Abstract
Background and objectives
Hyperphosphatemia in kidney transplant recipients has been shown to predict poorer graft and patient survival. However, studies examining hypophosphatemia are scarce.
Design, setting, participants, & measurements
To evaluate the association of serum phosphorus level with patient and graft survival, we performed a retrospective multicenter cohort study. Between January of 1997 and August of 2012, 2786 kidney transplant recipients (41.7±11.4 years; 59.3% men; 73.5% living donors; 26.1% with diabetes; 3.8% with prior history of cardiovascular disease) were classified into seven groups according to serum phosphorus levels 1 year after transplantation, with intervals of 0.5 mg/dl (lowest group, <2.5 mg/dl; highest group, ≥5.0 mg/dl; reference group, 3.5–3.99 mg/dl). Survival analysis was performed by defining baseline time point as 1 year after transplantation.
Results
During median follow-up of 78.5 months, 60 patient deaths and 194 cases of graft loss occurred. In multivariate analysis, both lowest and highest serum phosphorus groups were associated with higher mortality, compared with the reference group (hazard ratio [HR], 4.82; 95% confidence interval [95% CI], 1.36 to 17.02; P=0.01; and HR, 4.24; 95% CI, 1.07 to 16.84; P=0.04, respectively). Higher death-censored graft loss was observed in the lowest and highest groups (HR, 3.32; 95% CI, 1.42 to 7.79; P=0.01; and HR, 2.93; 95% CI, 1.32 to 6.49; P=0.01, respectively), despite eGFR exhibiting no difference between the lowest group and reference group (65.4±19.3 versus 61.9±16.7 ml/min per 1.73 m2; P=0.33). Moreover, serum phosphorus showed a U-shape association with patient mortality and graft failure in restricted cubic spline curve analysis.
Conclusions
Serum phosphorus level 1 year after transplantation exhibits a U-shape association with death-censored graft failure and patient mortality in kidney transplant patients characterized by relatively high rate of living donor transplant and low incidence of diabetes and prior cardiovascular disease compared with Western countries.
Keywords: mortality; graft survival; phosphorus; cardiovascular diseases; confidence intervals; diabetes mellitus; follow-up studies; glomerular filtration rate; graft survival; humans; hyperphosphatemia; hypophosphatemia; incidence; kidney; kidney transplantation; living donors; male; multivariate analysis; phosphorus; phosphorus, dietary; proportional hazards models; retrospective studies; survival analysis
Introduction
Among patients with ESRD, loss of renal function is often accompanied by disturbances in mineral metabolism, including decreased levels of serum calcium and elevated serum phosphorus, in addition to increased parathyroid hormone (PTH) resulting from secondary hyperparathyroidism (1). In several observational studies, biochemical parameters have been reported to be associated with poor clinical outcomes, such as mortality and cardiovascular disease (CVD), among patients with ESRD requiring dialysis (2–6). In a systematic review published in 2011, Palmer et al. (7) identified 47 cohort studies and described a linear relationship between high serum phosphorus and all-cause mortality in individuals with CKD. In contrast, some studies have demonstrated U-shape or J-shape (nonlinear) associations between serum phosphorus levels and mortality risk in patients with CKD (5,6,8–11). A number of recent studies have indicated that serum phosphorus levels are independently associated with CKD progression and kidney failure in nondialysis-dependent patients with CKD (12,13). Even in individuals with normal renal function, there is evidence that higher serum phosphorus levels are associated with an increased risk of CVD and mortality (14–16).
Hypophosphatemia and renal phosphorus wasting are common during several months after renal transplantation, and are associated with inappropriately high PTH and fibroblast growth factor-23 (FGF-23) levels. Renal phosphorus wasting is known to regress by 1 year after successful kidney transplantation (17). In addition, hyperphosphatemia or hypophosphatemia often persist at 1 year after renal transplantation as the result of deterioration of allograft function, or persistent hyper- or hypoparathyroidism. Studies concerned with the effect of hyper- or hypophosphatemia on graft and patient survival in kidney transplant recipients (KTRs) are scarce (18–20). Thus, this multicenter cohort study aimed to evaluate the relationship between post-transplant serum phosphorus levels and graft failure or patient mortality in KTRs.
Materials and Methods
Study Population and Data Collection
This study selected a total of 2786 KTRs with recorded serum phosphorus levels at 1 year after renal transplantation from Seoul National University Hospital, Boramae Medical Center, and Asan Medical Center, between January of 1997 and August of 2012. A total of 176 pediatric renal transplant patients and 111 patients with mortality or graft failure at <1 year after transplantation were excluded. Demographic and laboratory data were retrospectively reviewed from medical records. We obtained the laboratory data at 1 year after transplantation, because parameters of mineral metabolism are known to stabilize by 1 year after transplantation. The serum calcium level was adjusted for serum albumin, according to the following equation: corrected calcium = measured calcium + 0.8 × (4.0 − serum albumin in grams per deciliter). The serum phosphorus level was measured by reaction of inorganic phosphorus with ammonium molybdate under acidic conditions to produce the phosphomolybdate complex, which was quantified by spectrophotometry. The eGFR was calculated by the Modification of Diet in Renal Disease equation (21).
The primary outcome was overall mortality, and the secondary outcome was death-censored graft failure, which was defined as requirement for dialysis or a renal retransplantation. Death with a functioning graft (DWFG) was also evaluated as an outcome to exclude deaths which occurred after graft failures, because many uncontrolled factors could affect the mortality after graft failure. The cause of mortality was determined with an International Classification of Disease 10 code on the basis of the diagnosis of the patient at the time of discharge through medical records. Mortality data of some patients who were lost to follow-up were obtained from the Statistics Korea database. This study was approved by the institutional review board of each participating hospital, and was conducted in accordance with the 2008 Declaration of Istanbul and the 2000 Declaration of Helsinki.
