Abstract
Background and objectives
Experimental evidence suggests a role for monocytes in the biology of kidney disease progression; however, whether monocyte count is associated with risk of incident CKD, CKD progression, and ESRD has not been examined in large epidemiologic studies.
Design, settings, participants, & measurements
We built a longitudinal observational cohort of 1,594,700 United States veterans with at least one eGFR during fiscal year 2004 (date of last eGFR during this period designated time zero) and no prior history of ESRD, dialysis, or kidney transplant. Cohort participants were followed until September 30, 2013 or death. Monocyte count closest to and before time zero was categorized in quartiles: quartile 1, >0.00 to ≤0.40 thousand cells per cubic millimeter (k/cmm); quartile 2, >0.40 to ≤0.55 k/cmm; quartile 3, >0.55 to ≤0.70 k/cmm; and quartile 4, >0.70 k/cmm. Survival models were built to examine the association between monocyte count and risk of incident eGFR<60 ml/min per 1.73 m2, risk of incident CKD, and risk of CKD progression defined as doubling of serum creatinine, eGFR decline ≥30%, or the composite outcome of ESRD, dialysis, or renal transplantation.
Results
Over a median follow-up of 9.2 years (interquartile range, 8.3–9.4); in adjusted survival models, there was a graded association between monocyte counts and risk of renal outcomes. Compared with quartile 1, quartile 4 was associated with higher risk of incident eGFR<60 ml/min per 1.73 m2 (hazard ratio, 1.13; 95% confidence interval, 1.12 to 1.14) and risk of incident CKD (hazard ratio, 1.15; 95% confidence interval, 1.13 to 1.16). Quartile 4 was associated with higher risk of doubling of serum creatinine (hazard ratio, 1.22; 95% confidence interval, 1.20 to 1.24), ≥30% eGFR decline (hazard ratio, 1.18; 95% confidence interval, 1.17 to 1.19), and the composite renal end point (hazard ratio, 1.19; 95% confidence interval, 1.16 to 1.22). Cubic spline analyses of the relationship between monocyte count levels and renal outcomes showed a linear relationship, in which risk was higher with higher monocyte count. Results were robust to changes in sensitivity analyses.
Conclusions
Our results show a significant association between higher monocyte count and risks of incident CKD and CKD progression to ESRD.
Keywords: chronic kidney disease; clinical epidemiology; end stage kidney disease; Epidemiology and outcomes; ESRD; renal function decline; renal progression; white blood cell; chemokine; chemokine receptor; eGFR decline; eGFR slope; creatinine; Disease Progression; Epidemiologic Studies; Follow-Up Studies; kidney; Kidney Failure, Chronic; kidney transplantation; Monocytes; renal dialysis; Renal Insufficiency, Chronic; United States; Veterans
Introduction
Emerging evidence suggests that monocyte count may be an important predictor of atherosclerotic vascular disease (1,2), in which it is thought that increased number of circulating monocytes—in the presence of a repertoire of cues—may increase the probability of their differentiation into lipid-laden macrophages, thus contributing to progression of atherosclerotic lesions (3,4). Monocytes were the only white blood cell type to associate with peripheral artery disease and small cerebral vessel disease (5,6).
The observations establishing an association between monocyte count and vascular disease led to further research. In a cross-sectional analysis of 4581 cohort participants free of cardiovascular disease at baseline, Ganda et al. (7) reported that, compared with quintile 1 of renal function, in which average eGFR was 80.54 ml/min per 1.73 m2, quintile 5, in which average eGFR was 68.84 ml/min per 1.73 m2, had significantly increased odds of having higher monocyte count, therefore establishing—for the first time—a relationship between number of monocytes and a measure of renal function. The investigators concluded that mild decrease in renal function was associated with higher monocyte counts. Others have reported similar associations between measures of renal function and monocyte counts (8). The epidemiologic observations are complemented by experimental evidence suggesting a significant role of monocyte-derived macrophages, chemokines, and chemokine receptors that entrain monocytes in renal injury and repair (9–12). Studies from nonrenal literature, for example, suggest that circulating monocytes are elevated in patients with chronic liver disease and also suggest their functional contribution to the perpetuation of intrahepatic inflammation and profibrogenic hepatic stellate cells in activation in liver cirrhosis (13,14).
However, whether higher monocyte count is associated with risk of development of CKD and progression to ESRD is not known. An enhanced understanding of the relationship of monocyte count and renal outcomes may illuminate the clinical relevance of experimental and epidemiologic findings and further inform prediction models of CKD progression. We hypothesized that higher monocyte counts are associated with higher risk of developing incident CKD, CKD progression, and ESRD. We, therefore, built a cohort of 1,594,700 United States veterans to examine the association of monocyte count with risk of incident CKD, CKD progression, and ESRD.
