asian, Aboriginal, Pacific Islander) and who have an abnormal fasting or random blood sugar Offer OGTT early in pregnancy to women with one of the risk factors below or who have both a BMI ≥25kg/m2 and are from an ethnicity at high risk of diabetes (e.g. Asian, Aboriginal, Pacific Islander) |
| • Previous GDM | |
| • Previously elevated blood glucose level | |
| • Age ≥40 years | |
| • High-risk race/ethnicity | |
| • Family history of diabetes | |
| • Pre-pregnancy BMI > 35 kg/m2 | |
| • Previous macrosomia | |
| • Polycystic ovarian syndrome | |
| • Medications: corticosteroids, antipsychotics | |
| Offer OGTT to all women at 24 to 28 weeks gestation not already identified as having GDM |
IGT = impaired glucose tolerance test; IFG = impaired fasting glucose; BMI = body mass index; A1C = glycated haemoglobin; CVD = cardiovascular disease
Risk factor assessment is recommended in many populations in early pregnancy. The presence of a risk factor therefore influences early assessment of hyperglycaemia and whether mid-trimester testing in selectively tested populations is conducted. The aim of this study was to evaluate the performance of risk factors in identifying women requiring diagnostic testing for GDM, utilising published studies and available individual participant data.
Methods
We conducted a systematic review and meta-analyses of published studies evaluating risk factors for the identification of women at high risk of GDM. The review was conducted in accordance with the Centre for Reviews and Dissemination’s guidance[18]. We also analysed individual participant data (IPD) from two large birth cohorts: Born in Bradford (BiB)[19] and Atlantic Diabetes in Pregnancy (Atlantic DIP)[20]. The methods and results are reported following the PRISMA guidelines (S1 File).[21]
Search strategy
Title, abstract screening and then full text screening was performed in duplicate by two reviewers (DF, MS, SG or MB) with disagreements resolved by consensus or by a third reviewer.
Search: identification of studies from the systematic review
Searches were undertaken up to August 2016 in MEDLINE, MEDLINE in-process, Embase, Maternity and Infant Care and CENTRAL with no date or country restrictions (S2 File). In addition to database searches, citation checking of included publications was undertaken.
Study selection: Inclusion and exclusion criteria
All eligible published and on-going observational, cohort, case-control or cross-sectional studies were included. Due to time and cost constraints only studies published in English were included. Studies had to report data from women in whom risk factors for GDM were recorded and who were tested for GDM using an OGTT. We included studies that evaluated readily available/routinely collected maternal characteristics: age, ethnicity, parity, previous GDM, macrosomia, family history of diabetes, BMI and blood pressure. We did not include studies that focused solely on biochemical tests such as the 50g oral glucose challenge test, as these tests are less commonly used in universal pre-diagnostic test screening programmes and are more costly than risk factor screening.[12] We examined the value of using combinations of risk factors for selecting pregnant women for OGTT. Studies had to report the accuracy of combinations of risk factors; such as, numbers of risk factors present, risk models or scores based or measuring multiple risk factors, or the use of guideline recommendations. Studies reporting the screening accuracy of a single risk factor, without examining combinations of risk factors, were excluded.
No formal quality assessment process was undertaken because of the lack of any validated quality assessment tool for studies evaluating the performance of risk factors as a screening test; however studies had to report adequate information and that information had to be in a format that allowed comparison with others (described below in statistical analysis).
Data extraction
Data were extracted by three reviewers (MS, SG, DF) and any disagreements resolved through discussion. Publication year, location, GDM diagnostic criteria, risk factors, cut-off levels of risk factors if appropriate and number of women included with risk factor combinations were recorded. The total number of women with and without GDM according to diagnostic test results and assessment of risk factor performance (sensitivity and specificity and positive predictive value, if reported) were recorded.
Statistical analysis
For each group of risk factor combinations, sensitivity (proportion of GDM cases correctly identified by the risk factor); specificity (proportion of women without GDM correctly identified) and positive rate (proportion of women who would be offered an OGTT if the risk factor combinations were present) were calculated. Statistics were plotted for each study in Receiver Operating Characteristic (ROC) space, by plotting screening performance—sensitivity against positive rate.[22] A ‘good’ test will have high sensitivity with small numbers needing to be tested (with results near the top left of the space). Meta-analysis methods for pooling of screening studies, such as the Hierarchical summary receiver-operator curves (HSROC) model [23] were considered, but not performed because of the different screening approaches and included risk factors used by studies.
Individual participant data (IPD) cohort analysis
Data from two birth cohorts were eligible and available. Born in Bradford (BiB) [19] is a prospective birth cohort (research ethics committee approval reference 07/H1302/112); the methods have been previously described.[19] The Atlantic Diabetes in Pregnancy study (Atlantic DIP) is a multi-centre cohort study comprising of a partnership of five hospitals at the Irish Atlantic seaboard (research ethics committee approval was obtained from participating centres); study methods have been previously described.[20] Both cohorts offered all women a 75g OGTT irrespective of the presence of risk factors. The World Health Organization (WHO) 1999 (modified) criteria were used to diagnose GDM (fasting glucose ≥6.1mmol/l, two-hour post-load glucose ≥7.8mmol/l) in both cohorts.[24,25]
Statistical analysis
Risk factors recorded by the IPD cohorts were similar to those recorded by published studies included in the systematic review. We considered seven commonly used risk factors: age; BMI; parity (multiparous, primiparous); ethnicity (White, South Asian or Other), family history of diabetes; previous GDM or having had a previous macrosomic infant. We grouped women into white (British/Irish), south Asian or other, as these groupings most appropriately represent the ethnicities of the women in the included cohorts, it should be noted that these groupings may not be appropriate for other populations. Data on previous GDM or having had a previous macrosomic infant were not available in the Atlantic DIP cohort.