Statistical Analyses
The study population was classified into seven groups according to serum phosphorus at 1 year after transplantation, with intervals of 0.5 mg/dl (lowest group, <2.5 mg/dl; highest group, ≥5.0 mg/dl; reference group, 3.5–3.99 mg/dl). To compare clinical and laboratory parameters among the seven groups, the chi-squared test and one-way ANOVA test were used for categoric and continuous variables, respectively. To investigate the association of serum phosphorus level with patient and graft survival, Kaplan–Meier survival curves, stratified according to serum phosphorus, were compared with log-rank tests. Cox proportional hazard models adjusted with the age and sex of recipient; donor type; age of donor; diabetes mellitus; body mass index; acute rejection; and eGFR, serum albumin, hemoglobin, and total cholesterol levels at 1 year after transplantation, were used for multivariate survival analyses. To better represent the shape of the association between the levels of serum phosphorus and mortality or graft failure, serum phosphorus levels as a continuous variable and hazard ratios were modeled using restricted cubic spline curves. All statistical analyses were conducted using the SPSS version 18.0.0 (SPSS Inc., Chicago, IL) and the software package R version 3.2.1 (www.r-project.org; The R Foundation for Statistical Computing, Vienna, Austria). A P value of <0.05 was considered statistically significant.
Results
Baseline Characteristics and Clinical Parameters of the Study Population
Of 2786 KTRs in this study, the mean age was 41.7±11.4 (range, 18–73) years, and 59.3% of the recipients were men. Among KTRs, 84.2% had hypertension and 26.1% had diabetes mellitus, including new onset diabetes after transplantation. Additionally, 73.5% of the KTRs underwent living donor transplantation. The median duration of dialysis before transplantation was 17.9 months (25th–75th percentile, 4.6–48.5 months), and 10.1% of the patients underwent pre-emptive renal transplantation. The most common cause of ESRD was GN (20.9%), followed by diabetes mellitus (13.4%), and hypertension (7.3%). The median follow-up duration after transplantation was 78.5 months (range, 12.3–198.5 months), and 21.6% of the KTRs experienced biopsy-proven acute rejection episodes. A total of 60 patient deaths and 194 cases of graft loss occurred in the total 20,688 person-years of follow-up. A triple-drug immunosuppressive regimen, including a calcineurin inhibitor, an antimetabolic drug, and a steroid, was administered to most KTRs. At 1 year after kidney transplantation, the mean concentration of serum creatinine was 1.28±0.76 mg/dl, and the mean eGFR was 62.1±17.1 ml/min per 1.73 m2.
When comparing the age of recipients, sex of donors, human leukocyte antigen mismatches, acute rejection episodes, and history of CVD, no statistically significant differences were observed among the seven groups. However, the sex of recipients, age of donors, body mass index, diabetes mellitus including new onset diabetes after transplantation, duration of dialysis before transplantation, type of donors, and use of calcineurin inhibitors exhibited statistical differences among the seven groups. The eGFR at 1 year after transplantation was lower in the highest serum phosphorus group than other groups, and the concentrations of serum calcium and intact PTH were higher in the lowest serum phosphorus group. Clinical characteristics and laboratory parameters 1 year after kidney transplantation for each group, according to serum phosphorus levels, are summarized in Table 1.
Table 1.
Clinical characteristics and laboratory parameters at 1 yr after kidney transplantation in the study population according to the serum phosphorus levels at 1 yr after transplantation
| Parameters | Serum Phosphorus Levels at 1 yr after Transplantation | P Valuea | ||||||
|---|---|---|---|---|---|---|---|---|
| <2.5 mg/dl (n=115) | 2.5–2.99 mg/dl (n=351) | 3.0–3.49 mg/dl (n=690) | 3.5–3.99 mg/dl (n=848) | 4.0–4.49 mg/dl (n=523) | 4.5–4.99 mg/dl (n=190) | ≥5.0 mg/dl (n=69) | ||
| Age, yr | 43.4±9.7 | 41.6±10.7 | 41.5±11.1 | 41.5±11.6 | 42.2±11.8 | 42.4±12.2 | 39.0±12.1 | 0.20 |
| Men, % | 70.4% | 65.5% | 62.2% | 58.0% | 53.7% | 54.2% | 50.7% | <0.001 |
