Abstract
IgA nephropathy (IgAN) is a leading cause of CKD and renal failure. Recent international collaborative efforts have led to important discoveries that have improved our understanding of some of the key steps involved in the immunopathogenesis of IgAN. Furthermore, establishment of multicenter networks has contributed to rigorous design and execution of clinical trials that have provided important insights regarding immunotherapy in IgAN. In this article, we review emerging developments in clinical and translational IgAN research and describe how these novel findings will influence future strategies to improve the outcome of patients with IgAN.
Keywords: glomerulonephritis, IgA nephropathy, kidney, immunosuppression, review, corticosteroids, pathology, crescents, glomerulonephritis, IGA, humans, immunotherapy, renal insufficiency, renal insufficiency, chronic, translational medical research
Epidemiology of IgA Nephropathy
The most common GN globally is IgA nephropathy (IgAN). Prevalence varies geographically, and estimates of disease burden vary according to whether ascertained using biopsy registry data versus dialysis registries. Despite these caveats, biopsy and organ replacement registries suggest geographic variation in disease burden with a higher incidence in Pacific Asian regions.
Biopsy registry data underestimate disease burden as patients with mild disease may not undergo biopsy, and in countries lacking screening programs disease may not be detected. Distinguishing the contribution of ancestry and geography to differential disease susceptibility using biopsy registry data is further challenged by substantial geographic variation in biopsy practice patterns (e.g., for clinical indication versus mandatory military screening). A systematic review of biopsy-based studies spanning multiple countries suggests an overall population incidence of at least 2.5 per 100,000 (1). Only one study in this review was from Japan, and most were from Europe and North America. In children, the incidence in a Japanese biopsy registry study where broad screening programs were in place was eight-fold higher compared with a Tennessee program where biopsies were performed for-cause and routine screening was not in place (4.5 versus 0.57 per 100,000/yr) (2,3).
Compelling data from autopsy and donor registries support that there is a higher burden of IgAN in East and Pacific Asian countries. Lanthanic IgA deposition is detected in 1.3% of autopsies of trauma victims in Finland compared with 15.6% of deceased donor candidates in Japan (4,5), suggesting that regional variabilities in incidence are not simply attributable to variation in biopsy practices. These findings parallel geospatial differences in prevalence of genetic susceptibility loci reported in global genome-wide association studies (6).
Pooled cohorts from the European Validation Study of the Oxford Classification of IgA Nephropathy (VALIGA) and North American cohorts indicate rates ESRD or halving of eGFR of 27% at 10 years (7). There are regional differences in the progression of disease, however these are difficult to ascertain given lead-time biases introduced by variation in biopsy practice. In our cohort of 669 patients, multivariable analysis demonstrated that individuals of Pacific Asian origin have an increased risk of ERSD (adjusted hazard, 1.56; 95% confidence interval [95% CI], 1.1 to 2.22) (8).
Disease Pathogenesis in IgAN
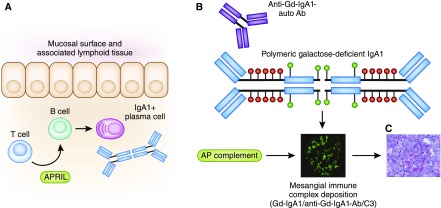
Susceptibility to IgAN and risk of disease progression are influenced by a confluence of genetic and environmental factors. The immunopathogenesis of this disease is described as a multi-“hit” process (9). These “hits” reflect knowledge derived from population genetic studies and careful characterization of the IgA moieties found in biopsy specimens and circulation of patients with IgAN. Figure 1 illustrates current understanding of the pathogenesis of IgAN and steps where therapeutic interventions may be targeted.
Figure 1.
Understanding the pathogenesis of IgA nephropathy reveals therapeutic targets. (A) Mucosal IgA production by plasma cells occurs by T cell–dependent and –independent processes. T cell cytokines such as APRIL promote B cell class switch to IgA1-producing plasma cells. Inhibitors of these cytokines are potential therapeutic targets. B cell and plasma cell inhibitors (e.g., rituximab, bortezomib) may result in decreased IgA production. (B) The IgA1 produced in patients with IgA nephropathy is underglycosylated (Gd-IgA1). Susceptible individuals will also produce anti–Gd-IgA1 autoantibodies (auto-Ab), reviewed in (9). Antiproliferative drugs may affect production of anti-glycan autoantibodies. The Gd-IgA1–auto-Ab immune complexes activate the alternative pathway of complement. Complement inhibitors may prevent formation of immune complexes. (C) The immune complexes are nephritogenic, contributing to local inflammation, cellular proliferation, and ultimately fibrosis. Broad-based targeting of processes implicated in glomerular and tubulo-interstitial injury (e.g., inflammation, fibrosis) target these final common pathways of kidney damage. Anti-Gd-IgA1-auto Ab, autoantibody directed at galactose-deficient IgA1.
A central finding in patients with IgAN is the presence of circulating and glomerular immune complexes comprised of galactose-deficient IgA1, an IgG autoantibody directed against the hinge region O-glycans, and C3. The presence of aberrantly glycosylated IgA1 is an heritable trait (10). Galactose-deficient IgA1 levels are elevated in 25% of blood relatives of IgAN patients and segregation analysis suggests a major dominant gene with additional polygenic background (11). A recent review outlines the cellular mechanisms responsible for IgA glycosylation (12). The relative levels of galactose-deficient IgA1 may also be partially influenced by environmental factors. For example, these antibodies are susceptible to bacteria-derived proteases (13). Recent data suggest that anti-glycan autoantibodies may be targeting IgA VH gene segments that occur as a result of somatic hypermutation, and not sequences present in the host germline (14).
These immune complexes are nephritogenic, contributing directly to glomerular inflammation and mesangial proliferation. Activation of the local and systemic renin angiotensin system and complement activation also ultimately contribute to glomerulosclerosis and tubulo-interstitial fibrosis, leading to loss of renal function. Coexistent risk factors such as hypertension and smoking contribute to disease progression, potentially through microvascular injury (15). Maladaptive hyperfiltration injury and glomerulomegaly attributed to obesity are also implicated in nonimmunologically-mediated disease progression (16).
The triggers responsible for production of galactose-deficient IgA1 are not known and the site of IgA production in IgAN is not established. Experimental models suggest environmental triggers may lead to excess production of aberrantly-glycosylated IgA in mucosal-associated lymphoid tissue. A B cell activation factor of the TNF family, BAFF, is critical but not sufficient for development of an experimental IgAN phenotype (17). In this model BAFF-overexpressing mice reared in pathogen-free conditions develop a phenotype similar to IgAN with detectable circulating and glomerular underglycosylated IgA, whereas those housed in germ-free cages do not. The related cytokine APRIL (a proliferation-inducing ligand) shares some signaling receptors important for B cell development with BAFF, is elevated in IgAN patients, and correlates with age, serum creatinine, and urine protein-to-creatinine ratio (18).