We classified BMI using the thresholds of 25kg/m2 (kilogramme/meter2) or over, or 30kg/m2 or over; because these are the recommended thresholds for overweight and obesity.[26,27] We used the age categories of 25 years or older, or 30 years or older, because they have been used previously [28–31] and are clinically relevant. This generated 287 combinations of risk factors. The sensitivity, specificity and positive rate were calculated for each combination of risk factors and those that were “dominated” by another in that class (i.e. a combination is dominated if there is one other related ‘test’ with both higher sensitivity and specificity which would be a better predictor) were removed. Sensitivity and positive rates for the remaining non-dominated tests were plotted in ROC space.
In addition we also examined screening performance based on a predicted risk of GDM, similar to screening strategies used to identify those at risk of cardiovascular disease.[32] A logistic regression model was fitted to data from each of the cohorts and to a pooled cohort dataset for comparison, regressing GDM incidence against the seven included risk factors. The resulting log odds ratios were used to calculate a predicted risk of GDM for each woman in the dataset. The sensitivity and positive rate for predicting GDM at each percentage point of risk from 1% to 80% was calculated and plotted in ROC space.
Results
Systematic review and meta-analysis
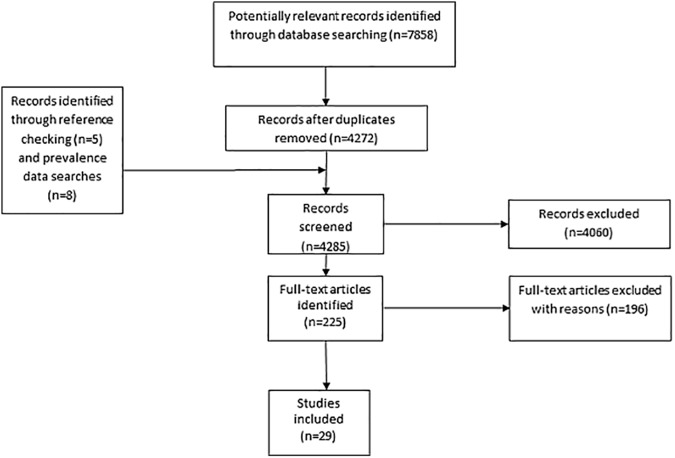
Searches identified 4272 unique citations (7858 before de-duplication). Thirteen additional publications were identified through reference checking. After title and abstract screening, 225 publications were retrieved for full-text screening. One hundred and ninety six full text papers were excluded because they did not meet eligibility criteria, leaving 29 studies (Fig 1), with 211,698 women. Six studies [33–38] assessed the screening performance of guideline recommendations (UK National Institute for Health and Care Excellence (NICE),[37] American Diabetes Association (ADA),[35–38] American College of Obstetricians and Gynecologists (ACOG),[36] Australasian Diabetes In Pregnancy Society (ADIPS),[37] Irish,[33] French[34]). Eight studies evaluated the screening performance of the number of risk factors (for example if two, three or four etc. risk factors were present),[39–46] six examined combinations of risk factors[28,47–51] and nine studies examined the ability of a risk prediction model or a risk score to predict GDM. [52–60]
Fig 1. Flow chart of the systematic review search process.
All studies were observational, consisting of a mix of prospective and retrospective cohort studies, with GDM diagnosed using an OGTT, using specified diagnostic criteria. Diagnostic criteria and glucose thresholds varied between studies, which influenced estimates of GDM prevalence. Studies were diverse in their included populations (Table 2).
Table 2. Characteristics of studies included in the systematic review.
| First author | Year | Country | GDM diagnosis criterion | Total women | No. with GDM | % with GDM | Risk factor screening strategy |
|---|---|---|---|---|---|---|---|
| Avalos[33] | 2013 | Ireland | IADPSG | 5500 | 521a |
9 |
Irish, NICE, ADA guideline recommendations |
| 491b | 9 | ||||||
| 585c | 11 | ||||||
| Caliskan[39] | 2004 | Turkey | NDDG | 422 | 14 | 3 | Number of risk factors |
| Cosson[34] | 2013 | France | WHO | 18755 | 2710 | 14 | French guideline recommendations |
| Cypryk[40] | 2008 | Poland | WHO | 2180 | 510 | 23 | Number of risk factors |
| Danilenko-Dixon[35] | 1999 | USA | NDDG | 18504 | 564 | 3 | ADA guideline recommendations |
| Erum[51] | 2015 | Turkey | ADA | 815 | 39 | 5 | ‘At least one risk factor’ |
| Gabbay-Benviz[58] | 2015 | USA | C&C | 924 | 63 | 7 | Risk score |
| Jensen[61] | 2003 | Denmark | DPSG | 2992d | 83 | 3 | Number of risk factors |
| Jiminez-moleon[36] | 2002 | Spain | NDDG | 1436 |
58c |
4 |
ADA and ACOG guideline recommendations |
| 2174 | 63e | 3 | |||||
| Kirke[59] | 2014 | Australia | WHO | 1636 | 73 | 4 | Risk score |
| Marquette[42] | 1985 | USA | C&C | 434 | 12 | 3 | Number of risk factors |
| Moses[47] | 1998 | Australia | ADIPS | 2907 | 183 | 6 | Age, BMI ethnicity |
| Nanda[52] | 2011 | UK | WHO | 11464 | 297 | 3 | Risk model |
| Naylor[53] | 1997 | US | NDDG or C&C | 1571 | 69 | 4 | Risk score |