Materials and Methods
Cohort Participants
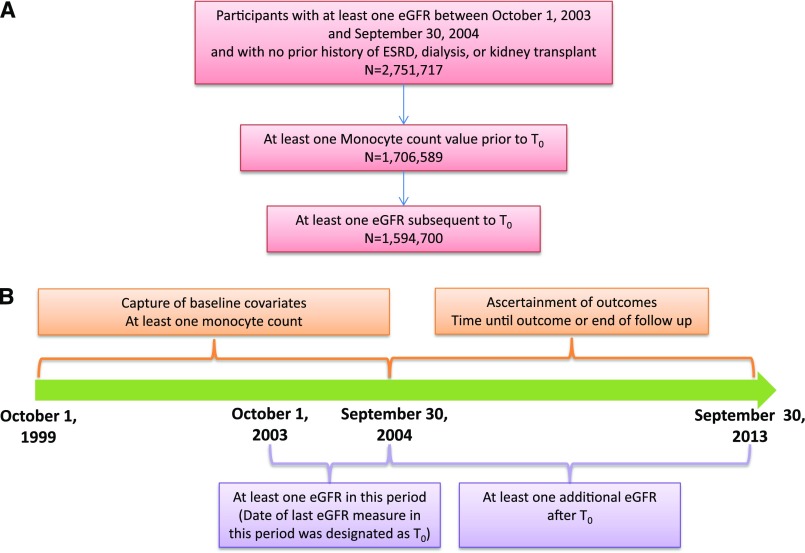
Using administrative data from the US Department of Veterans Affairs (VA), we identified users of the VA Healthcare System who had at least one eGFR value between October 1, 2003 and September 30, 2004, in which the date of last eGFR during this period was designated as time zero (T0), with no prior history of ESRD, dialysis, or kidney transplant (n=2,751,717). Additionally, cohort participants were selected on having at least one monocyte count value between October 1, 1999 and T0 (n=1,706,589) and at least one eGFR subsequent to T0 (n=1,594,700). Serum creatinine (and eGFR), monocyte levels, and other laboratory parameters were acquired during routine outpatient care. A flowchart and timeline for cohort selection are presented in Figure 1. The study was approved by the Institutional Review Board of the VA St. Louis Health Care System.
Figure 1.
Cohort assembly. (A) Diagram of participant flow. (B) Timeline for cohort selection. T0, time zero.
Data Sources
VA datasets were used to obtain patient’s demographics, inpatient and outpatient data, laboratory information (including serum creatinine), vital signs, and medications. Data from the US Renal Data System were used to supplement ESRD status information (15). Details on data sources are provided in Supplemental Material.
Primary Predictor Variable
The primary predictor variable for analyses was monocyte count. Monocyte count (1000 cells per cubic millimeter) closest to and before T0 was categorized into quartiles as quartile 1, from >0.00 to ≤0.40 (n=415,862 [26.1%]); quartile 2, from >0.40 to ≤0.55 (n=382,263 [24.0%]), quartile 3, from >0.55 to ≤0.70 (n=498,843 [31.3%]); and quartile 4, >0.70 (297,732 [18.7%]).
Outcomes
We evaluated the risk of incident eGFR<60 ml/min per 1.73 m2 in those with no prior history of eGFR<60 ml/min per 1.73 m2 at the time of cohort entry and additionally, risk of CKD among those with at least two eGFR values no <90 days apart. CKD was defined as two eGFR values <60 ml/min per 1.73 m2 at least 90 days apart (16,17). We additionally evaluated time until doubling of serum creatinine, time until ≥30% decline in eGFR, and time until ESRD, dialysis, or kidney transplant from creatinine at T0 (18,19). eGFR values were censored after onset of ESRD or at first record of dialysis or kidney transplant. All cohort participants were followed until September 30, 2013.
Covariates
Baseline covariates were ascertained from October 1, 1999 until cohort entry (T0). Covariates included age, race, sex, T0 eGFR, diabetes mellitus, hypertension, cardiovascular disease, chronic lung disease, peripheral artery disease, cerebrovascular accident, hepatitis C, HIV, dementia, hyperlipidemia, cancer, white blood cell count, microalbumin-to-creatinine ratio, angiotensin-converting enzyme inhibitors / angiotensin receptor blockers use, proton pump inhibitor use, and nonsteroidal anti-inflammatory drug use (17,20,21).
eGFR was calculated using the abbreviated Chronic Kidney Disease Epidemiology Collaboration equation (22). Covariate definitions are provided in Supplemental Material.
