Abstract
Background and objectives
Elevated serum triglyceride/HDL cholesterol (TG/HDL-C) ratio has been identified as a risk factor for cardiovascular (CV) disease and mortality in the general population. However, the association of this important clinical index with mortality has not been fully evaluated in patients with ESRD on maintenance hemodialysis (MHD). We hypothesized that the association of serum TG/HDL-C ratio with all-cause and CV mortality in patients with ESRD on MHD is different from the general population.
Design, setting, participants, & measurements
We studied the association of serum TG/HDL-C ratio with all-cause and CV mortality in a nationally representative cohort of 50,673 patients on incident hemodialysis between January 1, 2007 and December 31, 2011. Association of baseline and time-varying TG/HDL-C ratios with mortality was assessed using Cox proportional hazard regression models, with adjustment for multiple variables, including statin therapy.
Results
During the median follow-up of 19 months (interquartile range, 11–32 months), 12,778 all-cause deaths and 4541 CV deaths occurred, respectively. We found that the 10th decile group (reference: sixth deciles of TG/HDL-C ratios) had significantly lower risk of all-cause mortality (hazard ratio, 0.91 [95% confidence interval, 0.83 to 0.99] in baseline and 0.86 [95% confidence interval, 0.79 to 0.94] in time-varying models) and CV mortality (hazard ratio, 0.83 [95% confidence interval, 0.72 to 0.96] in baseline and 0.77 [95% confidence interval, 0.66 to 0.90] in time-varying models). These associations remained consistent and significant across various subgroups.
Conclusions
Contrary to the general population, elevated TG/HDL-C ratio was associated with better CV and overall survival in patients on hemodialysis. Our findings provide further support that the nature of CV disease and mortality in patients with ESRD is unique and distinct from other patient populations. Hence, it is vital that future studies focus on identifying risk factors unique to patients on MHD and decipher the underlying mechanisms responsible for poor outcomes in patients with ESRD.
Keywords: Lipids; triglyceride; high-density lipoprotein cholesterol; mortality; hemodialysis; Cholesterol; Cholesterol, HDL; Confidence Intervals; Follow-Up Studies; Humans; Hydroxymethylglutaryl-CoA Reductase Inhibitors; Kidney Failure, Chronic; Lipoproteins, HDL; renal dialysis; risk factors; Triglycerides
Introduction
The number of patients with ESRD requiring maintenance dialysis in the United States currently stands at approximately 460,000 (1). Despite many recent improvements in dialysis treatment, patients with ESRD continue to experience a lower quality of life, high hospitalization rates, and high annual mortality rates of approximately 20%, a rate worse than that of many cancers (1). Although the causes of death are diverse, approximately half are directly attributed to cardiovascular (CV) disease (1). In spite of this enormous CV disease burden and high mortality, the main contributors to mortality risk have not been clearly identified in the ESRD population. In fact, traditional CV risk factors, such as hypercholesterolemia and obesity, have not been found to be reliable predictors of mortality risk in these patients, as previous studies have shown these factors are paradoxically associated with better survival in the hemodialysis population (2–7). Therefore, it is imperative that the so-called traditional risk factors for CV disease be reassessed in patients with ESRD in order to help improve our ability to identify those patients with the highest risk of mortality who may stand to obtain the most benefit from our interventions.
It is well known that elevated serum triglyceride (TG) and reduced HDL cholesterol (HDL-C) levels are risk factors for CV disease in the general population, independent of LDL cholesterol (LDL-C) levels. This is reflected by the fact that treatment with hydroxymethylglutaryl–CoA reductase inhibitors (statins) only partly reduces the rate of CV disease in high-risk patients, and significant residual risk persists despite achievement of target LDL-C levels. Several observational studies have shown that the residual risk, which remains after maximal statin therapy, may be partly attributed to high TG and low HDL-C levels (8–11). More recent studies have found that combination of high TG and low HDL-C in the form of a ratio, and its use as a single marker has greater predictive value for detecting the risk of CV disease when compared with each of those individual markers alone (12–14). Moreover, serum triglyceride/HDL cholesterol (TG/HDL-C) ratio can be a good predictor of risk for the occurrence of nonfatal CV events (14–16), CV death (17,18), and all-cause mortality (19,20) among healthy individuals and those with a wide range of CV risk factors. However, there are significant limitations in the few studies that examine the association between TG/HDL-C ratio and mortality in patients on dialysis given the small sample size, pooling data from patients on prevalent hemodialysis and peritoneal dialysis, and failure to account for changes in TG/HDL-C ratios over time (21,22). It is important to note that the strength of the latter studies is that they were conducted in patients whose lipid levels were measured during a fasting state. However, there is a growing body of evidence indicating that postprandial TG levels are important predictors of CV disease and mortality (23–25). This is in agreement with the Zilversmit hypothesis that atherosclerosis is a postprandial phenomenon and evaluation of plasma lipids (especially TG containing lipoproteins) in a nonfasting state may also provide vital information about an individual’s CV risk (26–28). We have previously shown that the association of serum TG and HDL-C levels with outcomes in patients on maintenance hemodialysis (MHD) does not follow the pattern observed in the general population, and in a subset of patients can be paradoxic to what is expected (5,29). Although the information regarding the fasting status of patients in our database is lacking, it is important to note that in the general population, elevated fasting and nonfasting TGs are associated with worse CV outcomes. Hence, regardless of the fasting state, our previous findings are somewhat paradoxic to what is known in the general population (28). Therefore, in this study we set out to examine the association of baseline and time-varying TG/HDL-C ratio with all-cause and CV mortality among patients on incident hemodialysis.
Materials and Methods
Study Population and Data Source
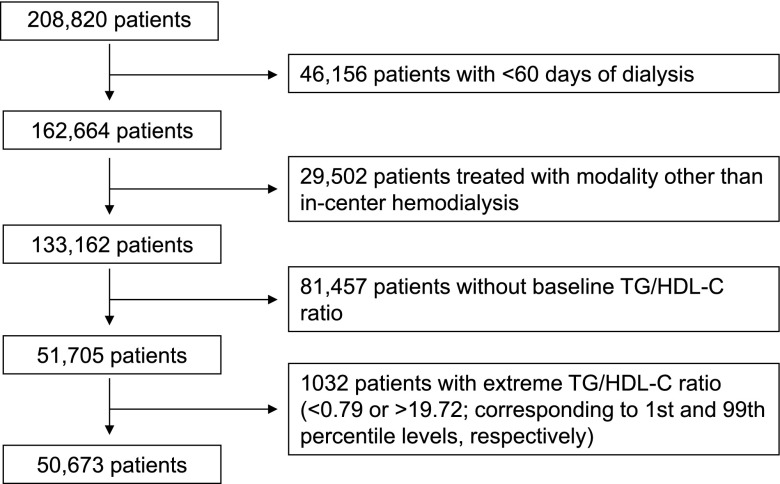
The study cohort was comprised of all patients with ESRD who were initiated on hemodialysis between January of 2007 and December of 2011 within one of the outpatient facilities of a large dialysis organization, and who were followed over a period of 5 years (30). Patients were included who were ≥18 years old, were treated with only in-center hemodialysis for at least 60 days, and had serum TG/HDL-C ratio measured during the first 91-day period of hemodialysis (baseline quarter). The comparison of baseline characteristics between patients in whom baseline TG/HDL-C ratio was missing and those in whom baseline TG/HDL-C ratio was available is summarized in Supplemental Table 1. Patients were further excluded if they had an outlier TG/HDL-C ratio value (below the first percentile or above the 99th percentile of observed values). Therefore, the final study population for all-cause mortality consisted of 50,673 patients (Figure 1).