| Nielsen[43] | 2016 | India | WHO | 3946 | 659 | 17 | Number of risk factors (1, 2 or 3) |
| Ostlund[28] | 2003 | Sweden | WHO | 3616 | 61 | 5 | "Traditional risk factors" |
| Phaloprakam[54] | 2009 | Thailand | C&C | 469 | 127 | 27 | Risk score |
| Pintaudi[48] | 2014 | Italy | IADPSG | 1015 | 113 | 11 | "Standard risk factors" |
| Sacks[44] | 1987 | USA | ADA | 4116 | 138 | 3 | Number of risk factors |
| Savona-Ventura[49] | 2013 | Mediterranean | ADA | 1368 | 119 | 9 | Based on age, obesity or diastolic BP |
| Shamsuddin[45] | 2001 | Malaysia | OGTT levels reported | 768 | 191 | 25 | Number of risk factors |
| Shirazian[55] | 2009 | Iran | ADA | 924 | 68 | 7 | Risk score |
| Sunsaneevithayakul[46] | 2003 | Thailand | Not reported | 9325 | 235 | 2 | Number of risk factors |
| Syngelaki[60] | 2015 | UK | WHO | 75161 | 1827 | 20 | Risk model |
| Teh[37] | 2011 | Australia | ADIPS | 2426 | 250 | 10 | NICE, ADA and ADIPS guideline recommendations |
| van Leeuwen[57] (A) | 2010 | Netherlands | OGTT/GCT levels reported | 995 | 24 | 2 | Risk model |
| van Leeuwen[56] (B) | 2009 | Netherlands | WHO | 1266 | 47 | 4 | Risk score |
| Williams[50] | 1999 | USA | NDDG | 25118 | 210f | 1 | Based on age, BMI ethnicity, family history |
| Yang[38] | 2002 | China | WHO | 9471 | 171 | 2 | ADA guideline |
aIrish guideline
bNICE guideline
cADA recommendations
dJensen (2003), 5235 women were included in the study, 2992 had an OGTT performed
eACOG recommendations
fWilliams (1999), number of women with GDM varied by the recorded risk factor (i.e. not all women had all risk factors recorded)
ACOG = American College of Obstetricians and Gynecologists
ADA = American Diabetes Association
ADIPS = Australasian Diabetes In Pregnancy Society
C&C = Carpenter and Coustan
NDDA = National Diabetes Data Group
NICE = National Institute for Health and Care Excellence
IADPSG = International Association of Diabetes in Pregnancy Study Groups
WHO = World Health Organization
Performance of risk factors in predicting GDM
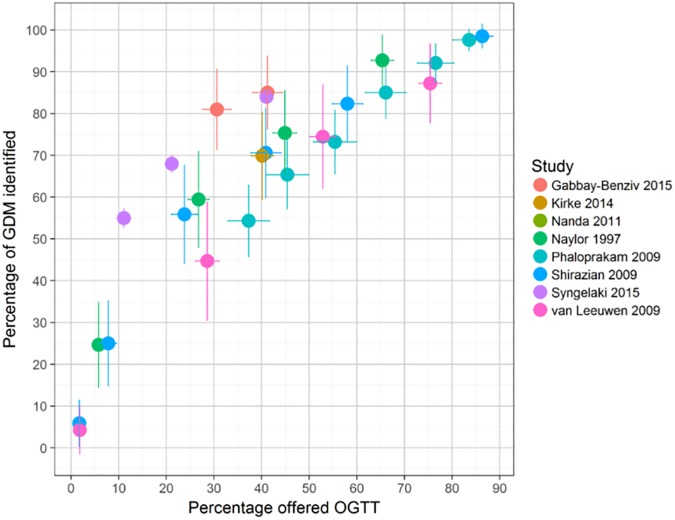
Figs 2 to 4 show estimates of sensitivity and proportion of women that would be offered an OGTT for each of the included studies, plotted in the ROC space. Fig 2 includes data from all 29 studies and shows, as one would expect, that the proportion of correctly identified GDM cases (sensitivity) increases with the number of women offered an OGTT, irrespective of the risk factor strategy used, there seems to be no obvious ‘best’ approach.
Fig 2. Screening performance (sensitivity and percentage offered an oral glucose tolerance test (OGTT)) by study and by risk factor method (guideline recommendations, number (No) of risk factors, ‘other method and risk model/score).
The colour of the points indicates the study. The shape of the points (circles, triangle, square, cross) indicates method used No. RF = number of risk factors (i.e. presence of one risk factor, two risk factors and so on). Studies may report more than one performance estimate, this is reflected in the number of coloured shapes for each study.
Fig 4. Screening performance of risk prediction or scoring models.
The colour of the points indicates the study. Vertical and horizontal lines show the 95% confidence intervals for sensitivity and positive rate respectively. Studies may report more than one performance estimate, this is reflected in the number of coloured shapes for each study
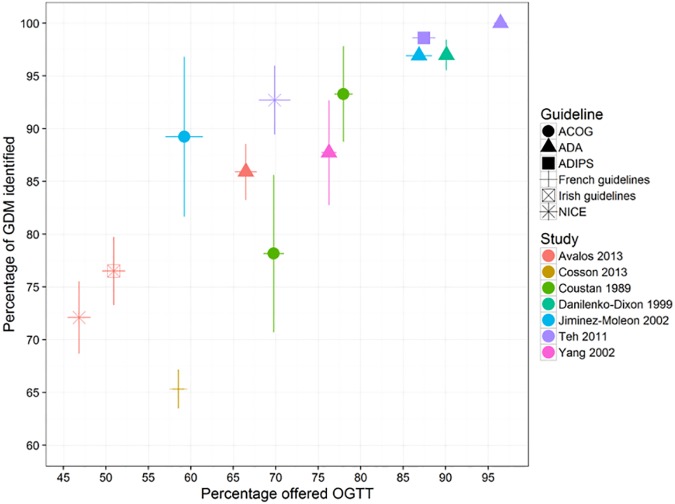
Fig 3 shows the proportion of correctly identified GDM cases and proportion offered an OGTT for different screening recommendations (American College of Obstetricians and Gynecologists (ACOG), American Diabetes Association (ADA), Australasian Diabetes in Pregnancy Society (ADIPS) and the UK National Institute for Health and Care Excellence (NICE)). There is considerable variation in both sensitivity and number of women offered an OGTT. The screening performance of guideline recommendations appears moderate at best, because generally at least 70% of women would need to be offered an OGTT to identify 80% of all women with GDM, with the exception of the ACOG guideline when applied to an Irish[33] or Spanish[36] population and the ADA guideline when applied to an Irish population.[33]
Fig 3. Screening performance of guidelines using a risk factor screening strategy.