Statistical Analyses
Kaplan–Meier curves were generated to show the survival of each outcome by monocyte count quartile. Incidence rates were calculated as number of events per 100,000 person-years, and 95% confidence intervals (95% CIs) were built on the basis of the normal distribution. Because of the censoring of eGFR values by occurrence of ESRD, dialysis, transplant, and death, competing risk Cox proportional hazard regression models were used in the assessment of survival outcomes. Specifically, ESRD, dialysis, transplant, and death were treated as competing risks in doubling of serum creatinine, >30% decline, incident eGFR<60 ml/min per 1.73 m2, and incident CKD models, whereas death was treated as a competing risk in the ESRD, dialysis, or transplant model. Such censoring was considered noninformative (23,24). Multiple models were built to assess the relation between monocyte count and outcomes, while sequentially controlling for demographics, comorbidities, and eGFR at T0. The proportional hazard assumption was assessed through use of log-negative log plots and deemed to have been met in all models. P values for trends were obtained by adding quartiles as a continuous variable to models. We repeated all analyses in a subcohort of participants with white blood cell counts between 4 and 10 thousand cells per cubic millimeter (counts considered in the normal range). Analyses were repeated with monocyte deciles, where decile 2 was the referent category. Cubic spline analyses—that allows for assessment of nonlinear relationships of continuous monocyte count with outcomes—of the association between monocytes and renal outcomes were performed (23). Details of the spline analyses may be found in Supplemental Material. Attributable fraction (AF) and population attributable fraction (PAF) were calculated using piecewise constant hazard models for disease incidence (25). Details of AF and PAF analyses are included in Supplemental Material.
In survival analyses, a 95% CI of a hazard ratio (HR) that does not include unity was considered statistically significant. In all analyses, a P value of ≤0.05 was considered statistically significant. Missing data were not imputed. All analyses were performed and graphs were made using SAS Enterprise Guide, version 7.1 and SAS 9.4 (SAS Institute, Cary, NC).
Sensitivity Analyses
We evaluated the consistency and robustness of study findings by performing a number of sensitivity analyses as described in Supplemental Material.
Results
