Abstract
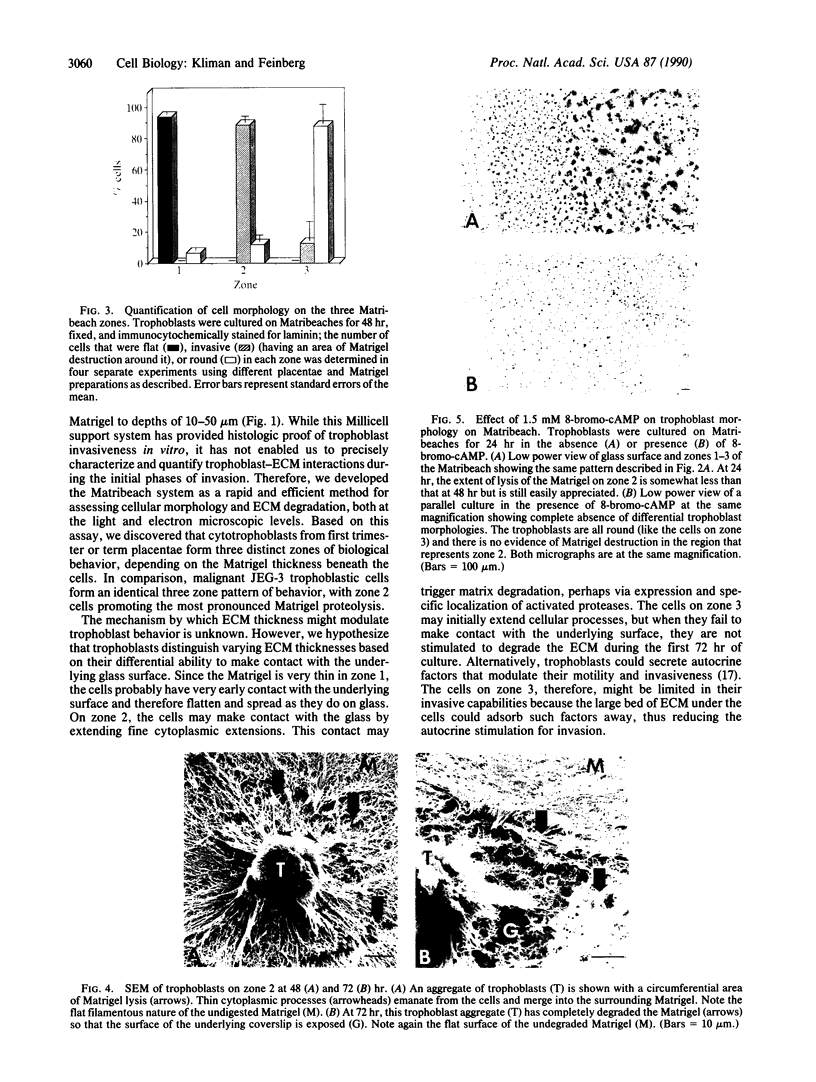
Trophoblast invasion of the uterine extracellular matrix, a critical process for human implantation and uteroplacental vascular development, is a striking example of controlled invasiveness. To examine cellular behavior relevant to this process, human trophoblasts were cultured on (i) Millicell filters prelayered with Matrigel and (ii) coverslips precoated with a gentle slope of Matrigel (Matribeach). Histologic sections of the Millicell system demonstrated significant invasion. However, on Matribeach the cells exhibited markedly different characteristics depending on the thickness of the Matrigel. On zone 1 (1-4 microns thick), flat aggregates and syncytia were seen. In contrast, cells on zone 2 (4-14 microns) formed rounded aggregates with intercellular processes. In this zone, prominent degradation of pericellular Matrigel proteins was assessed by both light microscopy and scanning electron microscopy. Treatment with 8-bromo-cAMP inhibited this proteolytic process. On zone 3 (14-60 microns), unicellular trophoblasts or small aggregates caused minimal matrix degradation. JEG-3 human choriocarcinoma cells exhibited similar morphologic and degradative properties on Matribeach, but zone 2 proteolysis was not affected by 8-bromo-cAMP. Our results suggest that extracellular matrix thickness has profound effects on cellular morphology and proteolytic activity. Furthermore, while both normal and malignant human trophoblasts can degrade extracellular matrix proteins, only normal trophoblast extracellular matrix degradation is inhibited by 8-bromo-cAMP.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- Feinberg R. F., Kao L. C., Haimowitz J. E., Queenan J. T., Jr, Wun T. C., Strauss J. F., 3rd, Kliman H. J. Plasminogen activator inhibitor types 1 and 2 in human trophoblasts. PAI-1 is an immunocytochemical marker of invading trophoblasts. Lab Invest. 1989 Jul;61(1):20–26. [PubMed] [Google Scholar]
- Feinman M. A., Kliman H. J., Caltabiano S., Strauss J. F., 3rd 8-Bromo-3',5'-adenosine monophosphate stimulates the endocrine activity of human cytotrophoblasts in culture. J Clin Endocrinol Metab. 1986 Nov;63(5):1211–1217. doi: 10.1210/jcem-63-5-1211. [DOI] [PubMed] [Google Scholar]
