Abstract
Background and objectives
A low total nephron number, which is associated with low birth weight (LBW), may indicate increased susceptibility to early-onset renal diseases in children. However, few studies have assessed renal biopsy findings in LBW children. We examined the relationship between LBW and glomerular density (GD) and/or glomerular volume (GV) in renal biopsy samples as a surrogate for total nephron number.
Design, setting, participants, & measurements
Renal biopsy findings of children of LBW were compared with those of age-matched control subjects of normal birth weight (NBW) who were histopathologically diagnosed with FSGS or minimal change nephrotic syndrome (MCNS) from 1995 to 2011. The GD and GV were estimated on the basis of measurements obtained by computerized image analysis.
Results
A total of 31 subjects (mean age 11 years; eight with low birth weight–FSGS [LBW-FSGS], 10 with normal birth weight–FSGS [NBW-FSGS], and 13 with normal birth weight–minimal change nephrotic syndrome [NBW-MCNS]) were analyzed. The mean birth weight of each group was 777 g (629–1000), 3110 g (2888–3358), and 3120 g (2748–3398), respectively (median [25th–75th percentile]). Age, body mass index, BP, and degrees of globally sclerotic glomeruli at biopsy were comparable between the groups. The GD was lower (LBW-FSGS, 1.4±0.6/mm2; NBW-FSGS, 3.3±1.2/mm2; and NBW-MCNS, 3.6±1.1/mm2; P<0.05) and the GV was greater (LBW-FSGS, 4.1 [3.1–5.1]×106 µm3; NBW-FSGS, 1.6 [1.5–2.1]×106 µm3; and NBW-MCNS, 1.3 [1.1–1.8]×106 µm3 [median, (25th–75th percentile)]; P<0.05) in patients with LBW-FSGS than in the other patient groups. The GD showed close positive correlations with birth weight (r=0.48) and gestational age (r=0.54), independent of renal function and degree of global glomerular sclerosis.
Conclusions
A low GD together with marked glomerular enlargement characterizes renal biopsy samples of children born with a LBW at an early stage of gestation.
Keywords: premature birth; biopsy; kidney glomerulus; birth weight; blood pressure; body mass index; child; female; gestational age; glomerulosclerosis, focal segmental; humans; infant, low birth weight; infant, newborn; kidney; kidney glomerulus; nephrons; nephrosis, lipoid; pregnancy; premature birth; proteinuria; sclerosis; segmental glomerulosclerosis
Introduction
Recent autopsy studies have demonstrated considerably greater variability in total nephron number (TNN) in normal populations than was previously suspected (1–4). TNN is correlated with the weight at birth, suggesting the importance of the intrauterine environment in determining the quantity of nephrons (1). Of note, other studies have reported that the marked variability in glomerular number has important implications for susceptibility to renal insufficiency and progression to ESRD, suggesting links among weight at birth, TNN, and susceptibility to progressive renal diseases (5,6). Importantly, however, all previous studies that have estimated TNN have been on the basis of detailed analyses of autopsy kidneys (1–3). Thus, at present, there is no established methodology to estimate TNN in living patients.
Recent studies have reported that individual glomerular density (GD; the number of glomeruli per renal cortical area) exhibits significant variation among patients with primary glomerular disease (7–11). Of note, GD is inversely correlated with mean glomerular volume (GV) in patients with IgA nephropathy and minimal change nephrotic syndrome (MCNS) (7,8). A low GD in renal biopsy samples is associated with a weaker response to corticosteroid therapy in adult patients with MCNS and a poor long-term renal outcome in patients with IgA nephropathy and with idiopathic membranous nephropathy (8–10). These results suggest that GD in renal biopsy specimens may serve as a surrogate for TNN in living patients. Interestingly, previous studies on autopsy kidneys have reported a close correlation between GD and weight at birth, further supporting this notion (1,12).