The demographic and health characteristics of the overall cohort and according to monocyte count quartile are presented in Table 1. Cohort participants in the highest monocyte quartile were more likely to be of white race and men; they also had a higher burden of comorbid illnesses, including diabetes mellitus, hypertension, cardiovascular disease, chronic lung disease, peripheral vascular disease, and lower eGFR (Table 1). Cohort participants were followed for a median of 9.2 years (interquartile range, 8.3–9.4).
Table 1.
Demographic and clinical characteristics of overall study cohort and according to quartiles of monocyte count
| Characteristic | Overall Cohort | Monocyte Quartile 1 >0.00 to ≤0.40 k/cmm | Monocyte Quartile 2 >0.40 to ≤0.55 k/cmm | Monocyte Quartile 3 >0.55 to ≤0.70 k/cmm | Monocyte Quartile 4 >0.70 k/cmm |
|---|---|---|---|---|---|
| No. (%) | 1,594,700 | 415,862 (26.1) | 382,263 (24.0) | 498,843 (31.3) | 297,732 (18.7) |
| Median monocyte count (IQR), k/cmm | 0.55 (0.40–0.70) | 0.40 (0.30–0.40) | 0.50 (0.50–0.50) | 0.60 (0.60–0.70) | 0.90 (0.80–1.00) |
| Median time from monocyte count to T0 (IQR), yr | 2.1 (0.6–3.6) | 2.1 (0.6–3.6) | 2.0 (0.6–3.6) | 2.2 (0.7–3.7) | 2.1 (0.6–3.7) |
| Race (%) | |||||
| White | 1,308,289 (82.0) | 312,045 (75.0) | 313,507 (82.0) | 425,010 (85.2) | 257,727 (86.6) |
| Black | 245,599 (15.4) | 90,462 (21.8) | 58,770 (15.4) | 62,491 (12.5) | 33,876 (11.4) |
| Other | 40,812 (2.6) | 13,355 (3.2) | 9986 (2.6) | 11,342 (2.3) | 6129 (2.1) |
| Men (%) | 1,512,680 (94.9) | 380,759 (91.6) | 361,818 (94.7) | 479,954 (96.2) | 290,149 (97.5) |
| Median age (IQR), yr | 62.0 (54.6–71.8) | 60.5 (53.2–70.9) | 62.1 (54.7–71.8) | 63.0 (55.2–72.2) | 62.6 (55.1–72.1) |
| Diabetes mellitus (%) | 456,591 (28.6) | 114,613 (27.6) | 108,237 (28.3) | 145,252 (29.1) | 88,489 (29.7) |
| Hypertension (%) | 1,087,788 (68.2) | 265,739 (63.9) | 257,155 (67.3) | 349,497 (70.1) | 215,397 (72.4) |
| Cardiovascular disease (%) | 502,250 (31.5) | 109,051 (26.2) | 115,026 (30.1) | 167,032 (33.5) | 111,141 (37.3) |
| Chronic lung disease (%) | 336,520 (21.1) | 70,301 (16.9) | 72,146 (18.9) | 110,466 (22.1) | 83,607 (28.1) |
| Peripheral artery disease (%) | 48,455 (3.0) | 9483 (2.3) | 10,350 (2.7) | 16,225 (3.3) | 12,397 (4.2) |
| Dementia (%) | 51,000 (3.2) | 13,141 (3.2) | 11,869 (3.1) | 15,756 (3.2) | 10,234 (3.4) |
| HIV (%) | 109,417 (6.9) | 28,482 (6.9) | 24,534 (6.4) | 33,164 (6.7) | 23,237 (7.8) |
| Hepatitis C (%) | 78,339 (4.9) | 20,929 (5.0) | 17,156 (4.5) | 22,899 (4.6) | 17,355 (5.8) |
| Cerebrovascular accident (%) | 8169 (0.5) | 1620 (0.4) | 1773 (0.5) | 2784 (0.6) | 1992 (0.7) |
| Hyperlipidemia (%) | 946,657 (59.4) | 233,221 (56.1) | 228,455 (59.8) | 306,461 (61.4) | 178,520 (60.0) |
| Cancer (%) | 202,957 (12.7) | 53,424 (12.9) | 47,192 (12.4) | 62,623 (12.6) | 39,718 (13.3) |