Figure 1.
Among a cohort of 208,820 dialysis patients, 50,673 patients who met the inclusion and exclusion criteria were included in the final analyses. HDL-C, HDL cholesterol; TG, triglyceride.
All data were obtained from electronic records of the dialysis organization. To minimize measurement variability, all repeated measures of every relevant variable within each 3-month period, starting from the date of first dialysis (patient quarters), were averaged to obtain one quarterly mean value. Blood samples were drawn using standardized techniques in all dialysis clinics and were transported to a central laboratory in Deland (Florida), typically within 24 hours. Information regarding the fasting state of the patients was not available in the database; therefore, it is conceivable that this cohort included a mixture of fasting and nonfasting patients. The following 12 preexisting comorbidities were obtained from International Classification of Diseases, Ninth Revision codes from the electronic records database: diabetes mellitus, hypertension, atherosclerotic heart disease (ASHD), congestive heart failure (CHF), other CV disease, cerebrovascular disease, dyslipidemia, HIV, chronic obstructive pulmonary disease, malignancy, alcohol dependence, and substance abuse. The study was approved by the University of California Irvine Institutional Review Board. Given the large sample size, anonymity of the patients studied, and nonintrusive nature of the research, the requirement for written consent was waived.
Exposure and Outcome Ascertainment
The exposures of interest were baseline and time-varying serum TG/HDL-C ratio levels. Given a possible nonlinear relationship with mortality rates, the TG/HDL-C ratio was treated as a categorical variable and divided into deciles (see Tables 1–3 for respective cutoff points). The reference TG/HDL ratio category for all analyses was the sixth decile. This category was chosen as the reference because the median ratio in this study was 3.64, which is similar to the ratio of 3.8 that was used in previous studies and is derived from Adult Treatment Panel recommendations (on the basis of normal fasting TG<150 mg/dl and HDL-C>40 mg/dl) (21,31,32).
Table 1.
Baseline characteristics of 50,673 patients stratified by serum triglyceride/HDL cholesterol ratio deciles
| Serum Triglyceride/HDL Cholesterol Ratio | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | <1.59 | 1.59–<2.09 | 2.09–<2.57 | 2.57–<3.07 | 3.07–<3.64 | 3.64–<4.30 | 4.30–<5.18 | 5.18–<6.41 | 6.41–<8.63 | ≥8.63 |
| (n=5067) | (n=5072) | (n=5062) | (n=5062) | (n=5072) | (n=5071) | (n=5061) | (n=5068) | (n=5070) | (n=5068) | |
| Age, yr | 63.9±15.5 | 64.4±15.4 | 64.1±15.2 | 64.1±14.7 | 63.9±14.9 | 63.0±14.7 | 62.8±14.5 | 62.1±14.2 | 61.1±14.3 | 59.2±14.1 |
| Sex, % women | 46.7 | 47.3 | 45.2 | 46.9 | 43.9 | 43.7 | 42.6 | 42.6 | 39.9 | 38.0 |
| Race, % | ||||||||||
| White | 35.4 | 41.1 | 43.3 | 43.9 | 46.2 | 48.4 | 49.9 | 50.0 | 52.0 | 55.3 |
| Black | 48.1 | 40.1 | 37.8 | 35.1 | 32.2 | 30.1 | 27.2 | 26.1 | 22.2 | 18.5 |
| Hispanic | 10.6 | 12.5 | 13.0 | 14.4 | 15.1 | 15.2 | 16.2 | 17.2 | 19.2 | 19.3 |
| Asian | 2.6 | 3.0 | 2.8 | 2.9 | 2.9 | 3.0 | 2.9 | 3.4 | 3.2 | 3.2 |
| Others | 3.1 | 3.4 | 3.1 | 3.8 | 3.7 | 3.3 | 3.8 | 3.3 | 3.5 | 3.6 |
| Primary insurance, % | ||||||||||
| Medicare | 54.8 | 55.0 | 55.0 | 55.0 | 54.8 | 53.5 | 52.9 | 52.0 | 50.8 | 49.8 |
| Medicaid | 6.5 | 6.1 | 6.4 | 5.5 | 6.7 | 6.3 | 6.5 | 6.5 | 6.8 | 6.9 |
| Others | 38.8 | 38.9 | 38.6 | 39.5 | 38.5 | 40.2 | 40.6 | 41.4 | 42.4 | 43.3 |
| Initial vascular access type, % | ||||||||||
| Central venous catheter | 70.7 | 72.4 | 72.4 | 74.1 | 74.8 | 74.4 | 75.3 | 75.6 | 76.8 | 76.4 |
| Arteriovenous fistula | 17.5 | 16.8 | 16.7 | 14.8 | 14.6 | 16.1 | 15.4 | 15.4 | 14.3 | 14.7 |
| Arteriovenous graft | 5.3 | 4.7 | 4.9 | 5.3 | 4.3 | 4.1 | 4.0 | 3.5 | 3.4 | 3.3 |