These findings suggest that, at least in mice, exposure to commensal or pathogenic bacteria is necessary for excess IgA production, and this is facilitated by the presence of mediators of B cell differentiation and proliferation BAFF and/or APRIL. Although application to the pathophysiology of IgAN in humans must be made with caution, this schema is further supported by genome-wide association studies and clinical studies of disease progression (19). It is hypothesized that APRIL contributes to IgAN by promoting B cell class switch to an IgA-producing plasma cell through its dominant actions on the TACI receptor.
A polymorphism in the APRIL gene confers IgAN susceptibility (20) and several risk alleles associated with IgAN are also associated with other diseases of mucosal immunity and defense against mucosal microbial pathogens (21). Elevated circulating APRIL levels were documented in a cohort of 166 patients with IgAN, and APRIL expression was correlated with a progressive clinical phenotype and elevated circulating levels of galactose-deficient IgA (19).
Recent studies suggesting immunotherapy primarily results in reduction in proteinuria (without change in long-term outcome) may call into question the role of anti-glycan antibodies in disease immunopathogenesis. However, one must consider that these are short-term treatment interventions, and the formation of anti-glycan antibodies is only one part of a complex disease process. Genetically-influenced and environmentally-determined defects in mucosal immune function (and microbiota profiles) may not be altered in a sustained fashion by a brief immunomodulatory intervention.
Activation of complement is regarded as an important pathogenic contributor in IgAN. The lectin pathway of complement activation is also implicated in IgAN pathogenesis. Polymeric IgA1 is capable of activation of this pathway in vitro, and mannose-binding lectin pathway elements are detected in glomerular deposits (22,23).
Clinical and genetic studies link defects in regulation of the alternative pathway of complement to IgAN immunopathogenesis (6). Circulating and immune complexes contain C3, and this is evident on immunofluorescence of kidney biopsies (24). Properdin and complement factor H (CFH) are also detected in immune deposits (reviewed by Gharavi et al. [25]). Genome-wide association studies identify an allele initially localized to the CFH gene that confers protection against development of IgAN. Further analysis indicate that deletion of complement factor H–related (CFHR) genes one and three are in linkage disequilibrium with the identified risk allele. The CFHR1 and CFHR3 proteins titrate CFH activity, and their absence is associated with altered CFH levels and enhanced CFH activity (25,26).
Clinical-Pathologic Correlation in IgAN: Mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), tubular atrophy (T), MEST and Crescents
Through rigorous design of a structured, standardized, and reproducible pathologic scoring system in IgAN, pathologic evaluation now adds important prognostic information beyond what is obtained from clinical variables alone.
The Oxford classification schema was derived studying patients with ≥0.5 g/d of proteinuria, eGFR≥30 ml/min per 1.73 m2 at renal biopsy, and ≥1 year of follow-up. Histologic variables selected for inclusion demonstrated a high degree of interobserver reproducibility and correlated with predetermined clinical endpoints including a composite outcome of ESRD or 50% reduction in eGFR and rate of renal function decline. Four key pathologic features were consistently independently-associated with renal outcome including mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), and tubular atrophy and interstitial fibrosis (T) now known collectively as the MEST or Oxford score (27). The absence/presence of >50% of glomeruli showing mesangial hypercellularity is denoted M0/M1, respectively; E1 indicates any endocapillary hypercellularity; S1 denotes any segmental glomerulosclerosis; and T0, T1, and T2 reflect fibrosis involving 1%–25%, 26%–50%, or >50% of the cortical area.
The clinical relevance and applicability of this score has now been validated across multiple populations including, most recently, the European (VALIGA) cohort (28). True validation studies (such as described by Herzenberg et al. [29]) applied the regression models derived in the original Oxford cohort and verified the independent contribution of each variable to the same clinical outcomes. Although the individual components were not always independently predictive in these validation studies the score overall consistently adds value to prognostication.
The largest validation study was performed in the VALIGA cohort of 1147 patients. Unlike the original Oxford study, entry criteria were less restrictive; patients at both ends of the spectrum of disease severity were included. The cohort was predominantly white (97.5%) and the combined end point of ESRD or decrease in eGFR by 50% occurred in 26% of patients at 10 years. Most of the histologic variables remained independently associated with outcome, when adjusted for baseline and longitudinal clinical variables. Interestingly, in the low risk group with proteinuria<0.5 g/d only endocapillary proliferation was associated with a rapid rate of renal function decline by univariate analysis (hazard ratio [HR], 5.2; 95% CI, 1.1 to 25.9), however, it was not significantly associated with outcome by multivariate analysis. Mesangial and endocapillary proliferation were associated with an increased risk of developing proteinuria≥1 g/d (HR, 2.3; 95% CI, 1.3 to 4.0) or ≥2 g/d (HR, 3.5; 95% CI, 1.5 to 8.4). In patients with eGFR<30 ml/min per 1.73 m2 M1 and T1–2 were associated with a lower renal survival (HR, 2.3; 95% CI, 1.2 to 4.2) (28).
An important goal of deriving a histologic scoring system is shortening the time frame of observation required to accurately predict which patients are at risk of adverse outcome. A recent pooled analysis of the 901 subjects from the VALIGA, Oxford, and North American cohorts demonstrated that incorporation of MEST score into predictive algorithms provides timely and accurate predictive accuracy to identify patients at highest risk of poor outcome (7). The addition of the MEST score to clinical data at biopsy provided significant improvement in prediction compared with considering only clinical data at biopsy (proteinuria, creatinine, mean arterial pressure). Importantly, the combination of MEST and cross-sectional clinical data at biopsy predicted adverse outcome as well as using 2 years of clinical data (proteinuria and mean arterial pressure averaged over a 2-year period). This was true regardless of treatment with renin-angiotensin system (RAS) blockade or immunosuppression.
Elucidating the relationship between histologic class and treatment response is an important next step. The MEST studies suggest that response to corticosteroids may differ according to histology. In the original derivation cohort, use of corticosteroids confounded the relationship between endocapillary proliferation, and adverse outcome (30). In the VALIGA cohort, half of subjects received immunosuppression, mostly including corticosteroids. In a VALIGA substudy, Tesar et al. (31) derived a propensity-matched cohort of 184 pairs to evaluate additive benefits of corticosteroids above RAS blockade. Use of corticosteroids afforded an improved renal survival, and the greatest reduction in rate of renal function afforded by corticosteroids occurred in subjects with mesangial proliferation, segmental glomerulosclerosis, and tubulo-interstitial fibrosis. Although it is difficult to make firm conclusions based upon observational data, future therapeutic trials in IgAN should include stratification based upon MEST score to determine if histology can be used to tailor treatment decisions.
