ABSTRACT
The outcome of acute myelogenous leukemia (AML) therapy depends on the propensity of leukemic blasts to accumulate ara-CTP, the active triphosphate of cytarabine (ara-C). We identified sterile α motif and HD domain-containing protein 1 (SAMHD1) as an ara-CTPase that protects cancer cells from cytarabine-induced toxicity. Therefore, we propose targeting SAMHD1 as a strategy to potentiate cytarabine and possibly other antimetabolite-based therapies.
KEYWORDS: ara-C, AML, cancer, leukemia, SAMHD1, Vpx
The annual burden of newly diagnosed leukemia patients worldwide is approximately 350,000, with the majority of cases being acute myelogenous leukemia (AML).1 The average 5-year overall survival (OS) rate of AML is approximately 20%, with good results for children but far worse for elderly patients.2,3 A significant improvement in the AML therapy with respect to survival was reported with the introduction of high-dose cytarabine (cytosine arabinoside, ara-C) courses during the consolidation phase; however, this study was reported more than 2 decades ago.4 Standard treatment still consists of cytotoxic therapy combining ara-C with an anthracycline.2,3 Even though it has been known for many years that the clinical outcome of AML treatment correlates critically with the ability of AML blasts to accumulate the cell-active triphosphate form of ara-C, ara-CTP,5 the underlying mechanism that distinguishes resistant from sensitive leukemic clones has remained unknown.
In 2011, Goldstone et al.6 were the first to recognize that the human immunodeficiency virus type 1 (HIV-1) restriction factor and mutated protein in Aicardi-Goutières syndrome, sterile α motif and HD domain-containing protein 1 (SAMHD1), contains a unique and allosterically activated deoxynucleoside triphosphate (dNTP) triphosphohydrolase activity toward all canonical dNTPs (i.e. dGTP, dATP, dTTP, and dCTP). In a later study, Arnold et al.7 provided proof-of-concept that artificial dNTP analogs like the triphosphate of the anti-cancer drug clofarabine, Cl-F-ara-ATP, can be substrates for SAMHD1. This prompted us to hypothesize that other nucleotide analogs could be SAMHD1 substrates, such as ara-CTP3 (Fig. 1), which differs only in the hydroxyl group configuration at the 2′-C atom from dCTP. We tested this hypothesis in vitro using purified human SAMHD1 protein, and showed that ara-CTP is a substrate but not an allosteric activator of SAMHD1. We subsequently demonstrated in AML and lymphoma cell lines that the removal of SAMHD1 could dramatically increase ara-C cytotoxicity. Firstly, we exploited virus-like particle (VLP)-delivery of the (lenti)viral protein X (Vpx), that is known to recruit SAMHD1 for degradation,3 and observed a dose-dependent sensitization of AML cells to ara-C up to ∼130-fold, with respect to cells treated with control VLPs lacking Vpx. Secondly, we used clustered regularly interspaced short palindromic repeats (CRISPR) /Cas9 (CRISPR associated protein 9)-mediated disruption of the SAMHD1 gene, which resulted in a substantial increase in ara-C toxicity that could be abrogated by ectopic expression of wild-type, but not catalytically inactive SAMHD1. In cells lacking SAMHD1 protein, we measured a ∼10-fold increase of intracellular ara-CTP compared with isogenic cell lines expressing SAMHD1, and importantly, incorporation of ara-CTP into DNA was equally increased in these cells. This was correlated with decreased DNA synthesis, as measured by thymidine incorporation, and a concomitant increase in DNA damage and apoptotic signaling (Fig. 1). Demonstrating the relevance of the ara-CTPase activity of SAMHD1 in vivo, xenograft AML mouse models treated with ara-C showed a dramatic increase in survival when SAMHD1 was absent in the transplanted cells. Furthermore, VLP-delivery of Vpx to primary AML patient blasts, sampled either at diagnosis or relapse, successfully depleted SAMHD1 protein and increased ara-C toxicity. Finally, retrospective analyses of SAMHD1 expression and survival in two well-characterized cohorts of adult and pediatric AML patients revealed a ∼20% absolute difference in OS at 18 and 12 months after diagnosis, respectively.3 Surprisingly, the mRNA expression levels of genes involved in ara-C metabolism that had been previously reported as potential biomarkers were not significantly correlated with survival in these patient cohorts.3 Taken together, our findings demonstrate that the therapeutic response of AML to ara-C is controlled by SAMHD1 (Fig. 1), and suggest this may be the case for other hematological malignancies. Furthermore, we provide a rationale to target SAMHD1 to potentiate antimetabolite-based therapies.