| Others and unknown | 6.5 | 6.1 | 6.0 | 5.8 | 6.3 | 5.4 | 5.3 | 5.5 | 5.5 | 5.6 |
| Comorbidities, % | ||||||||||
| Diabetes | 60.5 | 60.6 | 61.5 | 61.9 | 62.5 | 62.1 | 64.0 | 63.6 | 64.9 | 66.3 |
| Hypertension | 56.8 | 55.0 | 52.3 | 52.5 | 52.9 | 51.1 | 50.6 | 49.9 | 49.2 | 47.3 |
| Congestive heart failure | 37.7 | 36.5 | 36.4 | 36.6 | 35.9 | 37.3 | 36.3 | 35.9 | 36.0 | 37.5 |
| Atherosclerotic heart disease | 17.6 | 18.1 | 19.0 | 18.0 | 18.3 | 19.3 | 17.9 | 18.0 | 18.2 | 18.2 |
| Other cardiovascular disease | 17.3 | 16.3 | 16.8 | 16.7 | 17.1 | 16.9 | 16.2 | 16.2 | 17.0 | 16.7 |
| Cerebrovascular disease | 1.9 | 1.3 | 1.8 | 1.6 | 1.8 | 1.9 | 1.7 | 1.4 | 2.1 | 1.9 |
| Dyslipidemia | 35.4 | 33.9 | 35.0 | 33.2 | 34.3 | 34.7 | 35.2 | 33.9 | 34.8 | 36.4 |
| HIV | 0.4 | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 | 0.6 | 0.4 | 0.7 | 0.8 |
| COPD | 5.4 | 5.3 | 5.1 | 5.2 | 5.6 | 5.1 | 5.5 | 4.5 | 5.1 | 4.5 |
| History of malignancy | 2.0 | 2.1 | 2.1 | 2.6 | 2.3 | 2.2 | 2.2 | 2.3 | 2.5 | 2.5 |
| Alcohol dependence, % | 0.4 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 | 0.1 |
| Substance abuse, % | 0.5 | 0.4 | 0.4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Statin therapy, % | 37.0 | 40.1 | 39.2 | 41.2 | 41.9 | 41.6 | 42.9 | 42.6 | 42.8 | 42.8 |
| Dialysis dose: single pool Kt/V | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.4±0.3 |
| Body mass index, kg/m2 | 25.9±6.5 | 26.7±7.2 | 27.2±7.1 | 27.8±7.3 | 27.9±7.3 | 28.5±7.4 | 29.0±7.5 | 29.4±7.4 | 29.8±7.5 | 30.6±7.4 |
| Lipid parameters | ||||||||||
| Total cholesterol, mg/dl | 151.9±41.5 | 146.5±40.9 | 145.1±41.7 | 146.4±42.7 | 145.9±42.5 | 148.3±43.6 | 149.8±44.7 | 152.5±43.7 | 157.6±46.5 | 168.0±55.5 |
| LDL-C, mg/dl | 78.4±31.9 | 78.3±31.9 | 78.1±33.0 | 80.0±34.2 | 79.9±34.1 | 81.0±35.3 | 81.5±36.8 | 81.4±36.3 | 81.9±38.3 | 78.4±42.2 |
| TG, mg/dl | 73.2±18.6 | 91.8±22.1 | 106.2±24.9 | 119.7±28.1 | 132.7±30.8 | 148.6±34.7 | 165.9±38.1 | 190.3±42.7 | 225.5±52.1 | 310.4±91.7 |
| HDL-C, mg/dl | 59.0±14.5 | 49.9±11.9 | 45.7±10.7 | 42.6±9.9 | 39.7±9.2 | 37.6±8.7 | 35.2±8.0 | 33.1±7.4 | 30.6±6.9 | 27.0±6.8 |
| TG/HDL-C ratio | 1.3±0.2 | 1.8±0.1 | 2.3±0.1 | 2.8±0.1 | 3.3±0.2 | 4.0±0.2 | 4.7±0.2 | 5.8±0.4 | 7.4±0.6 | 11.7±2.6 |
| Other laboratory parameters | ||||||||||
| Hemoglobin, g/dl | 11.1±1.2 | 11.1±1.2 | 11.1±1.2 | 11.1±1.2 | 11.1±1.2 | 11.1±1.2 | 11.1±1.2 | 11.1±1.2 | 11.1±1.2 | 11.1±1.2 |
| White blood cells, ×103/µl | 7.2±2.3 | 7.4±2.4 | 7.6±2.4 | 7.7±2.7 | 7.8±2.4 | 7.8±2.5 | 7.9±2.5 | 8.1±2.8 | 8.1±2.8 | 8.2±3.0 |
| Albumin, g/dl | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 | 3.6±0.5 |
| Calcium, mg/dl | 9.1±0.6 | 9.1±0.5 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.5 | 9.1±0.6 | 9.1±0.6 |
| Phosphorus, mg/dl | 4.9±1.1 | 4.9±1.1 | 4.9±1.1 | 4.9±1.1 | 4.9±1.2 | 4.9±1.1 | 4.9±1.1 | 4.9±1.1 | 4.9±1.1 | 5.0±1.2 |
| Intact PTH, pg/ml | 330 | 317 | 317 | 313 | 313 | 315 | 312 | 316 | 314 | 312 |
| (202–516) | (201–499) | (198–486) | (199–479) | (196–488) | (201–478) | (198–481) | (196–486) | (196–474) | (196–474) | |
| Bicarbonate, mEq/L | 23.9±2.8 | 24.0±2.8 | 23.8±2.7 | 23.8±2.7 | 23.8±2.7 | 23.7±2.7 | 23.6±2.7 | 23.5±2.6 | 23.3±2.6 | 23.0±2.6 |
| TIBC, mg/dl | 224.6±45.4 | 223.4±46.1 | 222.3±46.8 | 222.4±47.6 | 223.2±47.8 | 223.9±49.1 | 226.0±48.7 | 226.8±48.7 | 229.1±50.0 | 234.8±52.7 |
| Ferritin, ng/ml | 253 | 260 | 249 | 280 | 283 | 293 | 296 | 304 | 316 | 312 |
| (146–429) | (154–438) | (163–465) | (168–482) | (170–469) | (171–508) | (174–496) | (184–506) | (185–535) | (182–535) | |
Data are presented as means±SD, median (interquartile range), or percentage. COPD, chronic obstructive pulmonary disease; LDL-C, LDL cholesterol; TG, triglyceride; HDL-C, HDL cholesterol; TG/HDL-C, triglyceride/HDL cholesterol ratio; PTH, parathyroid hormone; TIBC, total iron binding capacity.
Table 3.