The additive effect of crescents on prediction of outcome remains a challenging question in IgAN, and new information may shed some light on clinical implications of these findings. Although crescents are a common finding in IgAN biopsies, the presence of >30% of crescents is not. Early studies initially suggested that patients with crescents involving >50% of glomeruli are at high risk of progression, with 75% reaching ESRD by 10 years (32). Certainly, in the context of rapid progression of loss of kidney function, crescents portend a poor prognosis. Patients with rapidly progressive courses were not included in the Oxford study, therefore the study was underpowered to determine if a high percentage of crescents is independently-associated with adverse outcome (i.e., beyond the information that would have been derived from clinical data alone). A meta-analysis of the Oxford study and four validation studies did suggest that the presence of crescents is associated with a higher risk of adverse prognosis (HR, 2.3; 95% CI, 1.6 to 3.4; P<0.001) (33).
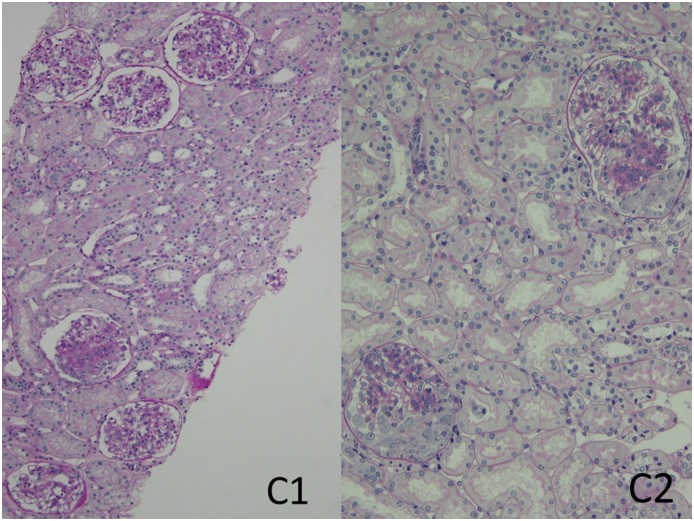
A working group formed in 2014 at the First Oxford Conference on IgA Nephropathy examined the association of crescents with renal outcomes in 3096 patients combined from four published studies: the original Oxford cohort, the VALIGA study cohort, and two large Asian cohorts, one from China and the other from Japan. Biopsies from 1118 (36%) of the patients contained any cellular or fibrocellular crescents; 440 (14%), 221 (7%), and 96 (3%) had crescents in ≥1/10, ≥1/6, and ≥1/4 of glomeruli, respectively. Overall, the presence of any such crescents was a significant, independent predictor of the likelihood of developing a combined event of ESRD or a ≥50% reduction in eGFR. Other independent predictors of the combined event were eGFR at biopsy, time-averaged proteinuria and mean arterial pressure, and Oxford M1, S1, and T1/T2 scores. The predictive value of all histologic parameters (including crescents) except T1/T2 was lost in patients receiving immunosuppression. However, the presence of cellular or fibrocellular crescents in ≥1/4 of glomeruli was significantly predictive of the combined event even in patients receiving immunosuppression. On the basis of these findings, the authors proposed adding crescent scores to the Oxford (MEST) classification as follows: C0—no cellular or fibrocellular crescents; C1—cellular/fibrocellular crescents in <25% of glomeruli, identifying patients at increased risk of a poor outcome (compared with C0) among those not given immunosuppressive therapy; and C2—crescents in ≥25% of glomeruli, identifying patients at increased risk of a poor outcome even if given immunosuppression (34) (see Figure 2).
Figure 2.
A novel addition to the MEST classification system identifies patients at increased risk of poor outcome based on crescents: C0 (no crescents), C1 (<25% left panel), and C2 (≥25% right panel). The biopsy specimen represented in the left panel had 21 total glomeruli of which two had segmental crescents; of the six complete glomeruli represented in the photomicrograph one shows a fibrocellular crescent with segmental solidification of the tuft adjacent to the crescent. There is also mild mesangial hypercellularity, and the MEST scores are M1, E0, S1, T0. The biopsy represented in the right panel contained 14 total glomeruli, of which five had segmental cellular crescents; two of the latter glomeruli are represented in the photomicrograph. The glomeruli also show mesangial hypercellularity, and the biopsy also showed focal and segmental endocapillary hypercellularity (not shown in the photomicrograph); the MEST scores are M1, E1, S0, T0. Periodic acid–Schiff stain; original magnification, ×100 (left panel), and original magnification, ×200 (right panel).
The MEST score does not replace the detailed and informative information provided by the full pathology report. One should be mindful that despite the statistically-significant association with progression, the pathology score combined with clinical data account for only a minority (<30%) of the variability in outcome in patients with IgAN (35). Therefore, a majority of the variability in outcome is attributable to unmeasured factors which would hopefully include therapeutic intervention.
Clinical Manifestations
The range of clinical manifestations of IgAN is broad, from asymptomatic microscopic hematuria to rapidly progressive GN. The typical mode of presentation varies according to age group and biopsy practice patterns.
Common Phenotypes: Asymptomatic Hematuria and Progressive Kidney Disease
By far the two most common clinical presentations are asymptomatic hematuria and progressive kidney disease. Asymptomatic hematuria with minimal proteinuria (i.e., <0.5 g/d) may be detected through screening programs, such as those in countries with school screening, military draft screens, or insurance programs. Isolated microscopic hematuria with minimal proteinuria is regarded as having a favorable prognosis, particularly in white populations (36). Nonetheless, a proportion will ultimately develop hypertension and significant proteinuria, suggesting that long-term follow-up of these patients should be instituted (37,38).
Progressive CKD is a common phenotype observed in multiple cohorts. Renal survival ranges greatly according to biopsy timing and introduction of lead-time bias. Actuarial renal 10-year survival is reported to be 57%–91% (39). In addition to pathologic findings, factors associated with poor prognosis include hypertension, proteinuria, and decreased eGFR at diagnosis (35,40). Race may also be an important determinant of outcome (8).
Less Frequent Manifestations
Synpharyngitic macroscopic hematuria is a classic clinical syndrome associated with first presentation of IgAN. The presence of gross hematuria at the time of pharyngitis or other infection is alarming to patients and often prompts immediate medical attention and diagnosis. This syndrome occurs in approximately 10%–15% of subjects (41) and predominantly in patients under the age of 40. Recurrent macroscopic hematuria is associated with a favorable prognosis in the short-term, potentially reflecting lead-time bias associated with this finding in younger populations. However, subsequent development of persistent proteinuria is well documented and associated with potentially progressive disease (42).
Although nephrotic-range proteinuria>3 g/d is not uncommon in IgAN, coexistence of the nephrotic syndrome is rare (43,44). A rare clinical presentation more in keeping with dual diagnoses of IgAN and minimal change disease is also well described. Renal function is often preserved, and overt nephrotic syndrome with edema, dyslipidemia, and hypoalbuminemia is present. Steroid responsiveness and favorable prognosis akin to minimal change lesions are reported (45), although a steroid-sparing agent is often required to maintain remission. In one cohort, only 306 patients of 11,885 with IgA met criteria for this diagnosis—diffuse IgA-dominant mesangial deposits with minimal changes in glomeruli on light microscopy and >50% podocyte foot process effacement. None of these patients reached ESRD at the end of a median of 2.6 years (45).