Hodgin et al. (13) reported that a very low birth weight (LBW) promotes the development of secondary FSGS in individuals born preterm. We recently demonstrated a relationship between LBW and the development of FSGS accompanying podocytopenia (14). Taken together, these results suggest that LBW associated with a low TNN may be a strong risk factor for the development of early-onset renal disease histopathologically characterized by FSGS. Therefore, we analyzed renal biopsy specimens from children with a diagnosis of FSGS or MCNS, with available records of weight at birth. We focused on the significance of the GD in these LBW children.
Materials and Methods
Patient Selection
This study included patients with FSGS and MCNS who underwent renal biopsies from 1995 to 2011 at the Department of Pediatrics, Niigata University Medical and Dental Hospital. The criteria for the decision to perform a biopsy were continuous proteinuria exceeding approximately 0.3 g/d, continuous hematuria and proteinuria, and a steroid-resistant nephrotic range of proteinuria in which proteinuria did not disappear for >4 weeks despite treatment with a high dose of prednisolone (2 mg/kg).
Biopsy specimens from 18 patients with FSGS were examined, and the morphologic appearance was compared between specimens from normal birth weight (NBW) children with FSGS (NBW-FSGS; n=10) and LBW children with FSGS (LBW-FSGS; n=8). Specimens from age-matched NBW patients with MCNS were used for comparison (NBW-MCNS; n=13). All patients with MCNS underwent renal biopsies because of their unfavorable clinical course, characterized by a high frequency of relapse or high dependency on corticosteroid treatment. Biopsies were performed before the administration of immunosuppressants and during corticosteroid-induced remission. We retrospectively reviewed the birth weights, gestational ages, and medical histories of all patients.
This study was approved by our local ethics board and all of the participants’ parents provided informed consent for use of excess renal biopsy tissue available after diagnostic examination for research purposes.
Definitions
The infants were divided into the following categories according to the World Health Organization classification scheme for birth weight: appropriate for gestational age (NBW), >2500 g; LBW, <2500 g; very LBW, <1500 g; and extremely LBW, <1000 g. The infants were also classified according to their gestational age: gestational age to full term; gestational age ≥37 weeks; preterm, 32–36 weeks; and very preterm, <32 weeks.
The eGFR was calculated by applying the following formula: eGFR = 0.413 × (height/serum creatinine [Cr]), with height expressed in centimeters (15). Nephrotic syndrome was defined as the presence of severe proteinuria (>40 mg/h per m2 in pooled night urine), or an early-morning urine protein-to-Cr ratio >2.0 g/g Cr, or hypoalbuminemia (serum albumin <2.5 g/dl) (16).
Histopathologic Analysis
All kidney tissue specimens were obtained via percutaneous needle biopsy. The tissues were embedded in paraffin, cut into 3–4 µm sections, and stained with periodic acid–Schiff and periodic acid–methenamine silver. Global glomerular sclerosis was defined when the entire glomerulus was involved in the sclerosis, and segmental sclerosis was defined as previously described (17). The degree of interstitial fibrosis was expressed as the percentage of affected area per total cortical area. Interstitial fibrosis was defined as an increased abundance of extracellular matrix separating tubules in the cortical area. It was scored as the percentage of involvement, with 1%–5% rounded to 5%, and other values rounded to the closest 10% (18).
The glomerular area (GA) and GD were measured using computerized image analysis (Leica IM500; Leica Microsystems, Germany). The GA was defined as the area within the outer capillary loops of the tuft. The mean GV was calculated from the measured GA as follows: GV = (GA) 3/2×β/d, where β is a dimensionless shape coefficient (β=1.38 for spheres) and d is a size distribution coefficient that is used to adjust for variation in the glomerular size (19). We used d=1.01 as in previous studies (20,21). The GD was determined by calculating the number of glomeruli that were not globally sclerotic per total renal cortical area. However, calculation of the GD is greatly influenced by the presence of global glomerular sclerosis. Therefore, we used two different definitions of the GD: the number of glomeruli that were not globally sclerotic per total cortical area, and the number of total glomeruli, including globally sclerotic glomeruli, per total cortical area.