Association of serum triglyceride/HDL cholesterol ratio with cardiovascular mortality, stratified by decile categories
| TG/HDL-C Levels | Unadjusted | Case-Mix | Case-Mix and MICS | Case-Mix, MICS, and Statin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Baseline model | ||||||||||||
| <1.59 | 1.06 | (0.94 to 1.20) | 0.32 | 1.12 | (0.99 to 1.27) | 0.07 | 1.09 | (0.96 to 1.24) | 0.17 | 1.07 | (0.93 to 1.23) | 0.33 |
| 1.59 to <2.10 | 0.98 | (0.86 to 1.11) | 0.74 | 0.99 | (0.87 to 1.12) | 0.86 | 0.95 | (0.84 to 1.08) | 0.45 | 0.90 | (0.78 to 1.03) | 0.13 |
| 2.10 to <2.57 | 0.95 | (0.84 to 1.08) | 0.48 | 0.94 | (0.82 to 1.06) | 0.32 | 0.92 | (0.81 to 1.05) | 0.21 | 0.87 | (0.75 to 1.00) | 0.05 |
| 2.57 to <3.08 | 1.07 | (0.95 to 1.21) | 0.28 | 1.06 | (0.93 to 1.20) | 0.40 | 1.04 | (0.91 to 1.17) | 0.59 | 1.06 | (0.92 to 1.21) | 0.44 |
| 3.08 to <3.65 | 0.94 | (0.82 to 1.06) | 0.31 | 0.90 | (0.79 to 1.03) | 0.12 | 0.88 | (0.77 to 1.00) | 0.06 | 0.90 | (0.78 to 1.03) | 0.13 |
| 3.65 to <4.32 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 4.32 to <5.19 | 0.94 | (0.83 to 1.07) | 0.35 | 0.91 | (0.80 to 1.04) | 0.16 | 0.92 | (0.81 to 1.05) | 0.24 | 0.93 | (0.80 to 1.07) | 0.29 |
| 5.19 to <6.43 | 0.85 | (0.74 to 0.97) | 0.01 | 0.84 | (0.74 to 0.96) | 0.01 | 0.87 | (0.76 to 0.99) | 0.04 | 0.84 | (0.73 to 0.98) | 0.02 |
| 6.43 to <8.64 | 0.83 | (0.73 to 0.95) | 0.01 | 0.82 | (0.72 to 0.94) | 0.003 | 0.84 | (0.73 to 0.96) | 0.01 | 0.85 | (0.73 to 0.98) | 0.03 |
| ≥8.64 | 0.76 | (0.67 to 0.87) | <0.001 | 0.79 | (0.69 to 0.91) | 0.001 | 0.83 | (0.72 to 0.96) | 0.01 | 0.84 | (0.72 to 0.98) | 0.03 |
| Time-varying model | ||||||||||||
| <1.38 | 1.10 | (0.97 to 1.25) | 0.12 | 1.21 | (1.07 to 1.37) | 0.003 | 1.21 | (1.06 to 1.38) | 0.004 | 1.19 | (1.03 to 1.37) | 0.02 |
| 1.38 to <1.84 | 1.09 | (0.97 to 1.24) | 0.16 | 1.14 | (1.00 to 1.29) | 0.04 | 1.11 | (0.98 to 1.26) | 0.11 | 1.11 | (0.96 to 1.27) | 0.16 |
| 1.84 to <2.29 | 1.13 | (1.00 to 1.28) | 0.06 | 1.15 | (1.01 to 1.30) | 0.03 | 1.10 | (0.96 to 1.24) | 0.16 | 1.08 | (0.94 to 1.24) | 0.30 |
| 2.29 to <2.77 | 1.05 | (0.93 to 1.19) | 0.42 | 1.06 | (0.93 to 1.20) | 0.40 | 1.02 | (0.89 to 1.16) | 0.80 | 1.03 | (0.89 to 1.18) | 0.71 |
| 2.77 to <3.33 | 1.00 | (0.88 to 1.14) | 0.97 | 1.01 | (0.88 to 1.14) | 0.94 | 0.98 | (0.86 to 1.11) | 0.74 | 0.97 | (0.84 to 1.12) | 0.70 |
| 3.33 to <4.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 4.00 to <4.89 | 0.92 | (0.81 to 1.05) | 0.23 | 0.93 | (0.82 to 1.06) | 0.29 | 0.96 | (0.84 to 1.09) | 0.52 | 0.88 | (0.76 to 1.02) | 0.10 |
| 4.89 to <6.19 | 0.81 | (0.71 to 0.93) | 0.002 | 0.82 | (0.72 to 0.94) | 0.004 | 0.84 | (0.74 to 0.97) | 0.02 | 0.84 | (0.72 to 0.98) | 0.02 |
| 6.19 to <8.59 | 0.81 | (0.71 to 0.93) | 0.002 | 0.83 | (0.73 to 0.95) | 0.01 | 0.88 | (0.77 to 1.01) | 0.07 | 0.88 | (0.76 to 1.03) | 0.11 |
| ≥8.59 | 0.66 | (0.57 to 0.76) | <0.001 | 0.70 | (0.61 to 0.81) | <0.001 | 0.77 | (0.66 to 0.90) | 0.001 | 0.78 | (0.66 to 0.92) | 0.004 |
Adjustments in case-mix model (n=48,728): age, sex, race/ethnicity, primary insurance, vascular access type, comorbid conditions, alcohol dependence, substance abuse, and single-pool Kt/V. Case-mix plus MICS model (n=47,185): case-mix adjusted model plus laboratory parameters including serum hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron binding capacity, ferritin, LDL cholesterol, and body mass index. Case-mix plus MICS plus statin model (n=42,605): statin therapy on the fully adjusted models. TG/HDL-C, triglyceride/HDL cholesterol ratio; MICS, malnutrition-inflammation-cachexia syndrome; HR, hazard ratio; 95% CI, 95% confidence interval.
The primary and secondary outcomes of interest were time to all-cause and CV death, respectively. For mortality analyses, patients remained at-risk until death, censoring for loss to follow-up, discontinuation of dialysis therapy, kidney transplantation, transfer to a nonaffiliated dialysis clinic, or end of the study period (December 31, 2011).
Statistical Analyses
Data were summarized using proportions, means (±SD), or median (interquartile range [IQR]) as appropriate, and were compared using paired t test, ANOVA, Kruskal–Wallis, and chi-squared tests, respectively.
Cox proportional hazard regression models were separately performed to study the associations of baseline and time-varying TG/HDL-C ratios with mortality. In baseline models, TG/HDL-C ratio and covariates were determined at baseline and their association with mortality was estimated. In time-varying models, TG/HDL-C ratio and covariates were calculated and updated at each patient quarter over the entire follow-up period to assess short-term associations between TG/HDL-C ratio and risk of death, assuming that TG/HDL-C ratio remained unchanged during the time interval before the next measurement. For each analysis, unadjusted and two additional models were constructed on the basis of the level of multivariate adjustment: (1) case-mix adjusted models, which adjusted for baseline characteristics of age, sex, race/ethnicity (white, black, Hispanic, Asian, or other), primary insurance (Medicare, Medicaid, and others), initial vascular access type (central venous catheter, arteriovenous fistula, arteriovenous graft, or other), 12 comorbid conditions, and dialysis dose as indicated by single-pool Kt/V; and (2) fully adjusted models, which included all covariates in the case-mix model plus malnutrition-inflammation-cachexia syndrome variables, including serum hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron binding capacity, ferritin, LDL-C, and body mass index (BMI, postdialysis dry body weight in kilograms/height in meters squared). Additional adjustment was also done for statin therapy (i.e., if statin therapy was ever used at any time during the follow-up period) in addition to the fully adjusted model. All mortality associations are expressed as hazard ratios and 95% confidence intervals.
For sensitivity analyses, we additionally explored the continuous, potentially nonlinear relationship between TG/HDL-C ratio and mortality by using fully adjusted, restricted cubic spline models with four knots. Given that TG and HDL-C metabolism may differ between sexes, we further assessed the association of TG/HDL-C ratio and mortality stratified by sex. To test the robustness of our findings, we also performed subgroup analyses on the basis of a priori selected variables; for example, age, sex, race/ethnicity, diabetes, hypertension, ASHD, CHF, statin therapy, serum LDL-C, hemoglobin, ferritin, intact parathyroid hormone, and albumin concentrations.
The frequency of missing data were low (≤0.5%, ascertained at baseline) for most covariates in multivariate adjusted models, except for statin therapy (10.2%), for which patients were excluded only from the analysis adjusting for statin therapy. As CV and non-CV death are competing events, sensitivity analyses were done using semiparametric baseline and time-varying competing risk regression evaluating the association of TG/HDL-C ratio, with the two outcomes noted (33). All analyses were implemented using Stata, version 13.1 (StataCorp., College Station, TX).
Results
Study Population
The baseline demographics, clinical, and laboratory characteristics of the patients according to serum TG/HDL-C ratio deciles are summarized in Table 1. The mean age of patients was 62.9±14.9 years, 44% were women, and 63% were diabetic. The mean±SD and median (IQR) baseline TG/HDL-C ratio values were 4.51±3.09 and 3.64 (2.33–5.74), respectively. Patients with elevated baseline TG/HDL-C ratios when compared with the reference group tended to be younger men who were more likely to be white and diabetic. They also had lower serum HDL-C and higher BMI, total cholesterol, LDL-C, and TG levels.