IgAN with rapidly progressive course is rare and most frequently associated with a pathologic finding of >50% of glomeruli exhibiting crescents. The largest case series of 113 subjects described a rate of ESRD of 42.5% at 1 year despite immunotherapy (46).
Treatment Strategies: Current Evidence and Novel Therapies
In this section, the focus of discussion will be on treating the most common clinical variants of IgAN.
Conservative Therapy
The importance of conservative therapy to reduce proteinuria and slow the rate of renal function decline in IgAN cannot be overemphasized. In the recent Supportive Versus Immunosuppressive Therapy of Progressive IgA Nephropathy (STOP-IgAN) trial, nearly one third of patients who completed a 6-month run-in phase of intensive optimization of conservative therapy no longer qualified for randomization to immunosuppressive therapy (47).
The role of RAS blockade in IgAN is now well established. Although dual RAS blockade may reduce proteinuria, regulatory agencies have recommended against use of combination therapy due to risks of hyperkalemia (48). Many trials have examined the effects of statins in patients with CKD, however few have focused on patients with IgAN. A small, randomized, placebo-controlled trial of 21 patients with serum creatinine <1.8 mg/dl, with absence of severe lesions on renal biopsy and without RAS blockade, received fluvastatin or placebo for 6 months. A decrease in proteinuria was seen after 6 months (baseline 837–1100 mg/24 hours in the placebo group versus 845–494 mg/24 hours, P<0.01) (49).
Obesity can contribute to proteinuria. In a cohort of 331 patients with IgAN in whom 44.1% were either overweight or obese, the overweight/obese group were more likely to be hypertensive (24.6% versus 52.9%, P<0.001), have worse renal function (mean eGFR 69.1 versus 80.2, P=0.003), and have proteinuria≥1 g/d (22.1% versus 39.7%, P=0.003). There was no difference in the endpoint of death/dialysis between those with normal body mass index (BMI) and the overweight/obese group, however the overweight/obese group had a worse eGFR (59.3 versus 73.4, P<0.001) and a greater prevalence of CKD stage 3 or greater (43.4% versus 21.0%, P 0.001) (50). Weight reduction may ameliorate proteinuria in IgAN (51). Given the link between smoking and progression, smoking cessation should also be promoted (15).
Corticosteroids
There are several clinical trials supporting that corticosteroids reduce proteinuria in IgAN. Although earlier studies supported that this was associated with an improvement in clinical outcome, more recent large trials question the sustained benefits of a brief period of immunosuppression.
One of the first randomized trials suggesting a benefit of corticosteroids was a study of 86 patients with biopsy-proven IgAN with proteinuria 1–3.5 g/d for at least 3 months and a serum creatinine ≤1.5 g/dl (52). Patients received steroid therapy (1 g intravenously for 3 days at months 1, 3, and 5, and oral prednisone at 0.5 mg/kg per day on alternate days for 6 months) or supportive therapy alone. Given the era of the trial, use of RAS blockade was not uniform and there was no run-in period of optimizing conservative therapy. In this study, 5-year renal survival (defined as 50% increase in serum creatinine) was greater in the corticosteroid group (81% versus 64%, P=0.05) as was survival without a 100% increase in creatinine (95% versus 74%, P=0.001). In the multivariate analysis, reduction in proteinuria from baseline to 6 months was significantly associated with renal survival (relative risk [RR], 0.48; 95% CI, 0.34 to 0.69; P<0.001). At 10 years, renal survival was better in the steroid group as compared with controls (97% versus 53%, P=0.003; RR, 0.06; 95% CI, 0.01 to 0.44; P=0.001). More patients in the steroid group had a reduction in proteinuria <0.5 g/d after 1 year (26% versus 5%; RR, 5.50; 95% CI, 1.3 to 23; number needed to treat=5; P=0.01) (53). This finding suggested that exposure to corticosteroids for a 6-month period may have a “legacy effect,” with sustained reduction in the risk of progressive renal dysfunction.
Similarly, a single-center, randomized, open-label trial of 97 patients with biopsy-proven IgAN, proteinuria≥1 g/d, and eGFR>50 ml/min per 1.73 m2 demonstrated benefits of corticosteroids (54). After a 3-month run-in phase during which they were required to discontinue RAS blockade patients were randomized to ramipril or ramipril plus prednisone 1.0 mg/kg per day for 8 weeks then tapered by 0.2 mg/kg per day every month. The corticosteroid group had a higher baseline proteinuria (2.5 versus 2 g/d). More patients in the combined group failed to reach the combined endpoint of ESRD or doubling of serum creatinine at 8 years (85.2% versus 52.1%, P=0.003). Similar results were demonstrated in a Chinese cohort of 63 subjects (55).
A subsequent meta-analysis of 536 patients in nine trials with proteinuria>1 g/d demonstrated corticosteroids decreased the risk of kidney failure (RR, 0.32; 95% CI, 0.15 to 0.67; P=0.002). The effects on renal outcome were dose-dependent, requiring >30 mg/d or high-dose pulse and were not surprisingly associated with an increased risk of adverse events (56). Major criticisms of these studies have been the lack of uniform use of RAS blockade for all patient groups (52) and small sample size, which may have been underpowered to detect adverse events associated with corticosteroids (56).
The STOP-IgAN study (47) recently further brought into question the long-term benefits of a 6-month course of corticosteroids. This study was designed to enroll approximately 380 subjects to determine the benefits of immunosuppression compared with conservative therapy in patients with persistent proteinuria and eGFR>30 ml/min per 1.73 m2 after a 6-month run-in period that involved adequate BP control and RAS blockade, as well as lipid control. Patients allocated to the immunotherapy would receive corticosteroids if the eGFR was >60 ml/min per 1.73 m2 whereas those with eGFR of 30–59 ml/min per 1.73 m2 would receive steroid and cyclophosphamide, followed by maintenance immunosuppression with azathioprine akin to one of the few randomized trials in patients with severe but not rapidly progressive disease (57). The study was powered to evaluate the effect of immunotherapy on primary endpoints: “full clinical remission” (protein-to-creatinine ratio of <0.2 and stable renal function with a decrease in the eGFR of <5 ml/min per 1.73 m2 from the baseline eGFR) and a decrease in the eGFR of >15 ml/min per 1.73 m2 from the baseline. Secondary endpoints related primarily to eGFR changes and slope.