Statistical Analyses
Continuous variables were expressed as mean±SD. The variables were assessed using the F-test, whether normally distributed or not. If the variables were normally distributed, paired t test was used to assess the significance of differences between two groups; when they were not normally distributed, the Mann–Whitney U test and Wilcoxon signed-rank test were used. The relationship between the continuous variables and GD was evaluated by univariate regression analyses. Multivariable analyses of the factors associated with GD were performed. The covariates included in the analyses were eGFR, birth weight, gestational age, and degree of globally sclerotic glomeruli. Because distribution of the birth weight, gestational age, and degree of globally sclerotic glomeruli were skewed, the log-transformed values were used in the regression analysis. Univariate and multivariate analyses were performed by using both definitions of the GD (excluding and including globally sclerotic glomeruli). Values of P<0.05 were considered to indicate statistical significance. All statistical analyses were performed using the SPSS software package (SPSS, Chicago, IL).
Results
Clinicopathologic Characteristics at the Time of Biopsy
We analyzed 31 patients in this study. The clinical characteristics at the time of renal biopsy are summarized in Table 1. The mean age was 11.3±3.6 years, the mean eGFR was 124±43 ml/min per 1.73 m2, the mean urinary protein excretion was 1.8±2.8 g/d, and 15 patients (49%) had nephrotic syndrome. We classified these patients into three groups on the basis of birth weight and histopathologic diagnosis. There were no significant differences in age, body mass index, or mean arterial pressure at the time of renal biopsy among the groups. Although there were slightly more boys in the MCNS group, the difference was not statistically significant. The patients with LBW-FSGS had lower eGFR than the other two groups. Of the patients with LBW-FSGS, seven (88%) were preterm births. All patients born with an NBW were full-term birth.
Table 1.
Characteristics of clinical findings at renal biopsy between the patients of low birth weight–FSGS, normal birth weight–FSGS, and normal birth weight–minimal change nephrotic syndrome
| Variables | All | LBW-FSGS | NBW-FSGS | P Value | NBW-MCNS | P Value |
|---|---|---|---|---|---|---|
| Cases, n | 31 | 8 | 10 | 13 | ||
| Boy/girl, n | 16/15 | 3/5 | 3/7 | 0.32 | 10/3 | 0.09 |
| Age, yr | 11.3±3.6 | 11.7±4.2 | 10.2±3.3 | 0.36 | 11.8±3.6 | >0.99 |
| Body mass index, kg/m2 | 19.7±4.3 | 17.3±5.7 | 19.2±3.3 | 0.12 | 82.6±7.6 | 0.37 |
| MAP, mmHg | 82.1±8.0 | 81.5±8.3 | 81.7±9.0 | 0.70 | 82.6±7.6 | 0.50 |
| Serum Cr, mg/dl | 0.6±0.3 | 0.8±0.3 | 0.5±0.4a | 0.02 | 0.5±0.1a | 0.002 |
| eGFR, ml/min per 1.73 m2 | 124±43 | 83±32 | 145±53a | 0.01 | 133±21a | 0.001 |
| Serum albumin, g/dl | 3.9±0.8 | 4.3±0.4 | 3.4±1.1 | 0.12 | 4.0±0.6 | 0.10 |
| Nephrotic syndrome at diagnosis (yes/no), n | 15/16 | 0/8 | 4/6 | 0.09 | 11/2a | <0.001 |
| Urinary protein excretion, g/g Cr | 1.8±2.8 | 2.3±2.3 | 3.0±3.9 | 0.97 | 0.7±1.5a | 0.01 |
| Birth weight, g | 2915 (2395–3255) | 777 (628–1000) | 3110 (2888–3358)a | <0.001 | 3120 (2748–3398)a | <0.001 |
| Gestational age, wk | 39.4 (37.4–40.0) | 25.4 (24.0–27.7) | 40.0 (39.3–40.1)a | <0.001 | 39.6 (39.1–40.0)a | <0.001 |
| Preterm birth/full term birth, cases | 7/24 | 7/1 | 0/10a | <0.001 | 0/13a | <0.001 |
Data are expressed as mean±SD or median with interquartile range (25th–75th percentile). LBW-FSGS, low birth weight–FSGS; NBW-FSGS, normal birth weight–FSGS; NBW-MCNS, normal birth weight–minimal change nephrotic syndrome; MAP, mean arterial pressure; Cr, creatinine.
