ABSTRACT
While chronic inflammation has been causally associated with several epithelial malignancies, whether it causally contributes to the development of prostate cancer has remained unclear. We recently reported that progenitor-like inflammation-associated luminal cells marked by low expression of Cluster of Differentiation 38 (CD38) can initiate human prostate cancer and predict poor outcome.
KEYWORDS: CD38, Luminal, Prostate cancer, stem cell
In 2016, an estimated 180,890 new cases of prostate cancer will be diagnosed, and approximately 26,120 men will die of the disease.1 Despite extensive research efforts, the prevalence of prostate cancer remains a mystery. Chronic inflammation has been causally associated with the development of epithelial malignancies such as stomach, large intestine, liver, and urinary bladder cancers. Recent studies have identified an association between inflammation and the development of prostate cancer.2
De Marzo, Nelson, and colleagues have reported changes in the morphology of epithelial cells associated with chronic inflammation in the human prostate known as proliferative inflammatory atrophy (PIA).3 Luminal cells in PIA have an atrophic appearance, exhibit an imbalance between proliferation and apoptosis, and show signs of oxidative stress. Additionally, these cells show an increase in the anti-apoptotic factor B-cell lymphoma 2 (BCL2) and a decrease in androgen receptor (AR) signaling. PIA is commonly observed in close spatial proximity to premalignant and malignant tissue, suggesting that PIA may represent a precursor to prostate cancer.3 However, the functional role of these cells in prostate cancer had not been investigated due to an inability to isolate PIA-like and non-PIA epithelial cells from viable human prostate tissue.
We identified a unique population of luminal cells marked by low expression of Cluster of Differentiation 38 (CD38lo) that exhibit many hallmarks of cells associated with PIA, including increased BCL2 expression and reduced androgen signaling.4 Gene expression analysis of CD38lo cells revealed enrichment of many inflammatory-related genes, and immunohistochemical staining of human prostate tissue confirmed that CD38lo luminal cells are predominantly localized in glands adjacent to inflammation.
Having established that CD38lo luminal cells represent an inflammation-associated cell population with hallmarks of PIA, we sought to assess their proliferative potential in three progenitor assays (colony-forming, sphere-forming, organoid-forming). CD38lo luminal cells isolated from freshly dissociated primary human prostate tissue exhibited a level of progenitor activity in between the stem-like basal cells and the differentiated CD38hi luminal cells. Importantly, the inflammation-associated CD38lo luminal cells represent an enriched luminal progenitor subset.
In previous studies, we have shown that basal cells from human prostate tissue can generate tumors following oncogenic transformation.5 In contrast, ex vivo-expanded luminal cells only give rise to indolent-like tumors with limited proliferative potential.6 In our recent study, CD38lo luminal progenitor cells were lentivirally transduced with oncogenes (Myc, AKT1, and AR), expanded in organoid culture, and transplanted subcutaneously with Matrigel and urogenital sinus mesenchyme cells into immune-deficient mice. Transplanted oncogene-expressing CD38lo luminal cells developed features of highly proliferative prostate adenocarcinoma, suggesting that they can initiate aggressive human prostate cancer in vivo.4
Our development of an approach to isolate PIA-like luminal cells based on low expression of CD38 allowed us to functionally test their capacity. Our demonstration that PIA-like luminal cells isolated from human prostate tissue can initiate prostate cancer3 provides functional evidence to support the model that PIA may represent a precursor to prostate cancer (Fig. 1). Furthermore, our ability to isolate PIA-like luminal cells allowed us to profile and characterize them in a deeper way than in previous studies. We can now show that PIA-like cells exhibit elevated nuclear factor kappa B (NF-kB) signaling,4, which has been associated with aggressive prostate cancer.7 Moreover, since CD38lo cells display decreased androgen signaling and elevated BCL2 expression, they would be predicted to respond poorly to hormonal therapy.
Figure 1.
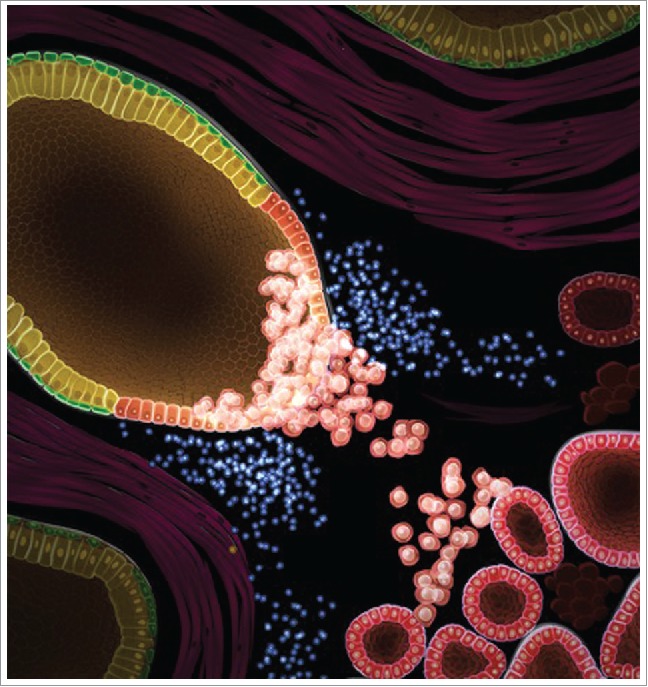
Initiation of aggressive prostate cancer by oncogene-expressing Proliferative Inflammatory Atrophy (PIA)-like luminal cells. PIA-like luminal cells (red) are associated with regions that contain inflammation (blue). When lentivirally transduced with oncogenes (Myc, AKT1, and androgen receptor), these cells expand and can initiate aggressive prostate cancer (bottom right corner). Visualization by Kandeo Studios.