| BMI, kg/m2 | 23.1±3.1 | 23.0±3.4 | 22.5±3.1 | 22.0±3.0 | 22.1±3.1 | 21.7±3.1 | 22.0±3.0 | <0.001 |
| Diabetes, %b | 24.3% | 23.7% | 24.3% | 26.9% | 27.9% | 31.1% | 23.2% | 0.03 |
| Dialysis duration, moc | 56.6 (17.8, 87.4) | 24.5 (6.3, 70.3) | 20.0 (5.0, 50.7) | 15.3 (4.3, 42.5) | 14.7 (3.0, 36.1) | 19.0 (3.9, 52.6) | 21.2 (5.6, 40.9) | <0.001 |
| Living donor, % | 53.6% | 71.3% | 71.5% | 80.8% | 75.7% | 75.8% | 66.2% | <0.001 |
| HLA mismatch, n | 3.0±1.8 | 3.2±1.7 | 3.1±1.7 | 3.1±1.7 | 3.0±1.7 | 3.1±1.7 | 3.3±1.7 | 0.45 |
| Tacrolimus % | 53.9% | 48.4% | 41.9% | 37.5% | 37.7% | 32.1% | 47.8% | <0.001 |
| Cyclosporin, % | 15.7% | 28.2% | 34.8% | 45.3% | 49.1% | 56.8% | 42.0% | |
| Acute rejection, % | 15.7% | 21.1% | 22.9% | 19.3% | 23.9% | 22.6% | 30.4% | 0.10 |
| BKVN, % | 2.6% | 1.1% | 2.0% | 2.1% | 1.7% | 2.6% | 2.9% | 0.87 |
| Donor age, yr | 39.6±11.7 | 40.4±11.4 | 38.7±12.0 | 39.6±12.4 | 38.6±12.8 | 38.5±11.8 | 34.0±12.5 | 0.003 |
| Male donor sex, % | 63.4% | 58.6% | 58.8% | 54.5% | 57.5% | 61.8% | 50.8% | 0.29 |
| eGFR, ml/min per 1.73 m2 | 65.4±19.3 | 65.1±17.8 | 63.1±15.6 | 61.9±16.7 | 61.5±15.8 | 59.5±17.4 | 47.5±25.7d | <0.001 |
| Creatinine, mg/dl | 1.22±0.42 | 1.21±0.36 | 1.22±0.33 | 1.25±0.46 | 1.24±0.47 | 1.31±0.62 | 2.97±3.70d | <0.001 |
| Ca, mg/dl | 10.0±0.9d | 9.6±0.8 | 9.5±0.6 | 9.3±0.5 | 9.3±0.5 | 9.3±0.5 | 9.2±0.5 | <0.001 |
| Corrected Cae | 10.1±0.9d | 9.7±0.8 | 9.5±0.6 | 9.4±0.5 | 9.4±0.5 | 9.4±0.5 | 9.4±0.6 | <0.001 |
| Ca-P product | 21.0±3.9d | 26.3±2.4d | 30.4±2.1d | 34.5±2.2d | 38.9±2.3d | 43.0±2.6d | 49.9±5.6d | <0.001 |
| Albumin, g/dl | 4.0±0.5 | 4.1±0.4 | 4.1±0.4 | 4.1±0.3 | 4.1±0.4 | 4.1±0.4 | 3.9±0.6 | <0.001 |
| Hemoglobin, g/dl | 13.5±2.3 | 13.7±1.9 | 13.6±2.0 | 13.3±1.9 | 13.1±1.8 | 12.8±1.8 | 12.2±2.3 | <0.001 |
| T.Chol, mg/dl | 175.6±32.9 | 173.5±32.7 | 179.3±32.0 | 180.5±32.8 | 182.5±33.7 | 182.9±36.0 | 174.7±41.1 | 0.002 |
| iPTH, ng/pg | 246.3±211.9d | 159.1±203.7 | 68.8±56.3 | 64.0±46.9 | 56.5±63.7 | 65.0±68.0 | 76.3±129.6 | <0.001 |
Most continuous values are expressed as the mean±SD, unless stipulated otherwise. BMI, body mass index; HLA, human leukocyte antigen; BKVN, BK virus nephropathy; Ca, calcium; Ca-P, calcium-phosphorus; T.Chol, total cholesterol; iPTH, intact parathyroid hormone.
The chi-squared test was used for categoric variables, whereas continuous variables were compared using one-way ANOVA, as appropriate.
Diabetes mellitus including new onset diabetes after transplantation.
Dialysis duration is expressed as median (25th, 75th percentile).
Post hoc analysis indicated that this group differed significantly from all other groups.
Corrected calcium level was calculated according to the following equation: corrected calcium = measured calcium + 0.8 × (4.0 − serum albumin in g/dl).
Association between Serum Phosphorus and Overall Mortality
Categoric Analysis.
Overall patient survival was 98.8% at 5 years, and 96.8% at 10 years. Causes of death included sudden cardiac death, septic shock, bacterial pneumonia, Pneumocystis jirovecii pneumonia, aspergillosis, cytomegalovirus pneumonitis, cerebral infarction and hemorrhage, fungal meningitis, fulminant hepatitis, bowel perforation, and malignancy, such as stomach cancer, renal cell carcinoma, post-transplant lymphoproliferative disorder, lymphoma, and common bile duct cancer. KTRs in the lowest group and the highest group had significantly higher rates of overall mortality than those in the reference group (P=0.04, log-rank test) (Figure 1A). In the multivariate Cox regression model, adjusted for multiple covariates, both the lowest and highest groups were significantly associated with patient mortality, when compared with the reference group (hazard ratio [HR], 4.82; 95% confidence interval [95% CI], 1.36 to 17.02; P=0.01; and HR, 4.24; 95% CI, 1.07 to 16.84; P=0.04, respectively). Other groups exhibited a tendency for higher mortality compared with the reference group, however, this tendency was not statistically significant (Figure 2D, Table 2). Additionally, older age (HR, 1.06; 95% CI, 1.03 to 1.10; P<0.001) and lower serum albumin (HR, 0.40; 95% CI, 0.23 to 0.70; P=0.001) were significantly correlated with higher mortality among KTRs.
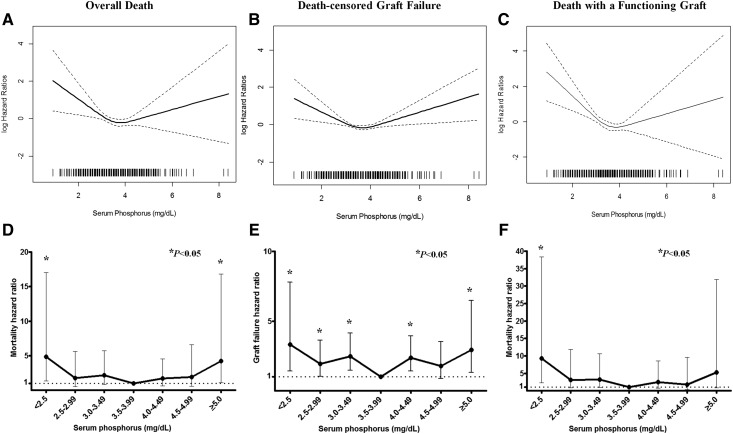
Figure 1.