During the mean follow-up of 22.7 months, 12,778 deaths occurred, with a crude mortality rate of 133 deaths per 1000 patient-years (95% confidence interval, 131 to 136). Of these, 11,391 individuals had data available on primary cause of death, in which 4541 (39.9%) were attributed to CV mortality. From the lowest (first) to highest (10th) deciles of baseline TG/HDL-C ratio, all-cause and CV mortality rates were 141, 142, 147, 141, 138, 137, 131, 126, 122, and 109, and 55, 51, 49, 55, 48, 52, 48, 44, 43, and 39 deaths per 1000 patient-years, respectively. The most common cause of CV death in this study was sudden cardiac death (n=2882, 62.1%), followed by acute myocardial infarction (n=492, 10.8%), CHF (n=296, 6.5%), cerebrovascular accident (n=231, 5.1%), arrhythmia (n=230, 5.1%), cardiomyopathy (n=219, 4.8%), ASHD (n=106, 2.3%), hypoxic brain damage (n=44, 1.0%), and pulmonary edema (n=35, 0.8%). Although there were some patients whose cause of death was unknown, when compared with patients who had available information about cause of death, their demographic, comorbidity, and laboratory characteristics were mostly similar (Supplemental Table 2).
All-Cause and CV Mortality
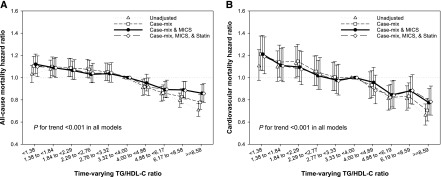
In contrast to the association between TG/HDL-C ratio and mortality that is seen in the general population, higher TG/HDL-C ratio was associated with better survival in patients on incident hemodialysis. The highest decile group on the basis of baseline TG/HDL-C ratio was associated with the lowest all-cause and CV mortality at all four levels of adjustment (Supplemental Figure 1, Tables 2 and 3). To account for changes in serum TG/HDL-C ratios over time and to examine short-term TG/HDL-C ratio and mortality associations, we used time-varying Cox regression models as shown in Figure 2. The median number of TG/HDL-C ratio that contributed to each quarterly-averaged metric per patient was three (IQR, 1–5 measurements). In the fully adjusted model, there was a graded inverse association between serum TG/HDL-C ratio and all-cause and CV mortality from the lowest to highest deciles, with significantly higher mortality in deciles 1–2 and lower mortality in deciles 8–10. It should be noted that these associations remained largely unchanged despite additional adjustment for statin therapy (Tables 2 and 3).
Table 2.
Association of serum triglyceride/HDL cholesterol ratio with all-cause mortality, stratified by decile categories
| TG/HDL-C Levels | Unadjusted | Case-Mix | Case-Mix and MICS | Case-Mix, MICS, and Statin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Baseline model | ||||||||||||
| <1.59 | 1.02 | (0.95 to 1.10) | 0.55 | 1.06 | (0.98 to 1.14) | 0.15 | 1.04 | (0.97 to 1.13) | 0.29 | 0.99 | (0.91 to 1.08) | 0.89 |
| 1.59 to <2.09 | 1.03 | (0.95 to 1.11) | 0.46 | 1.02 | (0.95 to 1.10) | 0.59 | 1.00 | (0.93 to 1.08) | 0.99 | 0.96 | (0.89 to 1.05) | 0.38 |
| 2.09 to <2.57 | 1.07 | (0.99 to 1.16) | 0.07 | 1.04 | (0.96 to 1.12) | 0.34 | 1.02 | (0.94 to 1.10) | 0.67 | 0.98 | (0.90 to 1.07) | 0.70 |
| 2.57 to <3.07 | 1.03 | (0.96 to 1.11) | 0.41 | 1.01 | (0.94 to 1.09) | 0.78 | 1.00 | (0.92 to 1.08) | 0.99 | 0.98 | (0.90 to 1.07) | 0.67 |
| 3.07 to <3.64 | 1.01 | (0.93 to 1.09) | 0.85 | 0.97 | (0.89 to 1.04) | 0.37 | 0.94 | (0.87 to 1.02) | 0.14 | 0.95 | (0.87 to 1.03) | 0.20 |
| 3.64 to <4.30 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 4.30 to <5.18 | 0.96 | (0.88 to 1.03) | 0.26 | 0.94 | (0.87 to 1.01) | 0.10 | 0.96 | (0.89 to 1.04) | 0.35 | 0.97 | (0.89 to 1.05) | 0.43 |
| 5.18 to <6.41 | 0.92 | (0.85 to 1.00) | 0.04 | 0.92 | (0.85 to 0.99) | 0.03 | 0.95 | (0.88 to 1.03) | 0.21 | 0.93 | (0.86 to 1.02) | 0.12 |
| 6.41 to <8.63 | 0.89 | (0.82 to 0.96) | 0.004 | 0.90 | (0.83 to 0.97) | 0.01 | 0.93 | (0.86 to 1.01) | 0.07 | 0.92 | (0.84 to 1.00) | 0.05 |
| ≥8.63 | 0.79 | (0.73 to 0.86) | <0.001 | 0.85 | (0.78 to 0.92) | <0.001 | 0.91 | (0.83 to 0.99) | 0.03 | 0.90 | (0.82 to 0.99) | 0.02 |
| Time-varying model | ||||||||||||
| <1.38 | 1.03 | (0.96 to 1.11) | 0.43 | 1.10 | (1.02 to 1.19) | 0.001 | 1.12 | (1.04 to 1.21) | 0.004 | 1.10 | (1.01 to 1.20) | 0.03 |
| 1.38 to <1.84 | 1.07 | (1.00 to 1.16) | 0.06 | 1.10 | (1.02 to 1.19) | 0.01 | 1.09 | (1.01 to 1.18) | 0.03 | 1.09 | (1.00 to 1.18) | 0.05 |
| 1.84 to <2.28 | 1.09 | (1.01 to 1.17) | 0.03 | 1.09 | (1.01 to 1.17) | 0.03 | 1.06 | (0.99 to 1.15) | 0.12 | 1.07 | (0.98 to 1.16) | 0.12 |
| 2.28 to <2.76 | 1.08 | (1.00 to 1.17) | 0.04 | 1.07 | (1.00 to 1.16) | 0.06 | 1.03 | (0.95 to 1.11) | 0.45 | 1.04 | (0.96 to 1.13) | 0.36 |
| 2.76 to <3.32 | 1.05 | (0.97 to 1.13) | 0.20 | 1.05 | (0.97 to 1.13) | 0.21 | 1.04 | (0.96 to 1.12) | 0.37 | 1.04 | (0.96 to 1.13) | 0.32 |
| 3.32 to <4.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 4.00 to <4.88 | 0.91 | (0.84 to 0.98) | 0.01 | 0.92 | (0.85 to 0.99) | 0.03 | 0.95 | (0.88 to 1.03) | 0.23 | 0.91 | (0.84 to 1.00) | 0.04 |
| 4.88 to <6.17 | 0.85 | (0.79 to 0.92) | <0.001 | 0.86 | (0.80 to 0.93) | <0.001 | 0.89 | (0.82 to 0.97) | 0.01 | 0.89 | (0.82 to 0.98) | 0.01 |
| 6.17 to <8.57 | 0.79 | (0.73 to 0.86) | <0.001 | 0.83 | (0.76 to 0.90) | <0.001 | 0.89 | (0.82 to 0.97) | 0.01 | 0.88 | (0.81 to 0.97) | 0.01 |
| ≥8.57 | 0.71 | (0.65 to 0.77) | <0.001 | 0.78 | (0.71 to 0.85) | <0.001 | 0.86 | (0.79 to 0.94) | 0.001 | 0.86 | (0.78 to 0.94) | 0.002 |
Adjustments in case-mix model (n=50,105): age, sex, race/ethnicity, primary insurance, vascular access type, comorbid conditions, alcohol dependence, substance abuse, and single-pool Kt/V. Adjustments in case-mix plus MICS model (n=48,527): case-mix adjusted model plus laboratory parameters including serum hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron binding capacity, ferritin, LDL cholesterol, and body mass index. Adjustments in case-mix plus MICS plus statin model (n=43,742): statin therapy on the fully adjusted models. TG/HDL-C, triglyceride/HDL cholesterol ratio; MICS, malnutrition-inflammation-cachexia syndrome; HR, hazard ratio; 95% CI, 95% confidence interval.