Vertical and horizontal lines show the 95% confidence intervals for sensitivity and positive rate respectively. The colour of the points indicates the study. The shape of the points (circles, triangle, square, cross) indicates method used. RF = Risk factor, No = number. ACOG = American College of Obstetricians and Gynecologists. ADA = American Diabetes Association. ADIPS = Australasian Diabetes In Pregnancy Society. NICE = National Institute for Health and Care Excellence. Studies may report more than one performance estimate, this is reflected in the number of coloured shapes for each study.
Fig 4 shows the results from eight studies that examined the sensitivity and number of women offered an OGTT after the application of a risk prediction model or risk score. [52–57] Each study has several points on the ROC curve because results are reported for various levels of risk. Results are reasonably consistent across studies with all points generally lying on a similar ROC curve.
Figs 2 to 4 clearly show a trade-off; as sensitivity increases (and more women are identified), the number needed to receive a diagnostic test also increases. For example Fig 4 shows that to identify 80% of women with GDM (sensitivity of 80%) using a risk prediction model or risk score, between 30% and 58% of women would need to undergo an OGTT (depending which risk model is used); to achieve a sensitivity of over 90%, nearly all women would need to undergo an OGTT.
Individual participant data analysis
Screening based on combinations of risk factors
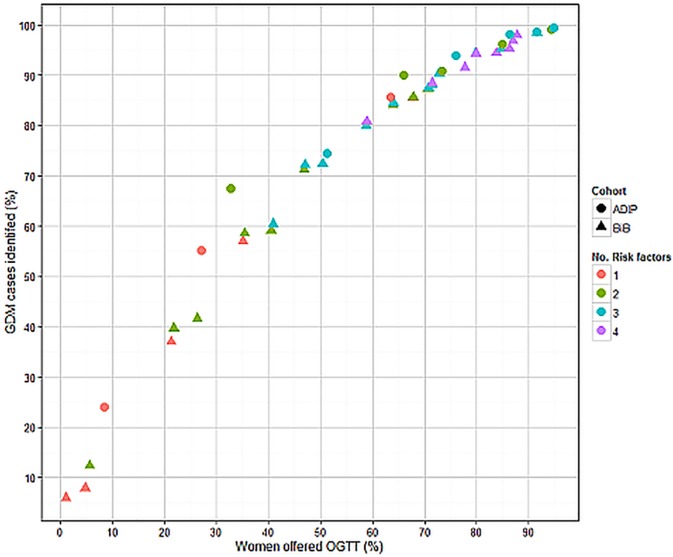
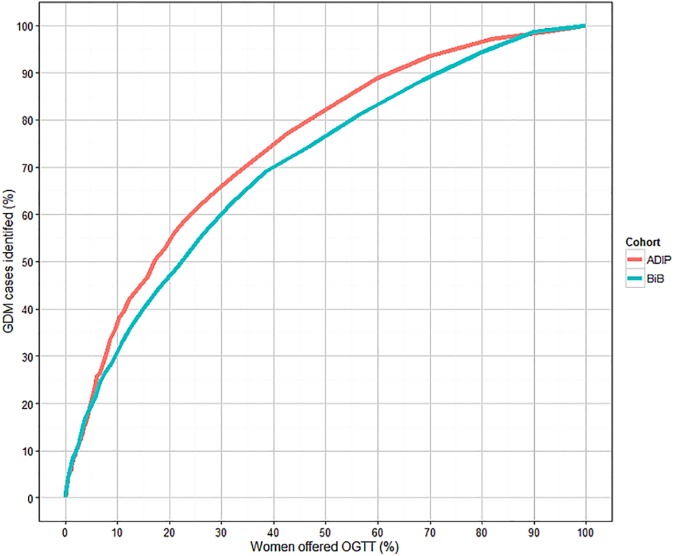
Fig 5 shows the percentage of GDM cases identified (sensitivity) against percentage of women offered an OGTT (positive rate) for each group of risk factors not ‘dominated’ by others. Irrespective of the number of risk factors included (one risk factor through to the use of four); all groups generally lay on the same ROC curve.
Fig 5. Screening performance of risk factor combinations for identifying GDM using IPD.
The colour of the points indicates the number (No) of risk factors included. Circles indicate results for Atlantic DIP and triangles represent results for BiB.
Fig 5 shows that using multiple risk factors is not superior to using just one or two, because the increase in sensitivity is only achieved by increasing the number of women offered an OGTT. Both cohorts demonstrate generally similar estimates of sensitivity and positive rate for each number of risk factors.
Table 3 shows examples of the performance of combinations of risk factors (two, through to four, not dominated) with sensitivity between 90% and 95% (detecting almost all cases of GDM) and for the UK NICE guideline recommended group of risk factors.[12] A woman is test positive (and therefore would be offered an OGTT) if she has one or more of the named risk factors in each group. Combining risk factors to produce the highest sensitivities, results in low specificities (and so higher false positives). Strategies that use only age and BMI categories however, perform similarly to others with additional risk factors. In our analyses, the NICE guideline recommended risk factor strategy was dominated by other strategies (other strategies had superior performance). For example, using combined cohort data, screening based on being either 25 years or older or having a BMI of 30 or over, achieved a higher sensitivity than using the combined NICE guideline recommended risk factors (Table 1) (93.2% and 78.2% respectively), but with a correspondingly higher positive rate (78.0% and 67.2% respectively) and lower specificity (23.3% and 31.7% respectively).