Figure 1.
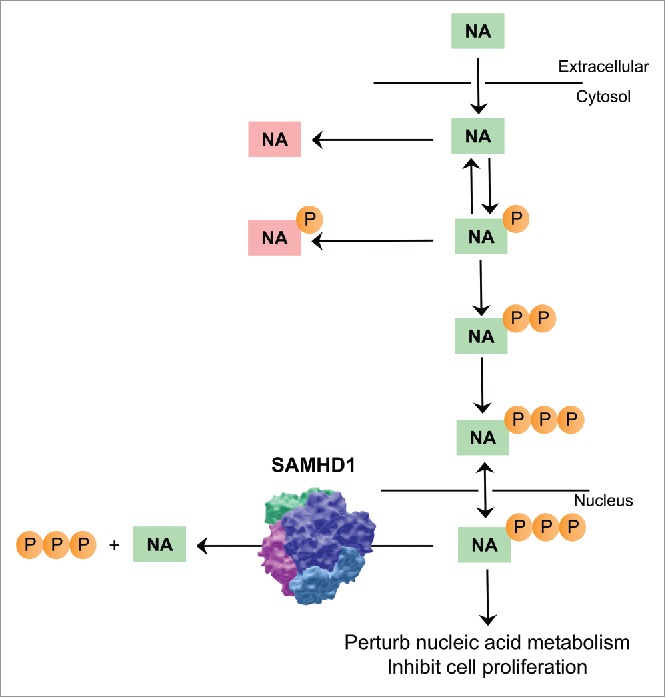
SAMHD1 detoxifies cells from nucleoside analogs by hydrolysing their triphosphorylated active metabolites. Nucleoside analogs (NAs) are actively taken up by the cell and sequentially phosphorylated to their active mono-, di-, and triphosphate forms (P, PP, and PPP, respectively), which can all compete with physiologic nucleotides in various metabolic processes. Nucleoside triphosphates can be incorporated into nucleic acids and perturb DNA and RNA metabolism to exert their anti-proliferative effects. Catabolic processes may reduce the level of active metabolites, such as removal of phosphate groups from nucleoside monophosphates by cytosolic 5′-nucleotidases, or deamination by deaminases (deaminated NAs shown in red). The nuclear protein sterile α motif and HD domain-containing protein 1 (SAMHD1), which is a promiscuous deoxynucleoside triphosphate (dNTP) triphosphohydrolase, removes all three phosphates from dNTP analogs leaving the non-toxic nucleoside core, thus providing a barrier to antimetabolite-based chemotherapies. Representation of SAMHD1 was created in Molecular Flipbook (www.molecularflipbook.org) using PDB file 4BZC (Ji et al., 2013, Nat Struct Mol Biol, DOI: 10.1038/nsmb.2692).
Two recent studies also identified ara-CTP as a SAMHD1 substrate.8,9 Schneider et al. began with an alternative hypothesis that had previously been shown for anti-HIV-1 drugs10: that SAMHD1 could increase therapeutic efficacy of nucleoside analog-based chemotherapies by depleting competing endogenous dNTPs. However, contrary to their hypothesis, these authors found an inverse correlation between SAMHD1 expression and ara-C cytotoxicity, and subsequently identified SAMHD1 as an ara-CTPase responsible for mediating therapeutic efficacy of ara-C in adult AML. Additionally, these authors provided evidence that SAMHD1 upregulation may be induced by long-term exposure to ara-C, suggesting that SAMHD1 may contribute to the development of ara-C resistance.9 Schneider et al.9 generated ara-C-resistant AML cell lines and, in addition to changes in expression of genes previously reported as ara-C metabolic enzymes, observed marked increases in SAMHD1 expression compared to the respective ara-C-sensitive parental cell lines. However, it should be noted that we did not observe a clear pattern of increased SAMHD1 mRNA expression in 31 paired AML samples at diagnosis and relapse,3 possibly reflecting the differences of resistance development in vivo. Hollenbaugh et al.8 asked a broader question of which dNTP modifications restrict or permit hydrolysis by SAMHD1. Using computational modeling, these authors predicted hydrolytic activity of SAMHD1 toward several clinically relevant nucleoside triphosphates, and subsequently demonstrated in vitro that several arabinose dNTP analogs are SAMHD1 substrates. Interestingly, Hollenbaugh et al. predicted that the triphosphate metabolite of gemcitabine, dF-dCTP, difluorodeoxycytosine triphosphate, is not a SAMHD1 substrate, which was demonstrated by both us3 and Schneider et al.9 They also identified dNTP analogs which inhibit hydrolysis of dGTP by SAMHD1 in vitro, and although these compounds lacked potency they could be used as starting points for rationale design of inhibitory nucleoside analogs. Taken together, these studies, along with our study, raise three important points (1) SAMHD1 may be a barrier to therapeutic efficacy of several clinically used nucleoside analogs (Fig. 1), (2) assessment of SAMHD1 levels may allow patient-specific dose adjustments, and (3) targeting SAMHD1 may increase the efficacy of these treatments; we are currently investigating all these points.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by an EMBO Long-Term Fellowship (ALTF-605–2014 to S.G.R.), the German Research Foundation (DFG) (SCHA1950/1–1 to T.S.), partially through the Federal Ministry of Education and Research of Germany (BMBF) supported Immunoquant project (0316170 C) as well as the HIVERA: EURECA project (01KI1307B), and the Swedish Children's Cancer Foundation (PR2016–0044 and TJ2016–0040 to N.H.).