P<0.05 versus LBW-FSGS.
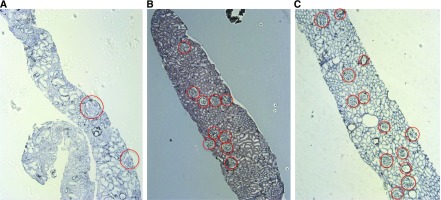
Table 2 shows the histopathologic characteristics of the patients. The morphologic data of each patient are also presented as supplementary data (Supplemental Table 1). There were no significant differences in the chronic histopathologic findings, including focal or global glomerulosclerosis and interstitial fibrosis/tubular atrophy. The GD was lower in patients with LBW-FSGS than in patients with NBW-FSGS and NBW-MCNS. In contrast, the GV was higher in patients with LBW-FSGS than in the other two groups. Representative images of the glomerular distribution in renal biopsy specimens are shown in Figure 1.
Table 2.
Comparison of histopathologic findings at biopsy between the patients of low birth weight–FSGS, normal birth weight–FSGS, and normal birth weight–minimal change nephrotic syndrome
| Variables | All | LBW-FSGS | NBW-FSGS | P Value | NBW-MCNS | P Value |
|---|---|---|---|---|---|---|
| Cases, n | 31 | 8 | 10 | 13 | ||
| Total glomerular number | 24.5±12.3 | 15.9±10.0 | 24.5±5.4a | 0.04 | 29.7±14.9a | 0.04 |
| Total cortical area, mm2 | 8.5±3.4 | 10.1±4.3 | 7.7±2.3 | 0.24 | 8.2±3.4 | 0.24 |
| IF/TA, % | 5.6±11.2 | 11.9±14.7 | 7.5±13.0 | 0.36 | 0.4±11.4 | 0.07 |
| Global glomerular sclerosis, % | 0 (0–0) | 0 (0–1.2) | 1.85 (0–4.5) | 0.46 | 0 (0–0) | 0.37 |
| Focal segmental sclerosis, % | 2.6±5.1 | 2.3±4.4 | 6.2±7.0 | 0.15 | 0 | 0.37 |
| GV (× 106 μm3) | 1.8 (1.4–2.6) | 4.0 (3.0–5.1) | 1.6 (1.5–2.1)a | <0.001 | 1.3 (1.1–1.8)a | <0.001 |
| GD excluding globally sclerotic glomeruli, /mm2 | 3.0±1.4 | 1.4±0.6 | 3.3±1.2a | 0.002 | 3.6±1.1a | <0.001 |
| GD including globally sclerotic glomeruli, /mm2 | 3.0±1.3 | 1.4±0.6 | 3.4±1.1a | 0.001 | 3.6±1.1a | <0.001 |
LBW-FSGS, low birth weight–FSGS; NBW-FSGS, normal birth weight–FSGS; NBW-MCNS, normal birth weight–minimal change nephrotic syndrome; IF/TA, interstitial fibrosis/tubular atrophy; GV, glomerular volume; GD, glomerular density. Data are expressed as mean±SD or median with interquartile range (25th–75th percentile).
P<0.05 versus LBW-FSGS.
Figure 1.