Figure 2.
Higher TG/HDL-C ratio was associated with improved survival in incident hemodialysis patients. Time-varying all-cause (A) and cardiovascular (B) mortality hazard ratios (and 95% confidence interval error bars) by serum triglyceride/HDL cholesterol (TG/HDL-C) ratio. Adjustments in case-mix model: age, sex, race/ethnicity, primary insurance, vascular access type, comorbid conditions, alcohol dependence, substance abuse, and single-pool Kt/V; case-mix plus malnutrition-inflammation-cachexia syndrome (MICS) models: case-mix adjusted model plus laboratory parameters, including serum hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron binding capacity, ferritin, LDL cholesterol, and body mass index; case-mix plus MICS plus statin models: statin therapy on the fully adjusted models. Higher TG/HDL-C ratio was associated with improved survival in incident hemodialysis patients.
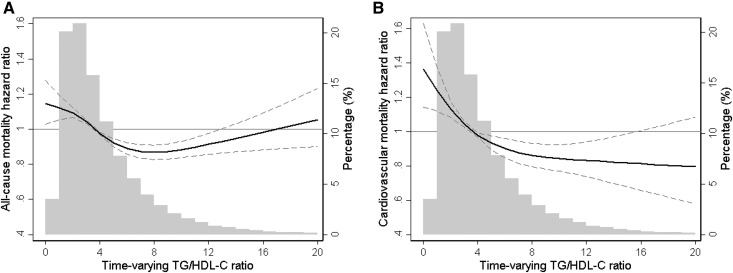
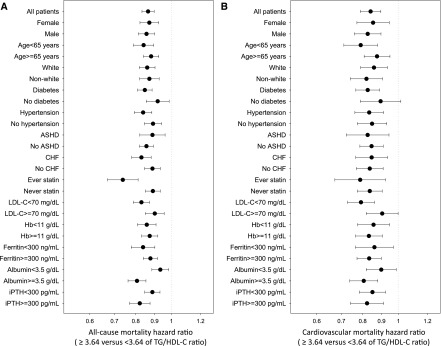
In sensitivity analyses using competing risk regression models in which non-CV death was assigned as a competing event, higher TG/HDL-C ratio was similarly associated with better survival and reduced CV mortality (Supplemental Table 3). In addition, a similar trend was observed for both all-cause and CV mortality in fully adjusted cubic spine models using baseline (Supplemental Figure 2) and time-varying (Figure 3) TG/HDL-C levels in which incrementally higher TG/HDL-C ratios were associated with lower risk of death. Although extreme TG/HDL-C levels tended to trend toward higher all-cause mortality risk, the small sample size in these categories makes these associations less reliable. Given the role of sex on lipids profile and metabolism, we also compared these association on the basis of patients’ sex. Although the overall trend remained similar between the two groups, there were small differences between men and women, as the association between higher TG/HDL-C ratio and better survival appeared to be stronger in men (Supplemental Figures 3 and 4). Further subgroup analyses confirmed the strong and consistent association between high TG/HDL-C ratio (median TG/HDL-C ≥3.64 versus <3.64) and lower all-cause and CV mortality across all prespecified subgroups (Figure 4).
Figure 3.
The data demonstrate that incrementally higher TG/HDL-C ratios were associated with lower risk of death. Multivariate adjusted hazard ratios of all-cause (A) and cardiovascular (B) mortality associated with time-varying triglyceride/HDL cholesterol (TG/HDL-C) ratios in a Cox model using restricted cubic spines, adjusted for age, sex, race/ethnicity, primary insurance, vascular access type, comorbidities, alcohol dependence, substance abuse, single-pool Kt/V, hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron binding capacity, ferritin, LDL cholesterol, and bodymass index. A histogram of observed time-varying TG/HDL-C ratio values and a hazard reference ratio of 1 (solid line) is overlaid.
Figure 4.
There is significant association between high TG/HDL-C ratio (≥3.64 versus <3.64 of median TG/HDL-C value) and lower all-cause and CV mortality across all prespecified subgroups. Time-varying hazard ratios of the level of or above median (‡3.64) versus the level below median (<3.64) of triglyceride/HDL cholesterol (TG/HDL-C) ratios for all-cause (A) and cardiovascular (B) mortality, adjusted for age, sex, race/ethnicity, primary insurance, vascular access type, comorbidities, alcohol dependence, substance abuse, single-pool Kt/V, hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron binding capacity, ferritin, LDL cholesterol, and body mass index. ASHD, atherosclerotic heart disease; CHF, congestive heart failure; Hb, hemoglobin; iPTH, intact parathyroid hormone; LDL-C, LDL cholesterol.
Discussion
In a large contemporary cohort of 50,673 patients treated with thrice-weekly hemodialysis and followed for up to 5 years, we found that higher TG/HDL-C ratios were associated with better survival and reduced CV mortality. To the best of our knowledge, this is the first published study to demonstrate an inverse association between TG/HDL-C ratio and mortality in the dialysis population. These findings are in contrast to the associations seen in the general population, in whom higher TG/HDL-C ratio are associated with increased risk of mortality. The large sample size, consideration of changes in TG/HDL-C ratios over time, and extensive adjustment and subgroup analyses are strengths of this study. These results are important in that they provide additional information about the unique and paradoxic associations between lipid levels and mortality in patients with ESRD on MHD. Furthermore, these findings highlight the distinct nature of hemodialysis- and ESRD-associated dyslipidemia, which is marked by abnormal TG production and metabolism and HDL-C deficiency and dysfunction.