Serum phosphorus concentration is significantly associated with mortality and death-censored graft failure among kidney transplant recipients in survival analyses. Kaplan–Meier survival curves showing the association between serum phosphorus levels at 1 year after transplantation and overall death, death-censored graft failure, or death with a functioning graft. Categoric analyses, as stratified by the level of serum phosphorus, are shown with Kaplan–Meier survival curves: (A) overall death, (B) death-censored graft failure, and (C) death with a functioning graft (only the reference, lowest, and highest groups are shown).
Figure 2.
Serum phosphorus levels have U-shape relationship with both mortality and death-censored graft failure among kidney transplant recipients. Association between serum phosphorus levels at 1 year after transplantation and overall death, death-censored graft failure, or death with a functioning graft. (A–C) Prediction of adjusted hazard ratios according to the continuous values of serum phosphorus was demonstrated with a restricted cubic spline curve in continuous analyses. (D–F) Hazard ratios and 95% confidence intervals from categoric analyses are shown. Cox proportional hazard model, adjusted with recipient age and sex, donor type, donor age, diabetes mellitus, body mass index, acute rejection, and eGFR, serum albumin, hemoglobin, and total cholesterol levels at 1 year after transplantation.
Table 2.
Association of serum phosphorus levels at 1 yr after renal transplantation and covariates with overall mortality
| Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Valuea | |
| Serum phosphorus | ||||
| <2.5 mg/dl (lowest) | 4.48 (1.42 to 14.12) | 0.01 | 4.82 (1.36 to 17.02) | 0.01 |
| 2.5–2.99 mg/dl | 1.49 (0.52 to 4.28) | 0.47 | 1.75 (0.54 to 5.62) | 0.35 |
| 3.0–3.49 mg/dl | 2.04 (0.95 to 4.39) | 0.07 | 2.19 (0.84 to 5.72) | 0.11 |
| 3.5–3.99 mg/dl | 1.0 (reference) | 0.16 | 1.0 (reference) | 0.29 |
| 4.0–4.49 mg/d | 1.77 (0.79 to 3.94) | 0.15 | 1.70 (0.64 to 4.53) | 0.31 |
| 4.5–4.99 mg/dl | 2.08 (0.77 to 5.63) | 0.004 | 1.90 (0.55 to 6.59) | 0.04 |
| ≥5.0 mg/dl (highest) | 4.24 (1.07 to 16.84) | 4.24 (1.07 to 16.84) | ||
| Age (per 1 yr) | 1.07 (1.05 to 1.10) | <0.001 | 1.06 (1.03 to 1.10) | <0.001 |
| Men (versus women) | 1.08 (0.64 to 1.81) | 0.78 | 1.32 (0.69 to 2.54) | 0.40 |
| Living donor (versus deceased) | 0.42 (0.25 to 0.69) | <0.001 | 0.62 (0.33 to 1.16) | 0.13 |
| Donor age (per 1 yr) | 1.02 (0.99 to 1.04) | 0.12 | 1.01 (0.99 to 1.04) | 0.31 |
| Diabetes (versus none) | 2.03 (1.21 to 3.42) | 0.01 | 1.07 (0.56 to 2.03) | 0.84 |
| eGFR (per 1 ml/min per 1.73 m2) | 0.98 (0.96 to 0.99) | 0.01 | 1.00 (0.98 to 1.02) | 0.97 |
| Albumin (per 1 g/dl) | 0.25 (0.17 to 0.35) | <0.001 | 0.40 (0.23 to 0.70) | 0.001 |
| BMI (per 1 kg/m2) | 1.04 (0.95 to 1.13) | 0.37 | 0.97 (0.87 to 1.07) | 0.54 |
| Hemoglobin (per 1 g/dl) | 0.81 (0.71 to 0.91) | <0.001 | 0.90 (0.76 to 1.07) | 0.24 |
| T.Chol (per 1 mg/dl) | 0.99 (0.99 to 1.01) | 0.92 | 1.00 (0.99 to 1.01) | 0.38 |
| Acute rejection (versus none) | 2.11 (1.26 to 3.53) | 0.004 | 1.62 (0.87 to 3.01) | 0.13 |
The study population was classified into seven groups according to the level of serum phosphorus at 1 yr after transplantation with intervals of 0.5 mg/dl (lowest group, <2.5 mg/dl; highest group, ≥5.0 mg/dl; reference group, 3.5–3.99 mg/dl). HR, hazard ratio; 95% CI, 95% confidence interval; BMI, body mass index; T.Chol, total cholesterol.
Multivariate Cox proportional hazard ratios, adjusted for recipient age and sex; donor type; donor age; diabetes mellitus; BMI; acute rejection; and eGFR, serum albumin, hemoglobin, and total cholesterol levels at 1 yr after transplantation.
Continuous Analysis.
Figure 2A shows the association of serum phosphorus as a continuous variable with overall mortality, as a restricted cubic spline curve. A bidirectional or U-shape association between serum phosphorus concentration and overall mortality was observed in the multivariate Cox model, as shown in the categoric analysis.
Association between Serum Phosphorus and Death-Censored Graft Failure
Categoric Analysis.