| Average eGFR at T0 (SD), ml/min per 1.73 m2 | 76.39 (18.7) | 77.68 (18.4) | 76.46 (18.5) | 75.59 (18.7) | 75.87 (19.5) |
| Median number of eGFR measures after T0 (IQR) | 12 (7–20) | 12 (7–19) | 12 (7–19) | 12 (7–20) | 13 (7–20) |
| Microalbumin-to-creatinine ratio (%),a mg/g | |||||
| 0–<30 | 88,187 (77.6) | 23,276 (79.3) | 20,513 (78.2) | 28,546 (77.4) | 15,852 (75.0) |
| 30–<300 | 21,792 (19.2) | 5297 (18.0) | 4899 (18.7) | 7087 (19.2) | 4509 (21.3) |
| ≥300 | 3617 (3.2) | 785 (2.7) | 819 (3.1) | 1229 (3.3) | 784 (3.7) |
| Median follow-up time (IQR), yr | 9.2 (8.3–9.4) | 9.2 (9.0–9.4) | 9.2 (9.0–9.4) | 9.2 (8.0–9.4) | 9.1 (6.5–9.4) |
| Death during follow-up (%) | 432,558 (27.1) | 94,039 (22.6) | 93,775 (24.5) | 140,676 (28.2) | 104,068 (35.0) |
k/cmm, 1000 cells per cubic millimeter; IQR, interquartile range; T0, time zero.
Numbers are for a subset of the cohort for which the corresponding data were available (n=113,596).
Monocyte Count and Risk of Incident eGFR<60 ml/min per 1.73 m2 and Incident CKD
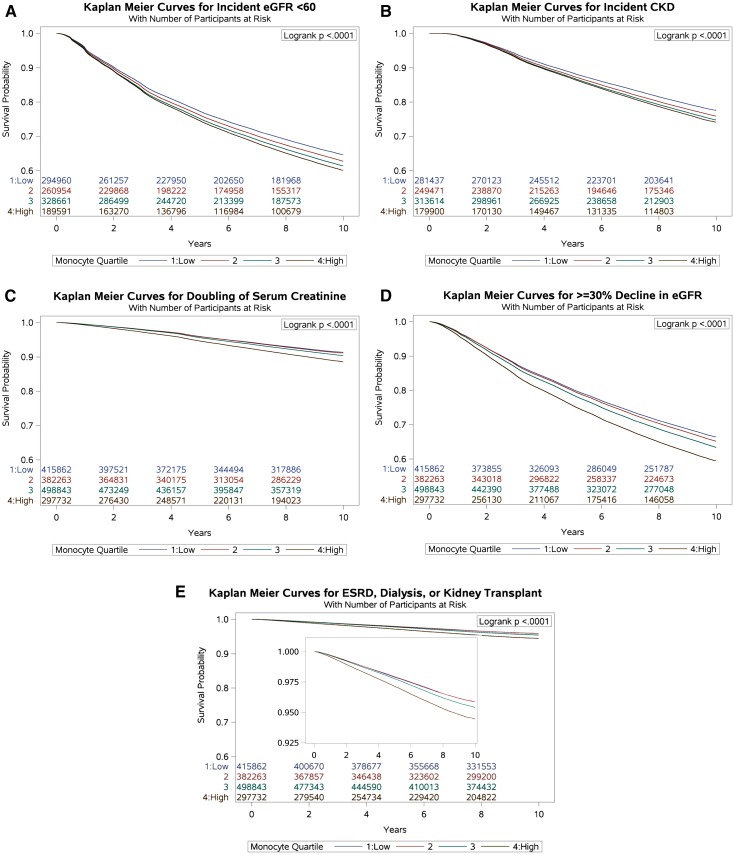
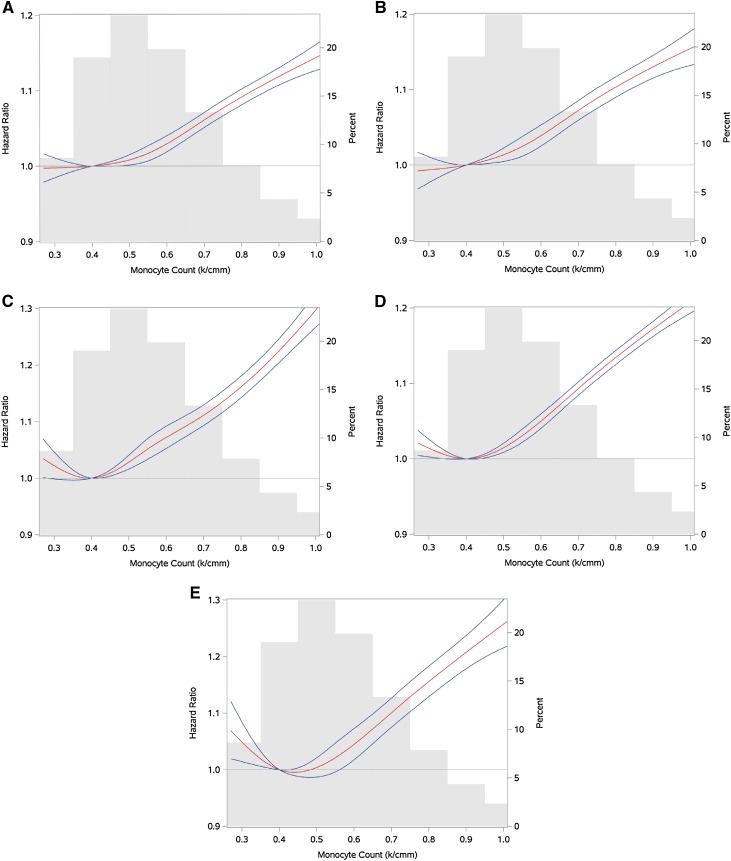
We evaluated the risk of incident eGFR<60 ml/min per 1.73 m2 among those who had no history of eGFR<60 ml/min per 1.73 m2 before the time of cohort entry (n=1,074,166). Among those, 366,191 (34.1%) experienced eGFR<60 ml/min per 1.73 m2 during the time in the cohort, and 95,830 (32.5%), 88,643 (34.0%), 114,906 (35.0%), and 66,812 (35.2%) experienced eGFR<60 ml/min per 1.73 m2 in monocyte quartiles 1–4, respectively (Figure 2A, Table 2). In adjusted survival models for demographics, comorbid conditions, and eGFR, there was a graded and significant association between quartiles of monocyte count and risk of incident eGFR<60 ml/min per 1.73 m2; compared with participants with a monocyte count in the lowest quartile (quartile 1), those with a monocyte count in quartiles 2–4 had HRs and 95% CIs of HR, 1.02 and 95% CI, 1.01 to 1.03; HR, 1.04 and 95% CI, 1.04 to 1.05; and HR, 1.13 and 95% CI, 1.12 to 1.14, respectively (Table 2). Cubic spline analyses of the relationship between monocyte count and the outcome of incident eGFR<60 ml/min per 1.73 m2 suggested a near-linear relationship, in which risk was higher among patients with higher monocyte count (Figure 3A).
Figure 2.
Kaplan–Meier curves representing the survival probability of renal outcomes by monocyte quartiles. (A) eGFR<60 ml/min per 1.73 m2. (B) Chronic Kidney Disease. (C) Doubling of serum creatinine. (D) >=30% decline in eGFR. (E) ESRD, dialysis, or transplant. Inset represents the same Kaplan-Meier curves on a narrower y-axis scale.
Table 2.