After the intensive 6-month optimization of conservative therapy, nearly one third of patients no longer qualified for randomization. Ultimately only 162 patients were randomized. At 3 years, a larger proportion of patients who received immunosuppression were in a full clinical remission compared with patients who received only supportive care (17% versus 5%, P<0.05). However, these differences in remission were not associated with significant difference in the rate of loss of kidney function. Not surprisingly, more infections occurred in the immunosuppression group, including one sepsis-related death. Impaired glucose tolerance and significant weight gain were also observed (47).
The interim results of the Therapeutic Evaluation of Steroids in IgA Nephropathy Global Study were recently presented as a late-breaking clinical trial abstract at the 53rd meeting of the European Renal Association—European Dialysis and Transplantation Association. This study was designed to evaluate the effects of a 6–8 months course of 0.6–0.8 mg/kg per day of oral methylprednisolone versus placebo, with a target sample size of 1300 and 5 years of longitudinal follow-up. The primary outcome was a composite of 40% decrease in eGFR, ESRD, or death. An interim analysis of 262 patients was presented. Although proteinuria was reduced with corticosteroid treatment, and the composite outcome occurred in a lower proportion of patients in the treatment arm (8% versus 15%, P=0.02), there were concerning signals of risk with corticosteroids at a mean follow-up of 1.5 years. Serious adverse events were reported more frequently in the corticosteroid arm (relative risk, 4.63; 95% CI, 1.63 to 13.18; P=0.001) mostly due to excess serious infections (14.7% versus 3.2%, P<0.001), including two fatal infections. Based upon the interim analysis the investigators concluded that renal benefit is likely but will need to be weighed carefully against risk. Recruitment is currently on hold pending protocol revision (ClinicalTrials.gov NCT01560052).
Mycophenolate
Data regarding the efficacy of mycophenolate are mixed, such that current clinical guidelines recommend against use of this agent in IgAN (58). At one end of the spectrum is applicability in patients with low-risk renal disease. In a randomized, double-blind, placebo-controlled clinical trial, 44 patients with persistent proteinuria>0.6–0.8 g/g and eGFR>50 ml/min per 1.73 m2 were randomly assigned to mycophenolate mofetil (MMF) or placebo in addition to conservative therapy. A significant number were excluded as they had a good response to conservative management during the run-in phase (36 of 184) and only seven patients in the treatment group and ten patients in the placebo group were analyzed at the end of the study period after the trial was stopped early due to lack of observed benefit (59).
At the other end of the spectrum is a study of mycophenolate in patients with more advanced and high-risk disease. A double-blind, controlled trial of 32 patients with ≥1g/d of proteinuria and at least two additional risk factors (male sex, hypertension, clearance<80 ml/min per 1.73 m2, glomerulosclerosis, tubulo-interstitial fibrosis, or ≥25% of glomeruli affected by crescents) (60). Patients received MMF or placebo in addition to RAS blockade. This study was terminated early due to the finding of a trend in worse renal outcome in the MMF group.
In contrast, in a small, randomized, nonblinded trial of 40 patients with >1 g/d of persistent proteinuria, more patients in the MMF group demonstrated remission of proteinuria (16 versus six) and had overall less proteinuria (1.14±0.23 g/24 hours versus 2.4±0.40 g/24 hours) (61). Long-term follow-up demonstrated improved renal survival in MMF-treated patients (90% versus 55%, P=0.01) (62).
These studies are limited by small sample size. It also remains possible there is race-specific variability in response to MMF as in lupus nephritis (63). However systematic reviews and meta-analyses of the randomized trials of MMF also suggest mixed results (64–66).
Rituximab
Although evidence for rituximab in other glomerular diseases is promising, early results in IgAN are not encouraging. A pilot trial evaluated the outcome of 34 patients with proteinuria>1 g/d and eGFR<90 ml/min per 1.73 m2 randomized to rituximab versus conservative management. No effects on proteinuria or renal function were seen (67).
Combination Therapy
Combination therapy with corticosteroid plus an additional agent is generally reserved for patients with progressive disease. A single-center, prospective, randomized, controlled trial of 38 patients with “high-risk” IgAN (hypertension, >15% rise in creatinine in prior year) demonstrated improved renal survival after 2 years in patients receiving prednisone and cyclosphosphamide/azathioprine as compared with no immunotherapy (57).
In contrast, dual-agent immunotherapy does not always afford an advantage. A multicenter, open-label, trial of 207 patients with creatinine<2.0 mg/dl and proteinuria≥1.0 g/d for at least 3 months randomized patients to corticosteroids alone (1 g intravenously for three consecutive days at months 1, 3, and 5, and then continued at 0.5 mg/kg) or corticosteroids in addition to azathioprine 1.5 mg/kg. There was no difference in primary outcome of time to 50% increase in creatinine or secondary outcome of change in proteinuria over time, however major side effects were seen more commonly in the azathioprine group (68). The same investigators conducted a separate randomization for an additional 46 patients with advanced disease (creatinine≥2.0 mg/dl) (69). There was no difference in renal survival at 6 years, although multivariable survival analysis identified that azathioprine therapy was associated with improved renal survival. A substantial number of patients did not complete the study due to complications of therapy, highlighting the potential toxicity of immunosuppression in patients with advanced renal insufficiency.
Novel Agents
A novel potential therapy for IgAN is a modified formulation of oral budesonide (Nefecon). This agent is proposed to act locally at the mucosal lymphoid tissue in the distal ileum and proximal large intestine to modulate IgA production. High first-pass metabolism also theoretically minimizes systemic effects (70). The results of the phase 2 double-blind, placebo controlled trial were recently presented. After a 6-month run-in phase, patients with proteinuria>0.5 g/g or 0.75 g/d and eGFR ≥45 ml/min per 1.73 m2 were randomized to receive placebo, 8, or 16 mg/d of budesonide. There was a significantly greater reduction in proteinuria in patients treated with the budesonide—up to 27% reduction with a 3% rise in proteinuria in the placebo group. In addition, there was a significant difference in a secondary endpoint; the change in eGFR was less in the budesonide group (−4.7 ml/min per 1.73 m2 for placebo versus 0.32 and 1.95 ml/min per 1.73 m2 for 8 mg and 16 mg/d, respectively P<0.05). More adverse events were seen in the budesonide group, necessitating treatment cessation in 22% of patients on this higher dose (ClinicalTrials.gov NCT01738035).
An open-label efficacy/safety study is currently recruiting patients with eGFR>30 ml/min per 1.73 m2 with IgAN and nephrotic syndrome who failed previous immunosuppression to treatment with Acthar gel, a purified form of adrenocorticotropic hormone (ClinicalTrials.gov NCT02382523). Another open-label pilot study is currently recruiting for efficacy/safety in patients with >1 g/d of proteinuria and no eGFR restriction of bortezomib, a proteasome inhibitor (ClinicalTrials.gov NCT01103778). Both studies examine proteinuria at 12 months as the primary outcome with varying requirements for RAS blockade. A double-blind safety/efficacy study in patients with >0.5 g/d of proteinuria and eGFR>30 ml/min per 1.73 m2 randomized patients to either fostamatinib, an oral spleen tyrosine kinase inhibitor, or placebo with the primary outcome of proteinuria at 24 weeks (ClinicalTrials.gov NCT02112838). Additional therapies are reviewed by Yeo et al. (71).