A total of 194 patients experienced allograft failure during the follow-up period. The overall graft survival rate was 96.3% at 5 years, and 89.8% at 10 years. KTRs in the lowest and highest groups exhibited a significantly greater rate of death-censored graft failure than those in the reference group (P<0.001, log-rank test) (Figure 1B). In the multivariate Cox regression model, both the lowest and highest groups were significantly associated with death-censored graft failure, compared with the reference group (HR, 3.32; 95% CI, 1.42 to 7.79; P=0.01; and HR, 2.93; 95% CI, 1.32 to 6.49; P=0.01, respectively). Other groups also showed significant increases in death-censored graft failure risk when compared with the reference group (Figure 2E, Table 3). The rate of death-censored graft failure was higher in the lowest group than in the reference group, even though the eGFR did not differ between these groups (65.4±19.3 versus 61.9±16.7 ml/min per 1.73 m2; P=0.33). Additionally, acute rejection (HR, 3.67; 95% CI, 2.62 to 5.15; P<0.001) was a significant risk factor for death-censored graft failure, whereas a higher eGFR at 1 year after transplantation (HR, 0.98; 95% CI, 0.97 to 0.99; P<0.001) was protective.
Table 3.
Association of serum phosphorus levels at 1 yr after renal transplantation and covariates with death-censored graft failure
| Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Valuea | |
| Serum phosphorus | ||||
| <2.5 mg/dl (lowest) | 2.70 (1.20 to 6.08) | 0.02 | 3.32 (1.42 to 7.79) | 0.01 |
| 2.5–2.99 mg/dl | 2.07 (1.21 to 3.56) | 0.01 | 1.93 (1.03 to 3.62) | 0.04 |
| 3.0–3.49 mg/dl | 1.92 (1.24 to 2.96) | 0.003 | 2.47 (1.47 to 4.15) | <0.001 |
| 3.5–3.99 mg/dl | 1.0 (reference) | <0.001 | 1.0 (reference) | <0.001 |
| 4.0–4.49 mg/dl | 2.09 (1.36 to 3.22) | 0.004 | 2.37 (1.42 to 3.94) | 0.10 |
| 4.5–4.99 mg/dl | 2.24 (1.30 to 3.87) | <0.001 | 1.77 (0.89 to 3.52) | 0.01 |
| ≥ 5.0 mg/dl (highest) | 4.74 (2.60 to 8.67) | 2.93 (1.32 to 6.49) | ||
| Age (per 1 yr) | 1.01 (0.99 to 1.02) | 0.24 | 0.99 (0.98 to 1.01) | 0.38 |
| Men (versus women) | 1.19 (0.89 to 1.60) | 0.25 | 1.87 (1.28 to 2.71) | 0.001 |
| Living donor (versus deceased) | 0.59 (0.44 to 0.79) | <0.001 | 0.76 (0.52 to 1.09) | 0.13 |
| Donor age (per 1 yr) | 1.01 (1.00 to 1.02) | 0.05 | 1.01 (0.99 to 1.02) | 0.31 |
| Diabetes (versus none) | 1.29 (0.94 to 1.77) | 0.11 | 1.24 (0.86 to 1.79) | 0.26 |
| eGFR (per 1 ml/min per 1.73 m2) | 0.97 (0.96 to 0.98) | <0.001 | 0.98 (0.97 to 0.99) | <0.001 |
| Albumin (per 1 g/dl) | 0.30 (0.23 to 0.41) | <0.001 | 0.29 (0.20 to 0.43) | <0.001 |
| BMI (per 1 kg/m2) | 1.03 (0.98 to 1.08) | 0.31 | 1.00 (0.95 to 1.06) | 0.96 |
| Hemoglobin (per 1 g/dl) | 0.82 (0.77 to 0.88) | <0.001 | 0.91 (0.83 to 1.01) | 0.06 |
| T.Chol (per 1 mg/dl) | 1.00 (0.99 to 1.01) | 0.69 | 1.01 (1.00 to 1.01) | 0.002 |
| Acute rejection (versus none) | 4.57 (3.44 to 6.08) | <0.001 | 3.67 (2.62 to 5.15) | <0.001 |
The study population was classified into seven groups according to the level of serum phosphorus at 1 yr after transplantation with intervals of 0.5 mg/dl (lowest group, <2.5 mg/dl; highest group, ≥5.0 mg/dl; reference group, 3.5–3.99 mg/dl). HR, hazard ratio; 95% CI, 95% confidence interval; BMI, body mass index; T.Chol, total cholesterol.
Multivariate Cox proportional hazard ratios, adjusted for recipient age and sex; donor type; donor age; diabetes mellitus; BMI; acute rejection; and eGFR, serum albumin, hemoglobin, and total cholesterol levels at 1 year after transplantation.
Continuous Analysis.
Figure 2B represents the association of serum phosphorus concentrations as a continuous variable with death-censored graft failure, as a restricted cubic spline curve. The graph shows a bidirectional or U-shape association between serum phosphorus level and death-censored graft failure in the multivariate Cox model, as shown in the categoric analysis.
Association between Serum Phosphorus and DWFG
Categoric Analysis.
The rate of DWFG in the lowest group was significantly higher than the reference group (P=0.001, log-rank test) (Figure 1C). However, the rate of DWFG in the highest group had a tendency to be higher than the reference group, and had no statistical significance. In the multivariate Cox regression model, adjusted for multiple covariates, the lowest group was significantly associated with DWFG compared with the reference group (HR, 9.32; 95% CI, 2.26 to 38.34; P=0.002). Other groups exhibited a tendency for higher risk of DWFG compared with the reference group, however, this tendency was not statistically significant (Figure 2F, Supplemental Table 1). Additionally, older age (HR, 1.07; 95% CI, 1.03 to 1.10; P<0.001) was significantly correlated with higher rates of DWFG among KTRs.