Risk of incident eGFR<60 ml/min per 1.73 m2, incident CKD, doubling of serum creatinine, ≥30% decline in eGFR, and ESRD, dialysis, or transplantation by monocyte count quartile
| Outcome and Measure | Monocyte Quartile 1 >0.00 to ≤0.40 k/cmm | Monocyte Quartile 2 >0.40 to ≤0.55 k/cmm | Monocyte Quartile 3 >0.55 to ≤0.70 k/cmm | Monocyte Quartile 4 >0.70 k/cmm |
|---|---|---|---|---|
| eGFR<60 ml/min per 1.73 m2a | ||||
| No. of events (%) | 95,830 (32.5) | 88,643 (34.0) | 114,906 (35.0) | 66,812 (35.2) |
| Incidence rate per 100,000 person-yr (95% CI) | 4560.4 (4531.5 to 4589.2) | 4854.1 (4822.1 to 4886.0) | 5105.7 (5076.2 to 5135.3) | 5327.8 (5287.4 to 5368.2) |
| HR (95% CI) | 1.00 | 1.02 (1.01 to 1.03) | 1.04 (1.04 to 1.05) | 1.13b (1.12 to 1.14) |
| Incident CKDc | ||||
| No. of events (%) | 56,419 (20.1) | 53,370 (21.4) | 69,306 (22.1) | 39,744 (22.1) |
| Incidence rate per 100,000 person-yr (95% CI) | 2522.3 (2501.5 to 2543.1) | 2728.7 (2705.5 to 2751.8) | 2865.6 (2844.3 to 5887.0) | 2949.2 (2920.2 to 2978.2) |
| HR (95% CI) | 1.00 | 1.03 (1.02 to 1.05) | 1.06 (1.05 to 1.07) | 1.15b (1.13 to 1.16) |
| Doubling of serum creatinine | ||||
| No. of events (%) | 30,463 (7.3) | 28,415 (7.4) | 38,885 (7.8) | 26,449 (8.9) |
| Incidence rate per 100,000 person-yr (95% CI) | 900.4 (890.2 to 910.5) | 922.1 (911.4 to 932.8) | 988.2 (978.4 to 998.0) | 1184.1 (1169.9 to 1198.4) |
| HR (95% CI) | 1.00 | 1.02 (1.00 to 1.04) | 1.07 (1.05 to 1.08) | 1.22b (1.20 to 1.24) |
| ≥30% Decline in eGFR | ||||
| No. of events (%) | 123,897 (29.8) | 116,637 (30.5) | 157,895 (31.7) | 101,973 (34.3) |
| Incidence rate per 100,000 person-yr (95% CI) | 4170.7 (4147.5 to 4193.9) | 4325.7 (4300.9 to 4350.5) | 4614.5 (4591.8 to 4637.3) | 5324.1 (5291.4 to 5356.7) |
| HR (95% CI) | 1.00 | 1.02 (1.01 to 1.03) | 1.06 (1.05 to 1.07) | 1.18b (1.17 to 1.19) |
| ESRD, dialysis, or transplant | ||||
| No. of events (%) | 14,875 (3.6) | 13,576 (3.6) | 19,141 (3.8) | 13,427 (4.5) |
| Incidence rate per 100,000 person-yr (95% CI) | 430.2 (423.3 to 437.1) | 430.7 (423.4 to 437.9) | 475.1 (468.3 to 481.8) | 584.7 (574.8 to 594.6) |
| HR (95% CI) | 1.00 | 0.98 (0.96 to 1.00) | 1.04 (1.02 to 1.06) | 1.19b(1.16 to 1.22) |
Models were adjusted for age, race, sex, cardiovascular disease, cerebrovascular accident, chronic lung disease, dementia, diabetes mellitus, hepatitis C, HIV, hypertension, hyperlipidemia, cancer, peripheral artery disease, and time zero eGFR. The referent category is monocyte quartile 1. k/cmm, 1000 cells per cubic millimeter; 95% CI, 95% confidence interval; HR, hazard ratio.
Incident eGFR<60 ml/min per 1.73 m2 was evaluated in a subcohort of people with no prior history of eGFR≤60 ml/min per 1.73 m2 at time of cohort entry (n=1,074,166).
P value for trend <0.001.
Incident CKD was evaluated in a subcohort of people with at least two eGFRs separated by at least 90 days who had no prior history of eGFR≤60 ml/min per 1.73 m2 at the time of cohort entry (n=1,024,422).
Figure 3.
Cubic spline analyses of risk of renal outcomes by monocyte count (median monocyte count as reference) in red, with monocyte count probability distribution in gray; blue lines represent 95% confidence intervals of the spline. (A) Risk of eGFR<60 ml/min per 1.73 m2. (B) Risk of Chronic Kidney Disease. (C) Risk of doubling of serum creatinine. (D) Risk of ≥30% decline in eGFR. (E) Risk of ESRD, dialysis, or transplant.