Reconciling Data and the Future of Clinical Trials in IgAN
Should corticosteroids be abandoned in IgAN? The current landscape of evidence falls short of providing a satisfactory answer to this question, and not surprisingly no single randomized trial can adequately address individual patient benefit. For example, one must be mindful that patients with persistent proteinuria>3.5 g/d were excluded from STOP-IgAN, limiting generalizability. The propensity-matching–based study of corticosteroids in the VALIGA cohort suggests that the greatest benefits of corticosteroids may be accrued in patients with the highest degree of proteinuria, particularly >3 g/d (31). Taken together, there are insufficient data to refute current Kidney Disease Improving Global Outcomes guidelines regarding corticosteroid use in patients with >3 g/d of proteinuria persisting despite conservative therapy, although treatment is not without risk.
On the other end of the disease spectrum, 20% of subjects ultimately randomized had under 1 g/d of proteinuria and the rate of renal function decline in the study population was low overall, as expected (72). The VALIGA analysis suggests a graded benefit of corticosteroids according to time-averaged proteinuria before treatment, with no difference in renal function decline below 1 g/d of proteinuria. Therefore, patients with persistent proteinuria<1 g/d likely do not derive significant benefit from addition of corticosteroids. The 1–3 g/d category remains a “gray zone.” Indeed, a long duration of follow-up will be required to demonstrate any potential benefit, based upon rates of renal function decline typically observed in patients with this degree of persistent proteinuria (72).
The rigor of design and execution of these multicenter trials bring a most welcome evolution in the landscape of clinical trials for IgAN. They have also highlighted urgent needs in this realm. A lack of sensitive and specific early surrogate markers of long-term outcome further challenges execution of these studies. Although substantial observational data support an association between complete or partial remission of proteinuria with improved renal survival, this remains to be validated in controlled trials.
None of the information regarding the pathogenesis of this disease suggests a curable time-limited event. Perhaps there is a role for treatment to be divided into induction and maintenance phases, with use of steroid-sparing maintenance therapy to achieve sustained reductions in time-averaged proteinuria that ultimately affect renal survival. Furthermore, studies of immunotherapy in IgAN consistently demonstrate significant risk of toxicity. Ultimately, it is anticipated that improved understanding of the immunopathogenesis of this disease will lead to development of more targeted and less toxic treatment options.
Disclosures
M.H. discloses receipt of consulting fees from Shire Viropharma (Lexington, MA) and Astra Zeneca (Wilmington, DE). These disclosures are unrelated to the material presented in this paper. H.N.R. has received speaker fees from Hoffman La-Roche (Mississauga, ON, Canada) and participated in an advisory board for Hoffman-La Roche and AMGEN (Mississauga, ON, Canada).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.McGrogan A, Franssen CF, de Vries CS: The incidence of primary glomerulonephritis worldwide: A systematic review of the literature. Nephrol Dial Transplant 26: 414–430, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Sehic AM, Gaber LW, Roy S 3rd, Miller PM, Kritchevsky SB, Wyatt RJ: Increased recognition of IgA nephropathy in African-American children. Pediatr Nephrol 11: 435–437, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Utsunomiya Y, Koda T, Kado T, Okada S, Hayashi A, Kanzaki S, Kasagi T, Hayashibara H, Okasora T: Incidence of pediatric IgA nephropathy. Pediatr Nephrol 18: 511–515, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Varis J, Rantala I, Pasternack A, Oksa H, Jäntti M, Paunu ES, Pirhonen R: Immunoglobulin and complement deposition in glomeruli of 756 subjects who had committed suicide or met with a violent death. J Clin Pathol 46: 607–610, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y: Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 63: 2286–2294, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M, Suzuki H, Eitner F, Snyder HJ, Choi M, Hou P, Scolari F, Izzi C, Gigante M, Gesualdo L, Savoldi S, Amoroso A, Cusi D, Zamboli P, Julian BA, Novak J, Wyatt RJ, Mucha K, Perola M, Kristiansson K, Viktorin A, Magnusson PK, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Boland A, Metzger M, Thibaudin L, Wanner C, Jager KJ, Goto S, Maixnerova D, Karnib HH, Nagy J, Panzer U, Xie J, Chen N, Tesar V, Narita I, Berthoux F, Floege J, Stengel B, Zhang H, Lifton RP, Gharavi AG: Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 8: e1002765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour SJ, Espino-Hernandez G, Reich HN, Coppo R, Roberts IS, Feehally J, Herzenberg AM, Cattran DC; The MEST score provides earlier risk prediction in IgA nephropathy. Kidney Int 89: 167–175, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Barbour SJ, Cattran DC, Kim SJ, Levin A, Wald R, Hladunewich MA, Reich HN: Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int 84: 1017–1024, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Magistroni R, D’Agati VD, Appel GB, Kiryluk K: New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int 88: 974–989, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiryluk K, Moldoveanu Z, Sanders JT, Eison TM, Suzuki H, Julian BA, Novak J, Gharavi AG, Wyatt RJ: Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int 80: 79–87, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, Mestecky J, Novak J, Julian BA: Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol 19: 1008–1014, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoppova B, Reily C, Maillard N, Rizk DV, Moldoveanu Z, Mestecky J, Raska M, Renfrow MB, Julian BA, Novak J: The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front Immunol 7: 117, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamm ME, Emancipator SN, Robinson JK, Yamashita M, Fujioka H, Qiu J, Plaut AG: Microbial IgA protease removes IgA immune complexes from mouse glomeruli in vivo: Potential therapy for IgA nephropathy. Am J Pathol 172: 31–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang ZQ, Raska M, Stewart TJ, Reily C, King RG, Crossman DK, Crowley MR, Hargett A, Zhang Z, Suzuki H, Hall S, Wyatt RJ, Julian BA, Renfrow MB, Gharavi AG, Novak J: Somatic mutations modulate autoantibodies against galactose-deficient IgA1 in IgA nephropathy. J Am Soc Nephrol 27: 3278–3284, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto R, Nagasawa Y, Shoji T, Iwatani H, Hamano T, Kawada N, Inoue K, Uehata T, Kaneko T, Okada N, Moriyama T, Horio M, Yamauchi A, Tsubakihara Y, Imai E, Rakugi H, Isaka Y: Cigarette smoking and progression of IgA nephropathy. Am J Kidney Dis 56: 313–324, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Kataoka H, Ohara M, Honda K, Mochizuki T, Nitta K: Maximal glomerular diameter as a 10-year prognostic indicator for IgA nephropathy. Nephrol Dial Transplant 26: 3937–3943, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML: An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 293: 2111–2114, 2001 [DOI] [PubMed] [Google Scholar]