Table 3. Performance of risk factors, grouped by age, BMI and UK NICE categories for the identification of GDM using IPD.
| Risk factors | Sensitivity | Specificity | Positive rate |
|---|---|---|---|
| BiB cohort | |||
| Age≥25 BMI≥30 | 90.4 | 28.7 | 72.7 |
| Age≥25 BMI≥30, prior GDM | 90.4 | 28.6 | 72.8 |
| Age≥25 BMI≥30, FH of diabetes | 91.6 | 23.2 | 77.7 |
| Age≥25 BMI≥30, FH of diabetes, prior GDM | 91.6 | 23.1 | 77.7 |
| Age≥30, BMI≥30, non-white ethnicity | 94.3 | 21.3 | 79.8 |
| Age≥30, BMI≥30, non-white ethnicity, prior GDM | 94.3 | 21.3 | 79.9 |
| Age≥25, BMI≥25, FH of diabetes | 94.4 | 16.9 | 83.8 |
| Age≥25, BMI≥25, FH of diabetes, prior GDM | 90.4 | 28.7 | 72.7 |
| Atlantic DIP cohorta | |||
| BMI≥25, non-white ethnicity | 90.1 | 36.8 | 66.0 |
| Age≥30, BMI≥30 | 90.8 | 28.6 | 73.4 |
| Age≥30, BMI≥30, non-white ethnicity | 93.9 | 26.0 | 76.0 |
| Cohorts combined | |||
| Age≥30, BMI≥30, FH of diabetes | 90.0 | 24.6 | 76.4 |
| Age≥30, BMI≥25, FH of diabetes, prior GDM | 90.3 | 24.6 | 76.5 |
| BMI≥25, non-white ethnicity | 92.0 | 24.0 | 77.3 |
| BMI≥25, non-white ethnicity, prior GDM | 92.1 | 24.0 | 77.3 |
| Age≥25, BMI≥30 | 93.2 | 23.3 | 78.0 |
| Age≥25, BMI≥30, prior GDM | 93.2 | 23.3 | 78.1 |
| Age≥30, BMI≥30, non-white ethnicity | 94.1 | 22.7 | 78.7 |
| Age≥30, BMI≥30, non-white ethnicity, prior GDM | 94.1 | 22.7 | 78.7 |
| Age≥25, BMI≥25 | 95.9 | 16.5 | 84.5 |
| Age≥25, BMI≥25, prior GDM | 95.9 | 16.5 | 84.5 |
| NICE guideline recommended risk factors[12] | 78.2 | 31.7 | 67.2 |
BMI = body mass index (kg/m2)
FH = family history
NICE = National Institute for Health and Care Excellence
aPrevious macrosomia and GDM not available in Atlantic DIP
Screening using risk prediction models
The odds ratios for the association between each risk factor and GDM for each cohort are shown in Table 4. All risk factors examined, apart from multiparity, were positively associated with GDM.
Table 4. The associations between risk factors and GDM using IPD.
| BiB | Atlantic DIP | |||
|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | Odds ratio | 95% Confidence interval | |
|
Risk factor Age (per year) |
1.09 | 1.08 − 1.1 | 1.10 | 1.07 − 1.12 |
| BMI (per kg/m2) | 1.06 | 1.05 − 1.08 | 1.13 | 1.11 − 1.15 |
| Ethnicity (non-white) | 2.32 | 1.90 − 2.83 | 5.16 | 3.85 − 6.91 |
| Multiparity | 0.89 | 0.73 − 1.08 | 0.74 | 0.58 − 0.96 |
| Family history of diabetes | 1.36 | 1.14 − 1.63 | 1.42 | 1.17 − 1.80 |
| Previous macrosomiaa | 1.54 | 1.12–2.13 | - | - |
| Previous GDMa | 5.90 | 3.78–9.22 | - | - |
anot available in Atlantic DIP
When considering risk factors available in both cohorts, the odds ratios were generally consistent, with the exception of non-white/Irish ethnicity, the strength of the association being more than twice that in Atlantic DIP than BiB (half the participants in BiB are of south Asian (non-white) origin, half are white British, whereas few women in Atlantic DIP are non-white). ‘Having had GDM in a previous pregnancy’ was most strongly associated with GDM in BiB (this risk factor was not available in Atlantic DIP).
The odds ratios shown in Table 4 were used to construct a predicted risk of GDM for each woman in each cohort. The ROC curves of sensitivity against positive rate are shown in Fig 6 and are similar for the two cohorts, though the performance seems marginally better for Atlantic DIP compared to BiB. The areas under the curves (AUCs) being 0.77 for Atlantic DIP and 0.72 for BiB, suggesting modest screening performance. Performance using a predictive risk model (Fig 6) seems similar to using a combination of several risk factors.
Fig 6. Sensitivity and positive rate when using a risk prediction model to predict GDM using IPD.
Discussion
To our knowledge, this is the first systematic review and meta-analysis to assess the predictive accuracy of different combinations of risk factors to identify women at high risk of GDM. We found that universal risk factor pre-diagnostic test screening can take a variety of forms, but whatever the form, this strategy did not appear effective for accurately identifying women with GDM. Furthermore we found no evidence that complex risk screening strategies using several risk factors or risk prediction models offered significant benefit over the simpler strategy of identifying one or two risk factors. Regardless of the methods used, correctly identifying most women with GDM, requires offering an OGTT to the majority of women and therefore does not vary considerably from offering all women an OGTT. For some populations however, limiting the offer of an OGTT to high risk women may result in important cost savings.
Our IPD analyses suggest that the risk factor combination of maternal age and BMI (25 years or older and BMI ≥25 kg/m2) would identify the majority of women with GDM, but consistent with our systematic review findings, would mean inviting most women for an OGTT. Although this is as effective as more complex strategies (risk prediction models for example) it may not vary greatly from offering all women an OGTT.
Strengths and limitations
This study examined published data identified by a systematic search, comprising 29 studies and including 211,689 women. We also conducted analyses using IPD from two large contemporary birth cohorts including 14,103 women. The findings from the published studies and IPD cohorts were consistent with each other. As well as triangulating findings from these two different designs we also compared findings from two different analytical approaches and also found consistency there, suggesting that our results are robust. Different populations based on geography and age were included suggesting that our results might be broadly generalisable to different antenatal populations in high income countries. Very few studies were from low income countries and it is therefore important to note that our findings may not generalise to those countries. Given the increase in non-communicable diseases in low and middle income countries and the scarcity of resources to be able to adequately deal with them, there is clearly a need to gain better understanding about how to screen for, diagnose and treat GDM in those countries.