Representative light micrographs of renal biopsy findings. Representative renal biopsy findings in cases of (A) LBW-FSGS (13-year-old boy with an eGFR of 68.4 ml/min per 1.73 m2), (B) NBW-FSGS (8-year-old boy with an eGFR of 170.7 ml/min per 1.73 m2), and (C) NBW-MCNS (14-year-old girl with an eGFR of 123.1 ml/min per 1.73 m2). Periodic acid–methenamine silver stain was used (original magnification, ×50). All glomeruli are indicated with a circle. LBW-FSGS, low birth weight–FSGS; NBW-FSGS, normal birth weight–FSGS; NBW-MCNS, normal birth weight–minimal change nephrotic syndrome.
Relationships among GD, GV, and Gestational Age
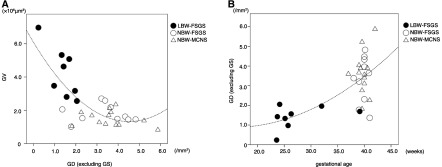
The continuous variables at biopsy were tested for their association with GD in all patients. GD was inversely correlated with GV (Figure 2A). The low GD in patients with LBW-FSGS was closely associated with early gestational age (Figure 2B). Univariate and multivariate regression analyses were performed to evaluate the factors associated with GD excluding globally sclerotic glomeruli (Table 3). The GD showed positive correlations with eGFR, birth weight, and gestational age. Birth weight and gestational age were associated with the GD, independently of renal function and degree of globally sclerotic glomeruli, in a multivariate analysis. Similar results were obtained by using the different definition of GD including globally sclerosed glomeruli in univariate and multivariable analyses (data not shown).
Figure 2.
Relationship between GD and mean GV/gestational age. GD showed a close inverse correlation with mean GV (A). GD showed close correlations with gestational age (B). Closed circle, LBW-FSGS; open circle, NBW-FSGS; open triangle, NBW-MCNS. The distributions are represented by a curve-fitting. GD, glomerular density; GS, globally sclerotic glomeruli; GV, glomerular volume; LBW-FSGS, low birth weight–FSGS; NBW-FSGS, normal birth weight–FSGS; NBW-MCNS, normal birth weight–minimal change nephrotic syndrome.
Table 3.
Factors associated with glomerular density by univariate and multivariate regression analyses at the time of renal biopsy
| Multivariate | ||||||
|---|---|---|---|---|---|---|
| Variables | Univariate | Model 1 | Model 2 | |||
| r | P Value | Difference, /mm2 | P Value | Difference, /mm2 | P Value | |
| eGFR, ml/min per 1.73 m2 | 0.68 | <0.001 | 0.36 | 0.02 | 0.39 | 0.01 |
| Birth weight, g | 0.48 | 0.01 | 0.43 | 0.01 | — | — |
| Gestational age, wk | 0.54 | 0.002 | — | — | 0.46 | 0.003 |
| Global glomerular sclerosis, % | −0.32 | 0.09 | −0.24 | 0.07 | −0.25 | 0.05 |
Model 1: eGFR, birth weight, and global glomerular sclerosis. Model 2: eGFR, gestational age, and global glomerular sclerosis. Because distribution of the birth weight and gestational age were skewed, the log-transformed values were used in the regression analysis.
Discussion
Nephrogenesis begins at week 9 of gestation, and the final number of human nephrons is determined at weeks 34–36. In particular, there is rapid development during the last trimester, and few viable nephrons are formed after preterm birth (22,23). Of the eight patients with LBW analyzed in this study, only one was a full-term birth; the other seven patients were born preterm. These seven preterm patients had a lower GD than most of the patients with NBW born at term. The low GD in the patients was likely established at the time of preterm birth, when they were in an active stage of nephrogenesis. These results are consistent with the recently established baboon model of preterm birth, in which low GD and glomerular enlargement is present in preterm kidneys (24).