Dyslipidemia is a well established traditional risk factor for CV disease in the general population. However, we have found that this relationship is much more complex and can even be paradoxic in some patients with ESRD on MHD (34,35). For instance, in contrast to the general population, serum LDL-C level has not been found to be a reliable indicator of CV risk in patients with ESRD (36,37). These epidemiologic observations are further supported by the recent randomized clinical trials that show that lowering serum LDL-C levels using statin therapy does not lead to reduction in CV events or mortality in patients on hemodialysis (38–40). Indeed, it is important to note that the hallmarks of dyslipidemia in CKD are impaired clearance of VLDL and chylomicrons, leading to hypertriglyceridemia, accumulation of intermediate-density lipoprotein and chylomicron remnants, and deficiency and impaired maturation of HDL, in addition to defective HDL antioxidant, anti-inflammatory, and reverse cholesterol transport activities (41). Clinically these molecular mechanisms manifest as high serum TG and low HDL-C levels, whereas serum total cholesterol and LDL-C concentrations are typically within the normal or reduced range (42). Previously, we found that increasing serum concentrations of TG are not associated with adverse outcomes in the hemodialysis population (5). Furthermore, in a more recent study we found that elevated serum levels of HDL-C can be associated with worse CV and all-cause mortality in a cohort of patients with prevalent ESRD on MHD (29). However, in both studies the associations mentioned were J-shaped, and the lowest and highest levels were associated with increased mortality. This is in contrast to the association between TG/HDL-C ratio and outcomes, which follows a more linear pattern in most of our analyses, as increasing ratios are mostly associated with improvement in survival. However, there does seem to be some difference in the association of this ratio and outcomes, depending on the patients’ sex: higher ratio seems to be more strongly associated with better outcome in men. Furthermore, as noted earlier, information regarding the fasting state of the patients in these cohorts is not available, and therefore one can argue that those with elevated TG levels may have been in a postprandial state.
Although the underlying mechanisms responsible for these observations are not clear at this time, these findings further demonstrate the limitations of serum lipid profile in predicting outcomes in patients on MHD. In this regard, paradoxic associations between high TG/HDL-C ratio and better survival can be explained by a growing body of evidence that indicates that nontraditional risk factors, such as oxidative stress and inflammation, may play a more critical role in ESRD-associated mortality than conventional indices, such as dyslipidemia. It is now becoming clear that in the setting of uremia, oxidative stress, and inflammation, HDL can be transformed from an antioxidant and anti-inflammatory lipoprotein to a pro-oxidant, proinflammatory particle, known as acute-phase HDL (43–46). Therefore, measurement of serum HDL-C concentrations does not provide any information regarding the nature of the HDL structure present in a given patient, or its function or properties (47,48). In fact, a study in a cohort of Japanese patients on MHD found that higher HDL-C concentrations were associated with higher levels of oxidized HDL-C, which were also associated with increased CV mortality (49). The same concept can be applied to TG and TG-rich lipoproteins because serum TG levels do not reflect the qualitative characteristics of the TG-carrying lipoproteins. Therefore, it is possible that the make-up or nature of a particular lipoprotein is much more important than its quantity in determining its effect on CV disease and mortality. Another important potential explanation for these seemingly paradoxic associations is the time-dependency of adverse effects imparted by dyslipidemia. Because the deleterious effects of dyslipidemia in atherogenesis typically take effect over a longer time period, it is possible that serum lipid levels in the short term are reflective of other factors (i.e., those with elevated serum TGs have a better nutritional status). This is also in line with so-called “reverse causation,” meaning it is also possible that lower TG/HDL-C ratio is not a cause but a consequence of underlying conditions, such as malnutrition, that concurrently lead to poor outcomes in this population (50). In fact, the role of malnutrition and protein energy wasting deserve close attention because our subgroup analyses revealed that the association of higher TG levels with improved outcomes may play a major role in the findings of this study. Although our findings remain significant despite adjustment for BMI, in order to better assess the role of malnutrition, future studies need to examine more objective nutritional assessments and their effect on the association of serum TG levels and outcomes in patients with ESRD. Regardless of the underlying mechanism, the interplay between these lipid abnormalities and their association with CV disease and mortality in patients on hemodialysis presents a unique challenge in clinical practice, as their effect on outcomes still remains to be fully clarified.
Several limitations of our study should be mentioned. First, the present findings should be qualified, given the observational nature of our study design, which precludes conclusions about causality. In addition, it should be mentioned that not all of the patients in our database had baseline TG/HDL-C ratio and 11% of patients did not have data available on CV mortality, which raises concern for selection bias. However, we did compare all baseline characteristics between the participants included and excluded in the cohort or those with and without data on cause of death, and found no meaningful differences between the two groups (Supplemental Tables 1 and 2). Furthermore, given that we did not have complete data on all lipid-modifying medications and traditional and nontraditional CV disease risk factors (such as serum high sensitivity C-reactive protein and other nutritional parameters, such as subjective global assessment or lean body mass), our studies may have been limited by residual confounding. Nonetheless we did try to address this shortcoming, at least in part, by vigorous adjustment for measured covariates such as demographic, clinical, and laboratory parameters including BMI.
In conclusion, elevated baseline and time-varying TG/HDL-C ratios were associated with reduced CV mortality and better survival in patients treated with hemodialysis. This relationship remained significant even after extensive adjustment for relevant clinical and laboratory covariates and subgroup analyses. These observations further highlight the need for a more in-depth and qualitative, in addition to quantitative, evaluation of lipids and lipoproteins and their outcomes in patients with ESRD, given the unique nature of dyslipidemia and CV disease in this population.
Disclosures
K.K.-Z. has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, and ZS Pharma. C.P.K. has received honoraria from Sanofi-Aventis, Relypsa, and ZS Pharma. H.M. has received grant funding from National Institutes of Health, Veterans Affairs Office of Research and Development, and Novartis.
Supplementary Material
Acknowledgments
We thank DaVita Clinical Research for providing the clinical data for this research.
K.K.-Z. is supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIH NIDDK) grants K24-DK09141, R01-DK078106, and R01-DK095668, and philanthropic grants from Mr. Harold Simmons, Mr. Louis Chang, Dr. Joseph Lee, and AVEO, Inc. C.P.K. is supported by NIH NIDDK grants R01-DK096920 and U01-DK102163, and C.M.R. is supported by NIH NIDDK grant K23-DK102903. H.M. is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs (1 IK CX 001043-01A2). E.S. is supported by a career development 490 award from the Office of Research and Development of the Department of Veterans Affairs (IK2-CX001266-01).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08730816/-/DCSupplemental.