- Fisher S. J., Cui T. Y., Zhang L., Hartman L., Grahl K., Zhang G. Y., Tarpey J., Damsky C. H. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J Cell Biol. 1989 Aug;109(2):891–902. doi: 10.1083/jcb.109.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Kao L. C., Caltabiano S., Wu S., Strauss J. F., 3rd, Kliman H. J. The human villous cytotrophoblast: interactions with extracellular matrix proteins, endocrine function, and cytoplasmic differentiation in the absence of syncytium formation. Dev Biol. 1988 Dec;130(2):693–702. doi: 10.1016/0012-1606(88)90361-2. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Coutifaris C., Babalola G. O., Soto E. A., Kao L. C., Queenan J. T., Jr, Feinberg R. F., Strauss J. F., 3rd The human trophoblast: homotypic and heterotypic cell-cell interactions. Prog Clin Biol Res. 1989;294:425–434. [PubMed] [Google Scholar]
- Kliman H. J., Nestler J. E., Sermasi E., Sanger J. M., Strauss J. F., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986 Apr;118(4):1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Mandler R., Murano G., Katz D. A., Gordon R. K., Chiang P. K., Schiffmann E. Tumor cell autocrine motility factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R., Robertson W. B., Brosens I., Dixon G. Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981 Jan-Mar;2(1):71–91. doi: 10.1016/s0143-4004(81)80042-2. [DOI] [PubMed] [Google Scholar]
- Queenan J. T., Jr, Kao L. C., Arboleda C. E., Ulloa-Aguirre A., Golos T. G., Cines D. B., Strauss J. F., 3rd Regulation of urokinase-type plasminogen activator production by cultured human cytotrophoblasts. J Biol Chem. 1987 Aug 15;262(23):10903–10906. [PubMed] [Google Scholar]
- Reich R., Thompson E. W., Iwamoto Y., Martin G. R., Deason J. R., Fuller G. C., Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988 Jun 15;48(12):3307–3312. [PubMed] [Google Scholar]
- Ringler G. E., Kao L. C., Miller W. L., Strauss J. F., 3rd Effects of 8-bromo-cAMP on expression of endocrine functions by cultured human trophoblast cells. Regulation of specific mRNAs. Mol Cell Endocrinol. 1989 Jan;61(1):13–21. doi: 10.1016/0303-7207(89)90185-8. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A., August A. M., Golos T. G., Kao L. C., Sakuragi N., Kliman H. J., Strauss J. F., 3rd 8-Bromo-adenosine 3',5'-monophosphate regulates expression of chorionic gonadotropin and fibronectin in human cytotrophoblasts. J Clin Endocrinol Metab. 1987 May;64(5):1002–1009. doi: 10.1210/jcem-64-5-1002. [DOI] [PubMed] [Google Scholar]