We evaluated the risk of incident CKD (defined as two eGFR values <60 ml/min per 1.73 m2 at least 90 days apart) in a subcohort of people with at least two eGFR measurements separated by at least 90 days who had no history of eGFR<60 ml/min per 1.73 m2 (n=1,024,422) before the time of cohort entry. Among those, 218,839 (21.4%) developed incident CKD, and 56,419 (20.1%), 53,370 (21.4%), 69,306 (22.1%), and 39,744 (22.1%) developed incident CKD in monocyte quartiles 1–4, respectively (Figure 2B, Table 2). Compared with participants with a monocyte count in the lowest quartile (quartile 1), those with a monocyte count in quartiles 2–4 had higher risk of CKD, with HR, 1.03 and 95% CI, 1.02 to 1.05; HR, 1.06 and 95% CI, 1.05 to 1.07; and HR, 1.15 and 95% CI, 1.13 to 1.16, respectively (Table 2). Cubic spline analyses suggested a linear relationship between monocyte count and the outcome of incident CKD (Figure 3B).
Monocyte Count and Risk of CKD Progression to ESRD
In the overall cohort of 1,594,700 participants, 124,212 (7.8%) experienced a doubling of serum creatinine, and 30,463 (7.3%), 28,415 (7.4%), 38,885 (7.8%), and 26,449 (8.9%) experienced a doubling of serum creatinine in monocyte quartiles 1–4, respectively (Figure 2C, Table 2). There were 500,402 (31.4%) who experienced ≥30% decline in eGFR in the overall cohort; 123,897 (29.8%), 116,637 (30.5%), 157,895 (31.7%), and 101,973 (34.3%) experienced ≥30% decline in eGFR in monocyte quartiles 1–4, respectively (Figure 2D, Table 2). In the overall cohort, 61,019 (3.8%) developed ESRD, required dialysis, or required kidney transplant; 14,875 (3.6%), 13,576 (3.6%), 19,141 (3.8%), and 13,427 (4.5%) developed ESRD, required dialysis, or required kidney transplant in monocyte quartiles 1–4, respectively (Figure 2E, Table 2).
In models adjusted for demographic variables, comorbid conditions, and baseline eGFR (eGFR at the time of cohort entry), there was a graded and significant association between monocyte count and risk of doubling of serum creatinine, risk of eGFR decline ≥30%, and risk of the composite outcome of ESRD, dialysis, or kidney transplant (Table 2). Cubic spline analyses of the relationship between monocyte count and the outcomes of doubling of serum creatinine, ≥30% decline in eGFR, and the composite outcome of ESRD, dialysis, and transplantation showed a linear relationship (Figure 3, C–E).
Monocyte Count and Risk of Chronic Renal Outcomes in Participants with Normal White Blood Cell Count
We repeated all analyses in participants with normal white blood cell counts, and results remained consistent (Supplemental Table 1). There was a graded association between monocyte count and risk of chronic renal outcomes.
AF and PAF
AFs for incident eGFR<60 ml/min per 1.73 m2 and incident CKD were 2.45% (95% CI, 1.95 to 2.94) and 3.63% (95% CI, 2.91 to 4.34), respectively (Table 3). AFs for doubling of serum creatinine, eGFR decline ≥30%, and ESRD, dialysis, or kidney transplant were 5.4% (95% CI, 4.2 to 6.6), 3.6 (95% CI, 3.2 to 4.1), and 2.2 (95% CI, 0.5 to 3.8), respectively (Table 3).
Table 3.
Attributable fraction and population attributable fraction
| Measure | eGFR<60 ml/min per 1.73 m2a | Incident CKDb | Doubling of Serum Creatinine | ≥30% Decline in eGFR | ESRD, Dialysis, or Transplant |
|---|---|---|---|---|---|
| Attributable fraction (95% CI), % | 2.45 (1.95 to 2.94) | 3.63 (2.91 to 4.34) | 5.41 (4.24 to 6.57) | 3.64 (3.16 to 4.14) | 2.19 (0.53 to 3.82) |
| Population attributable fraction (95% CI), % | 1.80 (1.43 to 2.17) | 2.69 (2.15 to 3.22) | 4.08 (3.18 to 4.50) | 2.74 (2.37 to 3.11) | 1.65 (0.39 to 2.90) |
Models were adjusted for age, race, sex, cerebrovascular accident, chronic lung disease, diabetes, dementia, hepatitis C, HIV, hypertension, time zero eGFR, cardiovascular disease, hyperlipidemia, cancer, and peripheral artery disease. Monocyte quartile 1 was used as the theoretical minimum risk exposure level. 95% CI, 95% confidence interval.