Table 4 showed the cause of death of our study population according to the serum phosphorus levels. In the highest group, cardiovascular overall mortality was significantly higher than other groups (P=0.04). On the other hand, infectious causes of DWFG were significantly higher in the lowest group than other groups (P=0.01). Other causes of death such as malignancy and others were not different among phosphorus groups in both overall mortality and DWFG.
Table 4.
Distribution of causes of death according to the serum phosphorus levels at 1 yr after transplantation
| Cause of death | Serum Phosphorus Levels at 1 yr after Transplantation | P Valuea | ||||||
|---|---|---|---|---|---|---|---|---|
| <2.5 mg/dl (n=115) | 2.5–2.99 mg/dl (n=351) | 3.0–3.49 mg/dl (n=690) | 3.5–3.99 mg/dl (n=848) | 4.0–4.49 mg/dl (n=523) | 4.5–4.99 mg/dl (n=190) | ≥5.0 mg/dl (n=69) | ||
| Overall death | ||||||||
| Cardiovascular, n (%) | 1 (0.9) | 4 (1.1) | 10 (1.4) | 5 (0.6) | 4 (0.8) | 5 (2.6) | 3 (4.3) | 0.04 |
| Infection, n (%) | 3 (2.6) | 0 (0) | 4 (0.6) | 4 (0.5) | 6 (1.1) | 1 (0.5) | 1 (1.4) | 0.07 |
| Malignancy + others, n (%) | 0 (0) | 1 (0.3) | 2 (0.3) | 2 (0.2) | 3 (0.6) | 0 (0) | 1 (1.4) | 0.55 |
| Death with a functioning graft | ||||||||
| Cardiovascular, n (%) | 1 (0.9) | 4 (1.1) | 8 (1.2) | 3 (0.4) | 2 (0.4) | 3 (1.6) | 2 (2.9) | 0.13 |
| Infection, n (%) | 3 (2.6) | 0 (0) | 4 (0.6) | 2 (0.2) | 3 (0.6) | 0 (0) | 0 (0) | 0.01 |
| Malignancy + others, n (%) | 0 (0) | 1 (0.3) | 1 (0.1) | 2 (0.2) | 3 (0.6) | 0 (0) | 1 (1.4) | 0.40 |
The chi-squared test was used for categoric variables.
Continuous Analysis.
Figure 2C shows the association of serum phosphorus as a continuous variable with DWFG, as a restricted cubic spline curve. A bidirectional or U-shape association between serum phosphorus concentration and DWFG risk was observed in the multivariate Cox model, as shown in the categoric analysis.
Association of Corrected Calcium, Calcium-Phosphorus Product, and Serum Albumin with Outcomes
Using the restricted cubic spline curve, the association of corrected calcium, calcium-phosphorus product, and serum albumin with overall mortality, DWFG, and death-censored graft failure were analyzed (Supplemental Figure 1, A–I). Although the concentrations of corrected calcium were not associated with mortality or death-censored graft failure (Supplemental Figure 1, A–C), the levels of calcium-phosphorus product showed a significant U-shape association with overall mortality, DWFG, and death-censored graft failure (Supplemental Figure 1, D–F), similar to that observed with serum phosphorus. Supplemental Figure 1, G–I shows the linear relationship observed between serum albumin levels and patient and graft survival; greater levels of serum albumin were associated with better patient and graft survival.
Discussion
In this study, we have demonstrated that serum phosphorus levels at 1 year after renal transplantation have a bidirectional association with long-term outcomes in KTRs, with both categoric and continuous analyses. To our knowledge, this is the first study that has identified a U-shape relationship between levels of serum phosphorus and both mortality and death-censored graft failure in KTRs. These associations persisted after adjustment for multiple covariates. Several previous studies have reported that a high serum phosphorus level predicts poorer graft and patient survival; however, no study has previously shown the association between hypophosphatemia and patient outcomes (18–20). Furthermore, the findings in this study are in contrast to those observed in a number of studies previously, where no associations between serum phosphorus and patient outcomes were found (22,23). There is a possibility that the U-shape association observed herein might reduce the statistical power of the negative results of previous studies.
It has been reported that hyperphosphatemia is independently correlated with higher cardiovascular and all-cause mortality among nondialysis patients with CKD as well as dialysis-dependent patients with ESRD (3,12,24,25). Suggested mechanisms by which hyperphosphatemia could increase the risk of death include vascular calcification, such as aortic and coronary artery calcification (16,26–28), and endothelial dysfunction (29). Giachelli et al. (30) demonstrated that hyperphosphatemic conditions may stimulate vascular smooth muscle cells to express osteogenic markers and a predisposition to calcification in their in vitro study. Because risk factors for atherosclerosis, such as diabetes, hypertension, dyslipidemia, and microinflammation, are coincident with CKD (31), hyperphosphatemia may have a synergistic effect on vascular calcification. Higher cardiovascular mortality rate of the highest phosphorus group in our study can support these findings. In addition, hyperphosphatemia could reflect elevated levels of FGF-23, which is a hormone produced by osteocytes, and is responsible for regulating the metabolism of vitamin D and phosphorus (32). Wolf et al. (32) demonstrated that increased levels of FGF-23 are independently associated with a higher risk of progression of CKD in a population of predialysis patients with CKD and KTRs. FGF-23 is also known to be independently associated with left ventricular hypertrophy, left ventricular mass index, development of cardiovascular events, incident heart failure, and all-cause mortality in patients with CKD in previous studies (32–36).