Recommendations regarding the identification of GDM vary and some institutions that previously recommended risk factor assessment now recommend offering all women an OGTT, however there is a lack of supporting evidence that this strategy improves maternal and offspring health compared to selective testing high risk women [8] and given the likely increase in associated costs, clinicians and commissioners may not be willing or able to accept universal testing for GDM. The risk factors that we were able to assess in published studies were limited by what was available, but they included a range of the commonly used risk factors for GDM. Studies used varying threshold criteria and this influences the numbers of women identified by risk factors and makes comparison complex. Applying the same criteria in dissimilar populations however will also produce varying results (see the NICE guideline results in Fig 3 and Table 3). A more consistent global approach to identifying women with GDM would reduce variation in practise and would likely improve care. Although our search did not identify any; it is possible that there may be eligible studies published in languages other than English.
Conclusions and implications for practice
Our results suggest that pre-diagnostic risk factor screening is a poor method for identifying women with GDM. Using this strategy will reduce the likely impact of antenatal GDM screening, testing and management programmes. Given these findings, there is an need for research to develop and evaluate (bio)markers that might more accurately identify women at high and low risk of GDM. Until then and if universal offer of an OGTT is not adopted, our results suggest that using age with a cut-off of 25 years (i.e. referring women at or older than 25 years for an OGTT) or who have a BMI of ≥25 or ≥30 kg/m2 would be currently the simplest and most accurate risk factor screening method. Ultimately though, the choice of whether and how to identify GDM should be informed by rigorous cost-effectiveness analysis.
Supporting information
(PDF)
(PDF)
Acknowledgments
Thank you to Julie Glanville and Mick Arber of the York Health Economics Consortium, University of York, and Judy Wright and Rocio Rodriguez of the Institute of Health Sciences, University of Leeds, who carried out the searches.
Data Availability
All relevant data related to published studies are within the paper and its supporting information. Born in Bradford cohort data are available from the BiB executive committee via the BiB project website www.borninbradford.nhs.uk/contact-us/ and Atlantic-Dip data from Fidelma Dunne (fidelma.dunne@nuigalway.ie), representing Institutional Data Access for researchers who meet the criteria for access to confidential data.
Funding Statement
This work was supported by the National Institute for Health Research (NIHR), Health Technology Assessment (HTA) programme, project number 11/99/02. DF holds a NIHR Post-doctoral Research Fellowship award (PD-2014-07-019). DAL works in a Unit that is supported by the University of Bristol and UK Medical Research Council (MC_UU_12013/5) and she holds a NIHR Senior Investigator award (NF-SI-0611-10196). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA, NIHR, MRC, United Kingdom National Health Service (NHS) or the Department of Health.
References
- 1.Casey BM, Lucas MJ, McIntire DD, Leveno KJ (1997) Pregnancy Outcomes in Women With Gestational Diabetes Compared With the General Obstetric Population. Obstet Gynecol 90: 869–873. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan JB, Mahan CM (1980) Insulin treatment and high risk groups. Diabetes Care 3: 482–485. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy L, Casas J-P, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373: 1773–1779. doi: 10.1016/S0140-6736(09)60731-5 [DOI] [PubMed] [Google Scholar]
- 4.Lawlor DA, Fraser A, Lindsay RS, Ness A, Dabelea D, Cantalano P, et al. (2010) Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 53: 89–97. doi: 10.1007/s00125-009-1560-z [DOI] [PubMed] [Google Scholar]
- 5.Shah BR, Retnakaran R, Booth GL (2008) Increased Risk of Cardiovascular Disease in Young Women Following Gestational Diabetes Mellitus. Diabetes Care 31: 1668–1669. doi: 10.2337/dc08-0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS (2005) Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 352: 2477–2486. doi: 10.1056/NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- 7.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. (2009) A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 361: 1339–1348. doi: 10.1056/NEJMoa0902430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrar D, Simmonds M, Griffin S, Duarte A, Lawlor DA, Sculpher M, et al. (2016) The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta-analyses and an economic evaluation. Health Technol Assess, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institutes of Health (2013) National Institutes of Health Consensus development conference statement. Obstet Gynecol 122: 358–369. doi: 10.1097/AOG.0b013e31829c3e64 23969806 [Google Scholar]
- 10.International Association of Diabetes and Pregnancy Study Groups Consensus panel (2010) International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 33: 676–682. doi: 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization; (2013) Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. World Health Organisation; http://apps.who.int/iris/bitstream/10665/85975/1/WHO_NMH_MND_13.2_eng.pdf?ua=1%2520%2520. [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence (2015) Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. National collaborating centre for Women's and Children's Health https://www.nice.org.uk/guidance/ng3. [PubMed]
- 13.Nagalla SR, Snyder CK, Michaels JE, Laughlin MJ, Roberts CT, Balaji MS, et al. (2015) Maternal serum biomarkers for risk assessment in gestational diabetes. A potential universal screening test to predict GDM status. Indian J Endocrinol Metab 19: 155–159. doi: 10.4103/2230-8210.140226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syngelaki A, Visser GHA, Krithinakis K, Wright A, Nicolaides KH (2016) First trimester screening for gestational diabetes mellitus by maternal factors and markers of inflammation. Metabolism 65: 131–137. doi: 10.1016/j.metabol.2015.10.029 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association (2014) Standards of Medical Care in Diabetes—2014. Diabetes Care 37: S14–80. doi: 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 16.Nankervis A, McIntyre H, Moses R, Ross G, Callaway L, Porter C, et al. (2014) ADIPS Consensus Guidelines for the Testing and Diagnosis of Gestational Diabetes Mellitus in Australia. http://adipsorg/downloads/ADIPSConsensusGuidelinesGDM-030513VersionACCEPTEDFINALpdf.
- 17.American Diabetes Association (2017) Standards of medical care in diabetes. Diabetes Care 40.
- 18.Centre for Reviews and Dissemination (2009) Systematic Reviews: CRD's guidance for undertaking reviews in health care. https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf.