References
- 1.US Renal Data System : USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 2.Lavie CJ, Milani RV, Ventura HO: Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 53: 1925–1932, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Milani RV, Artham SM, Patel DA, Ventura HO: The obesity paradox, weight loss, and coronary disease. Am J Med 122: 1106–1114, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, Greenland S: Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis 46: 489–500, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Kilpatrick RD, McAllister CJ, Kovesdy CP, Derose SF, Kopple JD, Kalantar-Zadeh K: Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol 18: 293–303, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Moradi H, Abhari P, Streja E, Kashyap ML, Shah G, Gillen D, Pahl MV, Vaziri ND, Kalantar-Zadeh K: Association of serum lipids with outcomes in Hispanic hemodialysis patients of the West versus East Coasts of the United States. Am J Nephrol 41: 284–295, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iseki K, Yamazato M, Tozawa M, Takishita S: Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int 61: 1887–1893, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, Chapman MJ, Dodson PM, Fioretto P, Ginsberg HN, Kadowaki T, Lablanche JM, Marx N, Plutzky J, Reiner Z, Rosenson RS, Staels B, Stock JK, Sy R, Wanner C, Zambon A, Zimmet P: The residual risk reduction initiative: A call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol 102[Suppl]: 1K–34K, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B; ILLUMINATE Investigators : Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357: 2109–2122, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr., Cushman WC, Simons-Morton DG, Byington RP; ACCORD Study Group : Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 362: 1563–1574, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W; AIM-HIGH Investigators : Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 365: 2255–2267, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Salazar MR, Carbajal HA, Espeche WG, Aizpurúa M, Leiva Sisnieguez CE, March CE, Balbín E, Stavile RN, Reaven GM: Identifying cardiovascular disease risk and outcome: Use of the plasma triglyceride/high-density lipoprotein cholesterol concentration ratio versus metabolic syndrome criteria. J Intern Med 273: 595–601, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Salazar MR, Carbajal HA, Espeche WG, Aizpurúa M, Leiva Sisnieguez CE, Leiva Sisnieguez BC, March CE, Stavile RN, Balbín E, Reaven GM: Use of the plasma triglyceride/high-density lipoprotein cholesterol ratio to identify cardiovascular disease in hypertensive subjects. J Am Soc Hypertens 8: 724–731, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Gaziano JM, Hennekens CH, O’Donnell CJ, Breslow JL, Buring JE: Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation 96: 2520–2525, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F: Low triglycerides-high high-density lipoprotein cholesterol and risk of ischemic heart disease. Arch Intern Med 161: 361–366, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Lee J, Ovbiagele B: Nontraditional serum lipid variables and recurrent stroke risk. Stroke 45: 3269–3274, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barzi F, Patel A, Woodward M, Lawes CM, Ohkubo T, Gu D, Lam TH, Ueshima H; Asia Pacific Cohort Studies Collaboration : A comparison of lipid variables as predictors of cardiovascular disease in the Asia Pacific region. Ann Epidemiol 15: 405–413, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF: Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med 62: 345–349, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Bittner V, Johnson BD, Zineh I, Rogers WJ, Vido D, Marroquin OC, Bairey-Merz CN, Sopko G: The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia: A report from the Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J 157: 548–555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan K, Zhao J, Huang H, Zhang Q, Chen X, Zeng Z, Zhang L, Chen Y: The association between triglyceride/high-density lipoprotein cholesterol ratio and all-cause mortality in acute coronary syndrome after coronary revascularization. PLoS One 10: e0123521, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen HY, Tsai WC, Chiu YL, Hsu SP, Pai MF, Yang JY, Peng YS: Triglyceride to high-density lipoprotein cholesterol ratio predicts cardiovascular outcomes in prevalent dialysis patients. Medicine (Baltimore) 94: e619, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, Xiong L, Xu Q, Wu J, Huang R, Guo Q, Mao H, Yu X, Yang X: Higher serum triglyceride to high-density lipoprotein cholesterol ratio was associated with increased cardiovascular mortality in female patients on peritoneal dialysis. Nutr Metab Cardiovasc Dis 25: 749–755, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM: Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 298: 309–316, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A: Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298: 299–308, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Eberly LE, Stamler J, Neaton JD; Multiple Risk Factor Intervention Trial Research Group : Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med 163: 1077–1083, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Zilversmit DB: Atherogenesis: A postprandial phenomenon. Circulation 60: 473–485, 1979 [DOI] [PubMed] [Google Scholar]
- 27.Zilversmit DB: Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clin Chem 41: 153–158, 1995 [PubMed] [Google Scholar]
- 28.Nordestgaard BG: Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ Res 118: 547–563, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Moradi H, Streja E, Kashyap ML, Vaziri ND, Fonarow GC, Kalantar-Zadeh K: Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant 29: 1554–1562, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, Cheung AK, Brunelli S, Heagerty PJ, Katz R, Molnar MZ, Nissenson A, Ravel V, Streja E, Himmelfarb J, Mehrotra R: Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant 30: 1208–1217, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanak V, Munoz J, Teague J, Stanley A Jr, Bittner V: Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol 94: 219–222, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults : Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 34.Pandya V, Rao A, Chaudhary K: Lipid abnormalities in kidney disease and management strategies. World J Nephrol 4: 83–91, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qunibi WY: Dyslipidemia in dialysis patients. Semin Dial 28: 345–353, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Tonelli M, Muntner P, Lloyd A, Manns B, Klarenbach S, Pannu N, James M, Hemmelgarn B; Alberta Kidney Disease Network : Association between LDL-C and risk of myocardial infarction in CKD. J Am Soc Nephrol 24: 979–986, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members : KDIGO clinical practice guideline for lipid management in CKD: Summary of recommendation statements and clinical approach to the patient. Kidney Int 85: 1303–1309, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F; AURORA Study Group : Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R; SHARP Investigators : The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 377: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaziri ND: Role of dyslipidemia in impairment of energy metabolism, oxidative stress, inflammation and cardiovascular disease in chronic kidney disease. Clin Exp Nephrol 18: 265–268, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Vaziri ND, Moradi H: Mechanisms of dyslipidemia of chronic renal failure. Hemodial Int 10: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M: In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int 76: 437–444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaziri ND, Navab K, Gollapudi P, Moradi H, Pahl MV, Barton CH, Fogelman AM, Navab M: Salutary effects of hemodialysis on low-density lipoprotein proinflammatory and high-density lipoprotein anti-inflammatory properties in patient with end-stage renal disease. J Natl Med Assoc 103: 524–533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, Bian A, Shintani A, Fogo AB, Linton MF, Fazio S, Kon V: Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol 60: 2372–2379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weichhart T, Kopecky C, Kubicek M, Haidinger M, Döller D, Katholnig K, Suarna C, Eller P, Tölle M, Gerner C, Zlabinger GJ, van der Giet M, Hörl WH, Stocker R, Säemann MD: Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol 23: 934–947, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaziri ND: HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol 12: 37–47, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Moradi H, Vaziri ND, Kashyap ML, Said HM, Kalantar-Zadeh K: Role of HDL dysfunction in end-stage renal disease: A double-edged sword. J Ren Nutr 23: 203–206, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honda H, Ueda M, Kojima S, Mashiba S, Michihata T, Takahashi K, Shishido K, Akizawa T: Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis 220: 493–501, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Ahmadi SF, Streja E, Zahmatkesh G, Streja D, Kashyap M, Moradi H, Molnar MZ, Reddy U, Amin AN, Kovesdy CP, Kalantar-Zadeh K: Reverse Epidemiology of traditional cardiovascular risk factors in the geriatric population. J Am Med Dir Assoc 16: 933–939, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.