Previous studies in mouse models have demonstrated that acute inflammation promotes an expansion of progenitor-like cells,8 and an increase in basal-to-luminal differentiation.9 While we show that PIA-like luminal cells are enriched in regions of inflammation, an inability to study kinetic processes in human tissue prevents us from uncovering the sequence of events that links inflammation and PIA-like luminal progenitor cells. Serial biopsies of benign human prostate tissue containing chronic inflammation may help uncover the precise order of events involved in PIA. Interestingly, our gene expression analysis revealed that PIA-like cells upregulate several pro-inflammatory cytokines and chemokines,4 suggesting that PIA-like cells may actively recruit inflammation. The assays used to measure progenitor activity require that epithelial cells be removed from their native environment, dissociated into single cells, and grown in a culture system lacking several of the key cell types found in the prostate microenvironment including inflammatory cells. We hope that future studies will be able to incorporate more of these microenvironmental cell types and may enable us to study the influence of inflammation on human prostate epithelial development and malignant transformation in culture.
A key goal of prostate cancer research is to uncover the biology that drives aggressive prostate cancer to allow us to distinguish indolent from aggressive disease and develop therapeutic targets to treat advanced stage disease. In this study, we illustrate that low CD38 mRNA levels in prostatectomy specimens from two separate cohorts are prognostic for biochemical recurrence, suggesting that CD38lo aggressive cancers may arise from PIA-like CD38lo luminal cells. Given that CD38lo luminal cells and CD38lo tumors exhibit progenitor-like features, we hypothesize that CD38 loss may be functionally significant. CD38 consumes cellular nicotinamide adenine dinucleotide (NAD),10 suggesting that loss of CD38 may increase the pool of NAD available for cellular metabolism in CD38lo luminal cells and CD38lo prostate cancer. Future studies should investigate if an increased pool of NAD provides CD38lo luminal cells with a proliferative advantage. Finally, NF-kB signaling and other signaling pathways found to be important in CD38lo luminal cells should be investigated as therapeutic targets to prevent the initiation and progression of CD38lo luminal cell-derived aggressive prostate cancer.
Disclosure of potential conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Angelo De Marzo for discussions about PIA and the role of inflammation in prostate cancer. Due to space limitations, many important studies and research articles were not able to be referenced.
References
- 1.Siegel RL, Miller KD and Jemal A. Cancer statistics, 2016. CA: Cancer J Clin 2016; 66:7-30; PMID: 26742998; http://dx.doi.org/ 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Gurel B, Lucia MS, Thompson IM Jr., Goodman PJ, Tangen CM, Kristal AR, Parnes HL, Hoque A, Lippman SM, Sutcliffe S, et al.. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol. Biomarkers Prev 2014; 23:847-56; PMID: 24748218; http://dx.doi.org/ 10.1158/1055-9965.EPI-13-1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Marzo AM, Marchi VL, Epstein JI, and Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol 1999. 155:1985-92; PMID: 10595928; http://dx.doi.org/ 10.1016/S0002-9440(10)65517-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Grogan TR, Hieronymus H, Hashimoto T, Mottahedeh J, Cheng D, Zhang L, Huang K, Stoyanova T, Park JW. et al.. Low CD38 identifies progenitor-like inflammation-associated luminal cells that can initiate human prostate cancer and predict poor outcome. Cell Rep 2016. 17(10):2596-606; PMID: 27926864; http://dx.doi.org/ 10.1016/j.celrep.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science 2010; 329:568-71; PMID: 20671189; http://dx.doi.org/ 10.1126/science.1189992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JW, Lee JK, Phillips JW, Huang P, Cheng D, Huang J, and Witte ON. Prostate epithelial cell of origin determines cancer differentiation state in an organoid transformation assay. Proc Natl Acad Sci USA 2016; 113:4482-7;PMID:27044116; http://dx.doi.org/24686169 10.1073/pnas.1603645113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin R, Yi Y, Yull FE, Blackwell TS, Clark PE, Koyama T, Smith JA Jr, Matusik RJ. NF-kB gene signature predicts prostate cancer progression. Cancer Res 2014; 74:2763-72; PMID: 24686169; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Wang L, Jerde TJ, Chan BD, Savran CA, Burcham GN, Crist S, and Ratliff TL. Characterization of autoimmune inflammation induced prostate stem cell expansion. Prostate 2015: 75(14):1620-31; PMID: 26174474; http://dx.doi.org/ 10.1002/pros.23043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon OJ, Zhang L, Ittmann MM, and Xin L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc Natl Acad Sci USA 2014; 111:E592-600; PMID:27044116; http://dx.doi.org/8235624 10.1073/pnas.1318157111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, and Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 1993; 262:1056-9; PMID: 8235624; http://dx.doi.org/ 10.1126/science.8235624 [DOI] [PubMed] [Google Scholar]