The loss of glomeruli with age occurs throughout adult life, with a mean predicted loss of approximately 4500 glomeruli per kidney per year from 18 to 70 years of age. In children, however, aging has little effect on renal histologic findings (25). Generally, pediatric patients have short disease duration and limited chronic, irreversible changes and/or renal compensatory changes compared with adults. Although renal function and degree of globally sclerosed glomeruli were independently associated with GD, the gestational age and the birth weight were also identified as independent factors associated with GD. In addition, similar results were obtained when age at biopsy was included or by using the different definition of GD including globally sclerosed glomeruli in multivariable analyses (data not shown). These results suggest that a low GD in patients with LBW-FSGS does not simply represent loss of functional nephrons after birth. Also, although biopsy specimens from patients with LBW-FSGS contained fewer glomeruli than those from the other two groups, there were no significant differences in cortical area among the groups. These results suggest that the low GD in patients with LBW-FSGS was not simply attributable to biopsy sampling error.
Patients with FSGS can be separated into primary or idiopathic cases, and those with secondary FSGS. The most common type of secondary FSGS is termed adaptive FSGS (26); this is thought to be triggered by structural and functional adaptations mediated by intrarenal vasodilatation, increased glomerular capillary pressure, and an enhanced plasma flow rate (27). In this study, the LBW-FSGS group exhibited the characteristic histopathologic findings and clinical courses that were consistent with secondary (adaptive) FSGS. These cases had relatively mild proteinuria and had been incidentally diagnosed; they did not have nephrotic syndrome. Using the classification of D’Agati et al. (17), perihilar variant was observed in three of eight (38%) patients in the LBW-FSGS group, characterized by perihilar sclerosis in >50% of glomeruli, accompanied by segmental lesions. Also, at least one glomerulus exhibited perihilar hyalinosis. Although such hyalinosis was not detected in the remaining five patients of the LBW-FSGS group, their glomerular findings were consistent with the presence of a perihilar variant of the disease, accompanied by segmental perihilar sclerosis.
Such pathologic changes may be associated not only with a low TNN and LBW, but also with other factors. Recent studies have suggested that such histologic features may be a result of not only the increased single GFR due to the small number of nephrons but also the absolute and/or relative decrease in the number of glomerular podocytes (14). A mismatch between low TNN and increased body size due to catch-up growth may also be applicable to the mismatch between low podocyte number and increased glomerular volume due to a higher single-nephron GFR. The vulnerabilities of glomerular structure in LBW kidneys warrant further investigation.
There were several limitations to this study. First, it is unclear whether the GD in renal biopsy specimens accurately reflects the number of nephrons in the whole kidney, because data on the total cortical volume of the kidney in these patients were not available. Second, the association between GD and long-term prognosis remains unknown because this was a cross-sectional study. Third, because the criteria for exclusion of renal biopsy specimens were not defined (i.e., the number of obtained glomeruli and the degree of globally sclerotic glomeruli), we cannot exclude the influence of sampling error in needle biopsies on the identification and/or diagnosis of FSGS.
In conclusion, we found that a low GD together with marked glomerular enlargement characterizes renal biopsy specimens of children born with a LBW at the early to middle stage of gestation. These results suggest a close relationship between GD in biopsy specimens and the congenitally determined number of nephrons in living patients.
Disclosures
None.
Supplementary Material
Acknowledgments
Portions of this study were presented at the American Society of Nephrology Kidney Week 2014, November 13, 2014, Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Can Renal Biopsy Be Used to Estimate Total Nephron Number?,” on pages 553–555.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05650516/-/DCSupplemental.