- 19.Wright J, Small N, Raynor P, Tuffnell D, Bhopal R, Cameron N (2012) Cohort profile: The Born in Bradford multi-ethnic family cohort study. Int J Epidemiol 4: 1–14. [DOI] [PubMed] [Google Scholar]
- 20.Dunne FP, Avalos G, Durkan M, Mitchell Y, Gallacher T, Keenan M, et al. (2009) ATLANTIC DIP: Pregnancy Outcome for Women With Pregestational Diabetes Along the Irish Atlantic Seaboard. Diabetes Care 32: 1205–1206. doi: 10.2337/dc09-1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preferred Reporting Items for Systematic Reviews and Meta-Analyses PRISMA. http://wwwprisma-statementorg/ http://www.prisma-statement.org/.
- 22.Hajian-Tilaki K (2013) Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med 4: 627–635. [PMC free article] [PubMed] [Google Scholar]
- 23.Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JAC (2007) A unification of models for meta-analysis of diagnostic accuracy studies. Biostat 8: 239–251. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization (1999) Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus. http://apps.who.int/iris/bitstream/10665/66040/1/WHO_NCD_NCS_99.2.pdf.
- 25.Lawlor DA, West J, Fairley L, Nelson SM, Bhopal RS, Tuffnell D, et al. (2014) Pregnancy glycaemia and cord-blood levels of insulin and leptin in Pakistani and white British mother–offspring pairs: findings from a prospective pregnancy cohort. Diabetologia 57: 2492–2500. doi: 10.1007/s00125-014-3386-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuttall FQ (2015) Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutrition Today 50: 117–128. doi: 10.1097/NT.0000000000000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryant M, Santorelli G, Farrar D, Lawlor DA, Tuffnell D, Bhopal R, et al. (2012) A comparison of South Asian specific and established BMI thresholds for determining obesity prevalence in pregnancy and predicting pregnancy complications: findings from the Born in Bradford cohort. Int J Obesity 38: 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostlund I, Hanson U (2003) Occurrence of gestational diabetes mellitus and the value of different screening indicators for the oral glucose tolerance test. Acta Obstet Gynecol Scand 82: 103–108. [DOI] [PubMed] [Google Scholar]
- 29.Schytte T, Jorgensen LGM, Brandslund I, Petersen PH, Andersen B (2004) The clinical impact of screening for gestational diabetes. Clinical Chemistry And Laboratory Medicine 42: 1036–1042. doi: 10.1515/CCLM.2004.209 [DOI] [PubMed] [Google Scholar]
- 30.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. (1997) A Prospective Study of Pregravid Determinants of Gestational Diabetes Mellitus. JAMA 278: 1078–1083. [PubMed] [Google Scholar]
- 31.Coustan DR, Nelson C, Carpenter MW, Carr SR, Rotondo LR, Widness JA (1989) Maternal Age and Screening for Gestational Diabetes: A Population-Based Study. Obst Gynecol 73: 557–561. [PubMed] [Google Scholar]
- 32.Simmonds MC, Wald N (2012) Risk estimation versus screening performance: A comparison of six risk algorithms for cardiovascular disease. J Med Screen 19: 201–205. doi: 10.1258/jms.2012.012076 [DOI] [PubMed] [Google Scholar]
- 33.Avalos GE, Owens LA, Dunne F (2013) Applying current screening tools for gestational diabetes mellitus to a european population: Is it time for change? Diabetes Care 36: 3040–3044. doi: 10.2337/dc12-2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cosson E, Benbara A, Pharisien I, Nguyen MT, Revaux A, Lormeau B, et al. (2013) Diagnostic and prognostic performances over 9 years of a selective screening strategy for gestational diabetes mellitus in a cohort of 18,775 subjects. Diabetes Care 36: 598–603. doi: 10.2337/dc12-1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danilenko-Dixon DR, Winter JTV, Nelson RL, et al. (1999) Universal versus selective gestational diabetes screening: application of 1997 American Diabetes Association recommendations. Am J Obstet Gynecol 181: 798–802. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez-Moleon JJ, Bueno-Cavanillas A, Luna-del-Castillo JD, Garcia-Martin M, Lardelli-Claret P, Galvez-Vargas R (2002) Prevalence of gestational diabetes mellitus: Variations related to screening strategy used. Eur J Endocrinol 146: 831–837. [DOI] [PubMed] [Google Scholar]
- 37.Teh WT, Teede HJ, Paul E, Harrison CL, Wallace EM, Allan C (2011) Risk factors for gestational diabetes mellitus: implications for the application of screening guidelines. Aust N Z J Obstet Gynaecol 51: 26–30. doi: 10.1111/j.1479-828X.2011.01292.x [DOI] [PubMed] [Google Scholar]
- 38.Yang X, Hsu-Hage B, Yu L, Simmons D (2002) Selective screening for gestational diabetes in Chinese women. Diabetes Care 25: 796. [DOI] [PubMed] [Google Scholar]
- 39.Caliskan E, Kayikcioglu F, Ozturk N, Koc S, Haberal A (2004) A population-based risk factor scoring will decrease unnecessary testing for the diagnosis of gestational diabetes mellitus. Acta Obstet Gynecol Scand 83: 524–530. doi: 10.1111/j.0001-6349.2004.00389.x [DOI] [PubMed] [Google Scholar]
- 40.Cypryk K, Szymczak W, Czupryniak L, Sobczak M, Lewinski A (2008) Gestational diabetes mellitus—an analysis of risk factors. Endokrynologia Polska 59: 393–397. [PubMed] [Google Scholar]
- 41.Jensen DM, Damm P, Sorensen B, Molsted-Pedersen L, Westergaard JG, Korsholm L, et al. (2003) Proposed diagnostic thresholds for gestational diabetes mellitus according to a 75-g oral glucose tolerance test. Maternal and perinatal outcomes in 3260 Danish women. Diabet Med 20: 51–57. [DOI] [PubMed] [Google Scholar]