This study indicated that hyperphosphatemia was associated with higher mortality and graft failure in KTRs. KTRs frequently have reduced renal function and complications of CKD, including mineral and bone disorders similar to native kidney disease. A decline in kidney function leads to phosphorus retention, which may facilitate the progression of vascular calcification and endothelial dysfunction via aforementioned mechanisms. Moreover, KTRs may have pre-existing vascular calcification formed before transplantation, which could establish a vulnerability to cardiovascular events and death. In our study, eGFR of the highest serum phosphorus group was significantly lower than other groups, and associated with higher mortality. However, the mechanism underlying the association between hyperphosphatemia and death-censored graft loss is unknown. In a uninephrectomized rat model, Haut et al. (37) reported that higher dietary phosphate was correlated with greater calcium and phosphate deposition and histologic changes in the renal tubules and interstitium. They hypothesized that high phosphate levels act as a direct tubular toxin, or that nephrotoxicity is the product of promoting the precipitation of calcium-phosphate products within the renal tubules. In keeping with the association between serum phosphorus levels and CKD progression (12,13), graft dysfunction and failure in KTRs could be associated with hyperphosphatemia. In contrast, hyperphosphatemia might be merely a consequence of decline in renal function in the deteriorating allograft; however, the association between hyperphosphatemia and death-censored graft failure persisted after adjustment for eGFR at 1 year after kidney transplantation in this study.
This study also revealed that hypophosphatemia was associated with higher mortality and graft failure in KTRs. Immediately after kidney transplantation, hypophosphatemia was frequently observed as the result of hyperphosphatoninism, i.e., urinary phosphorus wasting. Hyperphosphatoninism and hypophosphatemia may persist for up to 1 year after renal transplantation, and are known to be associated with increased levels of serum calcium and PTH (17). However, a mechanism explaining the association between hypophosphatemia and patient outcomes remains unknown. Association between hypophosphatemia and higher risk of death could be an indicator of poor nutritional status; that is, a relationship may be reflective of malnutrition-inflammation-cachexia syndrome. A higher rate of infectious causes of DWFG in the lowest phosphorus group in our study can support this hypothesis. Poor nutritional status could contribute to decreased defense against opportunistic infections and increased infectious death. In addition, persistent hyperparathyroidism has been reported to be an independent risk factor for all-cause mortality and graft loss in KTRs (38). In this study, the levels of intact PTH were significantly higher in the lowest phosphorus group, and this may explain the association between hypophosphatemia and patient outcomes. Post-transplant hyperparathyroidism is known to persist in 10%–50% of KTRs due to the acquired autonomy of parathyroid glands (39–42). Longer duration of dialysis time is a risk factor for persistent hyperparathyroidism after transplantation (43). Because of the phosphaturic effect of PTH, the KTRs with hyperparathyroidism could frequently have hypophosphatemia. Therefore, there was a strong correlation between hypophosphatemia and longer duration of dialysis time in this study. In addition, longer durations of dialysis time before transplantation in the lowest phosphorus group could be associated with severe persistent hyperparathyroidism, and could influence patient outcomes. However, this study was unable to elucidate the association between intact PTH itself and patient outcomes, as there were numerous missing intact PTH values.
The significant association between corrected calcium levels and patient outcomes was not observed in this study. However, similar to serum phosphorus, the levels of calcium-phosphorus product showed a U-shape association with both overall mortality and death-censored graft failure. Moreover, this study confirmed that decreased levels of serum albumin, a primary marker of malnutrition-inflammation-cachexia syndrome, had a significant association with higher overall mortality and death-censored graft failure.
This study has some limitations. First, management of biochemical abnormalities after kidney transplantation can vary between clinicians or institutions. We did not evaluate the management plan, such as the use of vitamin D, phosphate binder, or phosphorus replacement aimed at correcting or preventing out-of-range levels. Second, this study lacks laboratory markers for malnutrition-inflammation-cachexia syndrome, such as C-reactive protein, ferritin, and total iron-binding capacity. Additionally, FGF-23, klotho, and 1,25-hydroxyvitamin D were not measured; this is an inherent limitation of an observational, retrospective study. Therefore, large-scale, prospective, controlled studies are necessary to confirm our results. Third, our study population showed a relatively low incidence of diabetes and prior CVD and a high rate of living donor kidney transplantation, compared with Western countries. Such differences are an inherent fact of Korean KTRs, and can explain the relatively high patient and graft survival observed. Conversely, these aspects suggest that our patients may be an ideal group in which to look at the role of altered mineral metabolism. Fourth, a possibility of selection bias may exist, as we chose to exclude KTRs who had graft failure or mortality before 1 year after transplantation. Despite these limitations, this multicenter retrospective cohort study is the first to demonstrate a U-shape association between serum phosphorus levels and patient outcomes in a relatively large-scale study population.
In conclusion, the serum phosphorus level at 1 year after transplantation exhibits a U-shape association with death-censored graft failure and patient mortality in the kidney transplant cohort characterized by a relatively high rate of living donor kidney transplant and low incidence of diabetes and prior CVD compared with Western countries. Further studies are needed to assess whether evaluation and management of hypophosphatemia and hyperphosphatemia will improve outcomes.
Disclosures
None.
Supplementary Material
Acknowledgments
This research was supported by a grant of the Korea Health Technology Research and Development Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC15C1129).
A portion of this work was selected for a poster presentation, which was presented at the 2015 American Transplant Congress on May 5, at the Pennsylvania Convention Center in Philadelphia, Pennsylvania.