References
- 1.Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, Bertram JF: Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney Int 63: 2113–2122, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE: Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int 69: 671–678, 2006 [DOI] [PubMed] [Google Scholar]
- 3.McNamara BJ, Diouf B, Hughson MD, Douglas-Denton RN, Hoy WE, Bertram JF: Renal pathology, glomerular number and volume in a West African urban community. Nephrol Dial Transplant 23: 2576–2585, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoy WE, Bertram JF, Denton RD, Zimanyi M, Samuel T, Hughson MD: Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens 17: 258–265, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Hoy WE, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF: Reduced nephron number and glomerulomegaly in Australian Aborigines: A group at high risk for renal disease and hypertension. Kidney Int 70: 104–110, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Silverwood RJ, Pierce M, Hardy R, Sattar N, Whincup P, Ferro C, Savage C, Kuh D, Nitsch D: Low birth weight, later renal function, and the roles of adulthood blood pressure, diabetes, and obesity in a British birth cohort. Kidney Int 84: 1262–1270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koike K, Tsuboi N, Utsunomiya Y, Kawamura T, Hosoya T: Glomerular density-associated changes in clinicopathological features of minimal change nephrotic syndrome in adults. Am J Nephrol 34: 542–548, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Tsuboi N, Kawamura T, Ishii T, Utsunomiya Y, Hosoya T: Changes in the glomerular density and size in serial renal biopsies during the progression of IgA nephropathy. Nephrol Dial Transplant 24: 892–899, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Tsuboi N, Kawamura T, Koike K, Okonogi H, Hirano K, Hamaguchi A, Miyazaki Y, Ogura M, Joh K, Utsunomiya Y, Hosoya T: Glomerular density in renal biopsy specimens predicts the long-term prognosis of IgA nephropathy. Clin J Am Soc Nephrol 5: 39–44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuboi N, Kawamura T, Miyazaki Y, Utsunomiya Y, Hosoya T: Low glomerular density is a risk factor for progression in idiopathic membranous nephropathy. Nephrol Dial Transplant 26: 3555–3560, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Elsherbiny HE, Alexander MP, Kremers WK, Park WD, Poggio ED, Prieto M, Lieske JC, Rule AD: Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol 9: 1892–1902, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zidar N, Cör A, Premru Srsen T, Stajer D: Is there an association between glomerular density and birth weight in healthy humans. Nephron 80: 97–98, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Hodgin JB, Rasoulpour M, Markowitz GS, D’Agati VD: Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 4: 71–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikezumi Y, Suzuki T, Karasawa T, Yamada T, Hasegawa H, Nishimura H, Uchiyama M: Low birthweight and premature birth are risk factors for podocytopenia and focal segmental glomerulosclerosis. Am J Nephrol 38: 149–157, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Work DF: Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4: 1832–1843, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Ishikura K, Matsumoto S, Sako M, Tsuruga K, Nakanishi K, Kamei K, Saito H, Fujinaga S, Hamasaki Y, Chikamoto H, Ohtsuka Y, Komatsu Y, Ohta T, Nagai T, Kaito H, Kondo S, Ikezumi Y, Tanaka S, Kaku Y, Iijima K; Japanese Society for Pediatric Nephrology, Japanese Society for Pediatric Nephrology : Clinical practice guideline for pediatric idiopathic nephrotic syndrome 2013: Medical therapy. Clin Exp Nephrol 19: 6–33, 2015 [DOI] [PubMed] [Google Scholar]
- 17.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Weibel ER: Sterological Method: Practical Methods of Biological Morphometry, Vol. 1, London, Academic Press, 1979, pp 44–45, 131–134 [Google Scholar]
- 20.Fulladosa X, Moreso F, Narváez JA, Grinyó JM, Serón D: Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol 14: 2662–2668, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Hughson MD, Samuel T, Hoy WE, Bertram JF: Glomerular volume and clinicopathologic features related to disease severity in renal biopsies of African Americans and whites in the southeastern United States. Arch Pathol Lab Med 131: 1665–1672, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez MM, Gómez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE: Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 7: 17–25, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, Hoy WE, Bertram JF, Black MJ: Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol 22: 1365–1374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ: Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am J Physiol Renal Physiol 297: F1668–F1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyengaard JR, Bendtsen TF: Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992 [DOI] [PubMed] [Google Scholar]
- 26.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Rennke HG, Klein PS: Pathogenesis and significance of nonprimary focal and segmental glomerulosclerosis. Am J Kidney Dis 13: 443–456, 1989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.