- 42.Marquette GP, Klein VR, Niebyl JR (1985) Efficacy of screening for gestational diabetes. Am J Perinatol 2: 7–9. doi: 10.1055/s-2007-999901 [DOI] [PubMed] [Google Scholar]
- 43.Nielsen KK, Damm P, Kapur A, Balaji V, Balaji MS, Seshiah V, et al. (2016) Risk factors for hyperglycaemia in pregnancy in Tamil Nadu, India. PLoS ONE 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sacks DA, Abu FS, Karten GJ, Forsythe AB, Hackett JR (1987) Screening for gestational diabetes with the one-hour 50-g glucose test. Obstet Gynecol 70: 89–93. [PubMed] [Google Scholar]
- 45.Shamsuddin K, Mahdy ZA, Siti Rafiaah I, Jamil MA, Rahimah MD (2001) Risk factor screening for abnormal glucose tolerance in pregnancy. Int J Gynaecol Obstet 75: 27–32. [DOI] [PubMed] [Google Scholar]
- 46.Sunsaneevithayakul P, Boriboohirunsarn D, Sutanthavibul A, Ruangvutilert P, Kanokpongsakdi S, Singkiratana D, et al. (2003) Risk factor-based selective screening program for gestational diabetes mellitus in Siriraj Hospital: result from clinical practice guideline. J Med Assoc Thai 86: 708–714. [PubMed] [Google Scholar]
- 47.Moses RG, Moses J, Davis WS (1998) Gestational diabetes: do lean young caucasian women need to be tested? Diabetes Care 21: 1803–1806. [DOI] [PubMed] [Google Scholar]
- 48.Pintaudi B, Di Vieste G, Corrado F, Lucisano G, Pellegrini F, Giunta L, et al. (2014) Improvement of selective screening strategy for gestational diabetes through a more accurate definition of high-risk groups. Eur J Endocrinol 170: 87–93. doi: 10.1530/EJE-13-0759 [DOI] [PubMed] [Google Scholar]
- 49.Savona-Ventura C, Vassallo J, Marre M, Karamanos BG, group MGs (2013) A composite risk assessment model to screen for gestational diabetes mellitus among Mediterranean women. Int J Gynaecol Obstet 120: 240–244. doi: 10.1016/j.ijgo.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 50.Williams CB, Iqbal S, Zawacki CM, Yu D, Brown MB, Herman WH (1999) Effect of selective screening for gestational diabetes. Diabetes Care 22: 418–421. [DOI] [PubMed] [Google Scholar]
- 51.Erem C, Kuzu UB, Deger O, Can G (2015) Prevalence of gestational diabetes mellitus and associated risk factors in Turkish women: the Trabzon GDM Study. Arch Med Sci 11: 724–735. doi: 10.5114/aoms.2015.53291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nanda S, Savvidou M, Syngelaki A, Akolekar R, Nicolaides KH (2011) Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn 31: 135–141. doi: 10.1002/pd.2636 [DOI] [PubMed] [Google Scholar]
- 53.Naylor CD, Sermer M, Chen E, Farine D (1997) Selective screening for gestational diabetes mellitus. Toronto Trihospital Gestational Diabetes Project Investigators. New Engl J Med 337: 1591–1596. doi: 10.1056/NEJM199711273372204 [DOI] [PubMed] [Google Scholar]
- 54.Phaloprakarn C, Tangjitgamol S, Manusirivithaya S (2009) A risk score for selective screening for gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol 145: 71–75. doi: 10.1016/j.ejogrb.2009.04.016 [DOI] [PubMed] [Google Scholar]
- 55.Shirazian N, Emdadi R, Mahboubi M, Motevallian A, Fazel-Sarjuei Z, Sedighpour N, et al. (2009) Screening for gestational diabetes: usefulness of clinical risk factors. Arch Gynecol Obstet 280: 933–937. doi: 10.1007/s00404-009-1027-y [DOI] [PubMed] [Google Scholar]
- 56.van Leeuwen M, Opmeer BC, Zweers EJ, van Ballegooie E, ter Brugge HG, de Valk HW, et al. (2009) External validation of a clinical scoring system for the risk of gestational diabetes mellitus. Diabet Res Clin Prac 85: 96–101. [DOI] [PubMed] [Google Scholar]
- 57.van Leeuwen M, Opmeer BC, Zweers EJ, van Ballegooie E, ter Brugge HG, de Valk HW, et al. (2010) Estimating the risk of gestational diabetes mellitus: a clinical prediction model based on patient characteristics and medical history. BJOG 117: 69–75. doi: 10.1111/j.1471-0528.2009.02425.x [DOI] [PubMed] [Google Scholar]
- 58.Gabbay-Benziv R, Esin S, Baschat AA (2015) Incorporating first trimester analytes to predict delivery of a large for gestational infant in women with impaired glucose tolerance. J Perinat Med 43: 299–303. doi: 10.1515/jpm-2014-0041 [DOI] [PubMed] [Google Scholar]
- 59.Kirke AB, Evans SF, Walters BN (2014) Gestational diabetes in a rural, regional centre in south Western Australia: predictors of risk. Rural & Remote Health 14: 2667. [PubMed] [Google Scholar]
- 60.Syngelaki A, Pastides A, Kotecha R, Wright A, Akolekar R, Nicolaides KH (2015) First-Trimester Screening for Gestational Diabetes Mellitus Based on Maternal Characteristics and History. Fetal Diagn Ther 38: 14–21. doi: 10.1159/000369970 [DOI] [PubMed] [Google Scholar]
- 61.Jensen DM, Molsted-Pedersen L, Beck-Nielsen H, Westergaard JG, Ovesen P, Damm P (2003) Screening for gestational diabetes mellitus by a model based on risk indicators: A prospective study. Am J Obstet Gynecol 189: 1383–1388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data related to published studies are within the paper and its supporting information. Born in Bradford cohort data are available from the BiB executive committee via the BiB project website www.borninbradford.nhs.uk/contact-us/ and Atlantic-Dip data from Fidelma Dunne (fidelma.dunne@nuigalway.ie), representing Institutional Data Access for researchers who meet the criteria for access to confidential data.