- 18.McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, Ward L, Lawson MA, Macpherson AJ, McCoy KD, Pei Y, Novak L, Lee JY, Julian BA, Novak J, Ranger A, Gommerman JL, Browning JL: Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 121: 3991–4002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai YL, Zhu L, Shi SF, Liu LJ, Lv JC, Zhang H: Increased APRIL expression induces IgA1 aberrant glycosylation in IgA nephropathy. Medicine (Baltimore) 95: e3099, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu XQ, Li M, Zhang H, Low HQ, Wei X, Wang JQ, Sun LD, Sim KS, Li Y, Foo JN, Wang W, Li ZJ, Yin XY, Tang XQ, Fan L, Chen J, Li RS, Wan JX, Liu ZS, Lou TQ, Zhu L, Huang XJ, Zhang XJ, Liu ZH, Liu JJ: A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet 44: 178–182, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, Fasel D, Lata S, Prakash S, Shapiro S, Fischman C, Snyder HJ, Appel G, Izzi C, Viola BF, Dallera N, Del Vecchio L, Barlassina C, Salvi E, Bertinetto FE, Amoroso A, Savoldi S, Rocchietti M, Amore A, Peruzzi L, Coppo R, Salvadori M, Ravani P, Magistroni R, Ghiggeri GM, Caridi G, Bodria M, Lugani F, Allegri L, Delsante M, Maiorana M, Magnano A, Frasca G, Boer E, Boscutti G, Ponticelli C, Mignani R, Marcantoni C, Di Landro D, Santoro D, Pani A, Polci R, Feriozzi S, Chicca S, Galliani M, Gigante M, Gesualdo L, Zamboli P, Battaglia GG, Garozzo M, Maixnerová D, Tesar V, Eitner F, Rauen T, Floege J, Kovacs T, Nagy J, Mucha K, Pączek L, Zaniew M, Mizerska-Wasiak M, Roszkowska-Blaim M, Pawlaczyk K, Gale D, Barratt J, Thibaudin L, Berthoux F, Canaud G, Boland A, Metzger M, Panzer U, Suzuki H, Goto S, Narita I, Caliskan Y, Xie J, Hou P, Chen N, Zhang H, Wyatt RJ, Novak J, Julian BA, Feehally J, Stengel B, Cusi D, Lifton RP, Gharavi AG: Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet 46: 1187–1196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, Stahl GL, Matsushita M, Fujita T, van Kooten C, Daha MR: Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol 17: 1724–1734, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR: Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol 167: 2861–2868, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, Novak J: Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol 26: 1503–1512, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ, Novak J, He JC, Wang H, Lv J, Zhu L, Wang W, Wang Z, Yasuno K, Gunel M, Mane S, Umlauf S, Tikhonova I, Beerman I, Savoldi S, Magistroni R, Ghiggeri GM, Bodria M, Lugani F, Ravani P, Ponticelli C, Allegri L, Boscutti G, Frasca G, Amore A, Peruzzi L, Coppo R, Izzi C, Viola BF, Prati E, Salvadori M, Mignani R, Gesualdo L, Bertinetto F, Mesiano P, Amoroso A, Scolari F, Chen N, Zhang H, Lifton RP: Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 43: 321–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu L, Zhai YL, Wang FM, Hou P, Lv JC, Xu DM, Shi SF, Liu LJ, Yu F, Zhao MH, Novak J, Gharavi AG, Zhang H: Variants in complement factor H and complement factor H-related protein genes, CFHR3 and CFHR1, affect complement activation in IgA nephropathy. J Am Soc Nephrol 26: 1195–1204, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society: The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, Roberts IS, Morando L, Camilla R, Tesar V, Lunberg S, Gesualdo L, Emma F, Rollino C, Amore A, Praga M, Feriozzi S, Segoloni G, Pani A, Cancarini G, Durlik M, Moggia E, Mazzucco G, Giannakakis C, Honsova E, Sundelin BB, Di Palma AM, Ferrario F, Gutierrez E, Asunis AM, Barratt J, Tardanico R, Perkowska-Ptasinska A; VALIGA study of the ERA-EDTA Immunonephrology Working Group: Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 86: 828–836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herzenberg AM, Fogo AB, Reich HN, Troyanov S, Bavbek N, Massat AE, Hunley TE, Hladunewich MA, Julian BA, Fervenza FC, Cattran DC: Validation of the Oxford classification of IgA nephropathy. Kidney Int 80: 310–317, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society: The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Tesar V, Troyanov S, Bellur S, Verhave JC, Cook HT, Feehally J, Roberts IS, Cattran D, Coppo R; VALIGA study of the ERA-EDTA Immunonephrology Working Group: Corticosteroids in IgA nephropathy: A retrospective analysis from the VALIGA study. J Am Soc Nephrol 26: 2248–2258, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abe T, Kida H, Yoshimura M, Yokoyama H, Koshino Y, Tomosugi N, Hattori N: Participation of extracapillary lesions (ECL) in progression of IgA nephropathy. Clin Nephrol 25: 37–41, 1986 [PubMed] [Google Scholar]
- 33.Lv J, Shi S, Xu D, Zhang H, Troyanov S, Cattran DC, Wang H: Evaluation of the Oxford Classification of IgA nephropathy: a systematic review and meta-analysis. Am J Kidney Dis 62: 891–899, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Haas M, Verhave JC, Liu ZH, Alpers CE, Barratt J, Becker JU, Cattran D, Cook HT, Coppo R, Feehally J, Pani A, Perkowska-Ptasinska A, Roberts IS, Soares MF, Trimarchi H, Wang S, Yuzawa Y, Zhang H, Troyanov S, Katafuchi R: A multicenter study of the predictive value of crescents in IgA nephropathy [published online ahead of print September 9, 2016]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbour SJ, Reich HN: Risk stratification of patients with IgA nephropathy. Am J Kidney Dis 59: 865–873, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Gutiérrez E, Zamora I, Ballarín JA, Arce Y, Jiménez S, Quereda C, Olea T, Martínez-Ara J, Segarra A, Bernis C, García A, Goicoechea M, García de Vinuesa S, Rojas-Rivera J, Praga M; Grupo de Estudio de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN): Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J Am Soc Nephrol 23: 1753–1760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szeto CC, Lai FM, To KF, Wong TY, Chow KM, Choi PC, Lui SF, Li PK: The natural history of immunoglobulin a nephropathy among patients with hematuria and minimal proteinuria. Am J Med 110: 434–437, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Shen P, He L, Huang D: Clinical course and prognostic factors of clinical early IgA nephropathy. Neth J Med 66: 242–247, 2008 [PubMed] [Google Scholar]
- 39.D’Amico G: Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 24: 179–196, 2004 [DOI] [PubMed] [Google Scholar]
- 40.D’Amico G: Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Ibels LS, Györy AZ: IgA nephropathy: Analysis of the natural history, important factors in the progression of renal disease, and a review of the literature. Medicine (Baltimore) 73: 79–102, 1994 [PubMed] [Google Scholar]