The funding sources had no role in the study design, analysis, and interpretation of the data or in the preparation, approval, or decision to submit the manuscript for review.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07090716/-/DCSupplemental.
References
- 1.Owda A, Elhwairis H, Narra S, Towery H, Osama S: Secondary hyperparathyroidism in chronic hemodialysis patients: Prevalence and race. Ren Fail 25: 595–602, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Slinin Y, Foley RN, Collins AJ: Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 16: 1788–1793, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Marco MP, Craver L, Betriu A, Belart M, Fibla J, Fernández E: Higher impact of mineral metabolism on cardiovascular mortality in a European hemodialysis population. Kidney Int Suppl 63(85): S111–S114, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF: Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 305: 1119–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, Kronenberg F, Marcelli D, Passlick-Deetjen J, Schernthaner G, Fouqueray B, Wheeler DC; ARO Investigators : Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 26: 1948–1955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D: Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 24: 1506–1523, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K: Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 88: 1511–1518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da J, Xie X, Wolf M, Disthabanchong S, Wang J, Zha Y, Lv J, Zhang L, Wang H: Serum phosphorus and progression of CKD and mortality: A meta-analysis of cohort studies. Am J Kidney Dis 66: 258–265, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW; PREPARE study group : High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22: 2909–2916, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr, Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA: Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20: 397–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang AR, Grams ME: Serum phosphorus and mortality in the Third National Health and Nutrition Examination Survey (NHANES III): Effect modification by fasting. Am J Kidney Dis 64: 567–573, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evenepoel P, Meijers BK, de Jonge H, Naesens M, Bammens B, Claes K, Kuypers D, Vanrenterghem Y: Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol 3: 1829–1836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benavente D, Chue CD, Moore J, Addison C, Borrows R, Ferro CJ: Serum phosphate measured at 6 and 12 months after successful kidney transplant is independently associated with subsequent graft loss. Exp Clin Transplant 10: 119–124, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Moore J, Tomson CR, Tessa Savage M, Borrows R, Ferro CJ: Serum phosphate and calcium concentrations are associated with reduced patient survival following kidney transplantation. Clin Transplant 25: 406–416, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Schaeffner ES, Födinger M, Kramar R, Sunder-Plassmann G, Winkelmayer WC: Prognostic associations of serum calcium, phosphate and calcium phosphate concentration product with outcomes in kidney transplant recipients. Transpl Int 20: 247–255, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Egbuna OI, Taylor JG, Bushinsky DA, Zand MS: Elevated calcium phosphate product after renal transplantation is a risk factor for graft failure. Clin Transplant 21: 558–566, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Roodnat JI, van Gurp EA, Mulder PG, van Gelder T, de Rijke YB, de Herder WW, Kal-van Gestel JA, Pols HA, Ijzermans JN, Weimar W: High pretransplant parathyroid hormone levels increase the risk for graft failure after renal transplantation. Transplantation 82: 362–367, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Stevens LA, Djurdjev O, Cardew S, Cameron EC, Levin A: Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: Evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol 15: 770–779, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM: Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 39: 695–701, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Tuttle KR, Short RA: Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clin J Am Soc Nephrol 4: 1968–1973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y, Nakaya Y, Takeda E: Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 20: 1504–1512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giachelli CM, Jono S, Shioi A, Nishizawa Y, Mori K, Morii H: Vascular calcification and inorganic phosphate. Am J Kidney Dis 38[4 Suppl 1]: S34–S37, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J: The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med 140: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Wolf M: Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 82: 737–747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M: Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 24: 125–135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG: Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 60: 200–207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdallah E, Mosbah O, Khalifa G, Metwaly A, El-Bendary O: Assessment of the relationship between serum soluble Klotho and carotid intima-media thickness and left ventricular dysfunction in hemodialysis patients. Kidney Res Clin Pract 35: 42–49, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haut LL, Alfrey AC, Guggenheim S, Buddington B, Schrier N: Renal toxicity of phosphate in rats. Kidney Int 17: 722–731, 1980 [DOI] [PubMed] [Google Scholar]
- 38.Pihlstrøm H, Dahle DO, Mjøen G, Pilz S, März W, Abedini S, Holme I, Fellström B, Jardine AG, Holdaas H: Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism. Transplantation 99: 351–359, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Evenepoel P, Claes K, Kuypers D, Maes B, Bammens B, Vanrenterghem Y: Natural history of parathyroid function and calcium metabolism after kidney transplantation: A single-centre study. Nephrol Dial Transplant 19: 1281–1287, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Alfrey AC, Jenkins D, Groth CG, Schorr WS, Gecelter L, Ogden DA: Resolution of hyperparathyroidism, renal osteodystrophy and metastatic calcification after renal homotransplantation. N Engl J Med 279: 1349–1356, 1968 [DOI] [PubMed] [Google Scholar]
- 41.Messa P, Sindici C, Cannella G, Miotti V, Risaliti A, Gropuzzo M, Di Loreto PL, Bresadola F, Mioni G: Persistent secondary hyperparathyroidism after renal transplantation. Kidney Int 54: 1704–1713, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Jeon HJ, Kim YJ, Kwon HY, Koo TY, Baek SH, Kim HJ, Huh WS, Huh KH, Kim MS, Kim YS, Park SK, Ahn C, Yang J: Impact of parathyroidectomy on allograft outcomes in kidney transplantation. Transpl Int 25: 1248–1256, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Apaydin S, Sariyar M, Erek E, Ataman R, Yiğitbaş R, Hamzaoğlu I, Serdengeçti K, Ulkü U: Hypercalcemia and hyperparathyroidism after renal transplantation. Nephron 81: 364–365, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.