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359-86; PMID:25220842; http://dx.doi.org/ 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Ossenkoppele G, Lowenberg B. How I treat the older patient with acute myeloid leukemia. Blood 2015; 125(5):767-74; PMID:25515963; http://dx.doi.org/ 10.1182/blood-2014-08-551499 [DOI] [PubMed] [Google Scholar]
- 3.Herold N, Rudd SG, Ljungblad L, Sanjiv K, Myrberg IH, Paulin CB, Heshmati Y, Hagenkort A, Kutzner J, Page BD, et al.. Targeting SAMHD1 with the Vpx protein to improve cytarabine therapy for hematological malignancies. Nat Med. 2017 Feb; 23(2):256-263; PMID:28067901; http://dx.doi.org/ 10.1038/nm.4265 [DOI] [PubMed] [Google Scholar]
- 4.Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, Omura GA, Moore JO, McIntyre OR, Frei E 3rd. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and leukemia group B. New Engl J Med 1994; 331(14):896-903; PMID:8078551; http://dx.doi.org/ 10.1056/NEJM199410063311402 [DOI] [PubMed] [Google Scholar]
- 5.Plunkett W, Iacoboni S, Estey E, Danhauser L, Liliemark JO, Keating MJ. Pharmacologically directed ara-C therapy for refractory leukemia. Semin Oncol 1985; 12(2 Suppl 3):20-30; PMID:4012338 [PubMed] [Google Scholar]
- 6.Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF, Yap MW, et al.. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 2011; 480(7377):379-82; PMID:22056990; http://dx.doi.org/ 10.1038/nature10623 [DOI] [PubMed] [Google Scholar]
- 7.Arnold LH, Kunzelmann S, Webb MR, Taylor IA. A continuous enzyme-coupled assay for triphosphohydrolase activity of HIV-1 restriction factor SAMHD1. Antimicrob Agents Chemother 2015; 59(1):186-92; PMID:25331707; http://dx.doi.org/ 10.1128/AAC.03903-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollenbaugh JA, Shelton J, Tao S, Amiralaei S, Liu P, Lu X, Goetze RW, Zhou L, Nettles JH, Schinazi RF, et al.. Substrates and inhibitors of SAMHD1. PLoS One 2017; 12(1):e0169052; PMID:28046007; http://dx.doi.org/ 10.1371/journal.pone.0169052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider C, Oellerich T, Baldauf HM, Schwarz SM, Thomas D, Flick R, Bohnenberger H, Kaderali L, Stegmann L, Cremer A, et al.. SAMHD1 is a biomarker for cytarabine response and a therapeutic target in acute myeloid leukemia. 2017 Feb; 23(2):250-255; PMID:27991919; http://dx.doi.org/ 10.1038/nm.4255 [DOI] [PubMed] [Google Scholar]
- 10.Amie SM, Daly MB, Noble E, Schinazi RF, Bambara RA, Kim B. Anti-HIV host factor SAMHD1 regulates viral sensitivity to nucleoside reverse transcriptase inhibitors via modulation of cellular deoxyribonucleoside triphosphate (dNTP) levels. J Biol Chem 2013; 288(28):20683-91; PMID:23744077; http://dx.doi.org/ 10.1074/jbc.M113.472159 [DOI] [PMC free article] [PubMed] [Google Scholar]