- 42.Le W, Liang S, Chen H, Wang S, Zhang W, Wang X, Wang J, Zeng CH, Liu ZH: Long-term outcome of IgA nephropathy patients with recurrent macroscopic hematuria. Am J Nephrol 40: 43–50, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Lai KN, Lai FM, Chan KW, Ho CP, Leung AC, Vallance-Owen J: An overlapping syndrome of IgA nephropathy and lipoid nephrosis. Am J Clin Pathol 86: 716–723, 1986 [DOI] [PubMed] [Google Scholar]
- 44.Herlitz LC, Bomback AS, Stokes MB, Radhakrishnan J, D’Agati VD, Markowitz GS: IgA nephropathy with minimal change disease. Clin J Am Soc Nephrol 9: 1033–1039, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li XW, Liang SS, Le WB, Cheng SQ, Zeng CH, Wang JQ, Liu ZH: Long-term outcome of IgA nephropathy with minimal change disease: A comparison between patients with and without minimal change disease. J Nephrol 29: 567–573, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo Z, Wang C, Li S, Zhang J, Zhang J, Liu L, Shi S, Wang S, Chen M, Cui Z, Chen N, Yu X, Zhao M, Wang H: Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. Am J Kidney Dis 24: 2118–2125, 2013 [DOI] [PMC free article] [PubMed]
- 47.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JF, Hilgers RD, Floege J; STOP-IgAN Investigators: Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373: 2225–2236, 2015 [DOI] [PubMed] [Google Scholar]
- 48.European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP): Restriction of combined use of medicines affecting the renin-angiotensin system (RAS). European Medicines Agency, London: 2014 [Google Scholar]
- 49.Buemi M, Allegra A, Corica F, Aloisi C, Giacobbe M, Pettinato G, Corsonello A, Senatore M, Frisina N: Effect of fluvastatin on proteinuria in patients with immunoglobulin A nephropathy. Clin Pharmacol Ther 67: 427–431, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Berthoux F, Mariat C, Maillard N: Overweight/obesity revisited as a predictive risk factor in primary IgA nephropathy. Nephrol Dial Transplant 28[Suppl 4]: iv160–iv166, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Kittiskulnam P, Kanjanabuch T, Tangmanjitjaroen K, Chancharoenthana W, Praditpornsilpa K, Eiam-Ong S: The beneficial effects of weight reduction in overweight patients with chronic proteinuric immunoglobulin a nephropathy: A randomized controlled trial. J Ren Nutr 24: 200–207, 2014 [DOI] [PubMed] [Google Scholar]
- 52.Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, Locatelli F: Corticosteroids in IgA nephropathy: A randomised controlled trial. Lancet 353: 883–887, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F: Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Manno C, Torres DD, Rossini M, Pesce F, Schena FP: Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 24: 3694–3701, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, Wang H: Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis 53: 26–32, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Lv J, Xu D, Perkovic V, Ma X, Johnson DW, Woodward M, Levin A, Zhang H, Wang H; TESTING Study Group: Corticosteroid therapy in IgA nephropathy. J Am Soc Nephrol 23: 1108–1116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ballardie FW, Roberts IS: Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol 13: 142–148, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Kidney Disease Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group: KDIGO clinical practice guideline for glomerulonephritis. Kidney Int 2: 139–274, 2012 [Google Scholar]
- 59.Hogg RJ, Bay RC, Jennette JC, Sibley R, Kumar S, Fervenza FC, Appel G, Cattran D, Fischer D, Hurley RM, Cerda J, Carter B, Jung B, Hernandez G, Gipson D, Wyatt RJ: Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. Am J Kidney Dis 66: 783–791, 2015 [DOI] [PubMed] [Google Scholar]
- 60.Frisch G, Lin J, Rosenstock J, Markowitz G, D’Agati V, Radhakrishnan J, Preddie D, Crew J, Valeri A, Appel G: Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: A double-blind randomized controlled trial. Nephrol Dial Transplant 20: 2139–2145, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Tang S, Leung JC, Chan LY, Lui YH, Tang CS, Kan CH, Ho YW, Lai KN: Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int 68: 802–812, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Tang SC, Tang AW, Wong SS, Leung JC, Ho YW, Lai KN: Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int 77: 543–549, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, Li LS, Mysler E, Sánchez-Guerrero J, Solomons N, Wofsy D; Aspreva Lupus Management Study Group: Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 20: 1103–1112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, Li Y, Yang S, Li Y, Liang M: Efficacy and safety of mycophenolate mofetil treatment in IgA nephropathy: A systematic review. BMC Nephrol 15: 193, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu G, Tu W, Jiang D, Xu C: Mycophenolate mofetil treatment for IgA nephropathy: A meta-analysis. Am J Nephrol 29: 362–367, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Tian L, Shao X, Xie Y, Wang L, Wang Q, Che X, Ni Z, Mou S: The long-term efficacy and safety of immunosuppressive therapy on the progression of IgA nephropathy: A meta-analysis of controlled clinical trials with more than 5-year follow-up. Expert Opin Pharmacother 16: 1137–1147, 2015 [DOI] [PubMed] [Google Scholar]
- 67.Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, Sethi S, Tumlin JA, Mehta K, Hogan M, Erickson S, Julian BA, Leung N, Enders FT, Brown R, Knoppova B, Hall S, Fervenza FC: A Randomized, Controlled Trial of Rituximab in IgA Nephropathy with Proteinuria and Renal Dysfunction [published online ahead of print November 7, 2016]. J Am Soc Nephrol doi:10.1681/ASN.2016060640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pozzi C, Andrulli S, Pani A, Scaini P, Del Vecchio L, Fogazzi G, Vogt B, De Cristofaro V, Allegri L, Cirami L, Procaccini AD, Locatelli F: Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol 21: 1783–1790, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pozzi C, Andrulli S, Pani A, Scaini P, Roccatello D, Fogazzi G, Pecchini P, Rustichelli R, Finocchiaro P, Del Vecchio L, Locatelli F: IgA nephropathy with severe chronic renal failure: A randomized controlled trial of corticosteroids and azathioprine. J Nephrol 26: 86–93, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Smerud HK, Bárány P, Lindström K, Fernström A, Sandell A, Påhlsson P, Fellström B: New treatment for IgA nephropathy: Enteric budesonide targeted to the ileocecal region ameliorates proteinuria. Nephrol Dial Transplant 26: 3237–3242, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Yeo SC, Liew A, Barratt J: Emerging therapies in immunoglobulin A nephropathy. Nephrology (Carlton) 20: 788–800, 2015 [DOI] [PubMed] [Google Scholar]
- 72.Reich HN, Troyanov S, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry: Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]