Incident eGFR<60 ml/min per 1.73 m2 was evaluated in a subcohort of people with no prior history of eGFR≤60 ml/min per 1.73 m2 at the time of cohort entry (n=1,074,166).
Incident CKD was evaluated in a subcohort of people with at least two eGFRs separated by at least 90 days who had no prior history of eGFR≤60 ml/min per 1.73 m2 at the time of cohort entry (n=1,024,422).
PAFs for incident eGFR<60 ml/min per 1.73 m2 and incident CKD were 1.8% (95% CI, 1.4 to 2.2) and 2.7% (95% CI, 2.2 to 3.2), respectively (Table 3). PAFs for doubling of serum creatinine, eGFR decline ≥30%, and ESRD, dialysis, or kidney transplant were 4.1% (95% CI, 3.2 to 4.5), 2.7 (95% CI, 2.4 to 3.1), and 1.7 (95% CI, 0.4 to 2.9), respectively (Table 3).
Sensitivity Analyses
We evaluated the robustness and consistency of study findings in a number of sensitivity analyses. High monocyte count was associated with higher risk of the alternative outcomes of severe CKD progression defined as eGFR loss >−5 ml/min per 1.73 m2 per year (HR, 1.32; 95% CI, 1.30 to 1.65) and ESRD, kidney transplant, or ≥50% decline in eGFR (HR, 1.23; 95% CI, 1.21 to 1.24) (Supplemental Tables 2 and 3) (15,17,21,26–29). When average monocyte count during the period at or before T0 was used as the primary predictor variable, the results were consistent with those in the primary analyses (Supplemental Table 4). The potential effect of censoring at ESRD, dialysis, or kidney transplant—in models in which it was not the outcome of interest—was found at both extremes of informative censoring to have results consistent with the primary analyses (Supplemental Table 5) (17,21). In bias analyses that assessed potential changes in associations using Monte Carlo sensitivity analysis to simulate accounting for the microalbumin-to-creatinine ratio as a potential confounder, results were consistent with those shown in primary analyses (Supplemental Table 6) (17). The results were consistent where monocyte count was categorized into deciles (Supplemental Table 7) and where monocyte count was grouped into 0.1 (1000 cells per cubic millimeter) increasing ordinal categories (Supplemental Table 8). After additionally controlling for angiotensin-converting enzyme inhibitor / angiotensin receptor blocker, proton pump inhibitor, and nonsteroidal anti-inflammatory drug use, results were found to be congruent with those in the primary analysis (Supplemental Table 9).
Discussion
In a large observational cohort study of 1,594,700 United States veterans followed for a median of 9.16 years, we show a graded association between monocyte count and risk of development of CKD (incident eGFR<60 ml/min per 1.73 m2 and incident CKD), risk of CKD progression (doubling of serum creatinine and >30% reduction in eGFR), and risk of ESRD. The results were consistent in analyses considering only participants with normal white blood cell count and robust to challenges in multiple sensitivity analyses. Spline analyses showed a linear relationship between monocyte count and risk of adverse renal outcomes.
This work sought to specifically examine the relationship between monocyte count and risk of incident CKD and its progression. However, elevated monocyte count may also be present in the context of elevated overall white blood cell count. Prior evidence suggested that total white blood cell count predicts mortality in patients with kidney disease (30,31). Higher total white blood cell count is generally present in the context of infection or inflammation and associated with higher risk of hospitalization, cardiovascular events, and a host of untoward outcomes (31). There are no data, however, specifically linking elevated total white blood cell count with risk of development of kidney disease and its progression. Our finding that, in participants with normal white blood cell count, there was a significant association between monocyte count and risk of chronic renal outcomes suggests that, in the absence of leukocytosis, monocyte count remains a significant predictor of kidney disease outcomes.
The mechanism underlying the association of monocytes and kidney outcomes is not clear. One simple explanation of the observed associations is that monocyte count may be a mere surrogate marker—a clinical predictor—of inflammation, poorer overall health, and thus, higher risk of adverse long-term renal outcomes. Epidemiologic findings suggest that higher monocyte count is associated with atherosclerotic vascular disease, small vessel disease, and cross-sectional measures of renal function; a plausible working hypothesis is that the renal manifestations associated with higher monocyte counts are likely related to progression of vascular disease and subsequently, reduced kidney function (1,3–6). Monocytes, chemokines, and chemokine receptors have also been implicated in the biology of renal disease progression, where it is plausible that higher monocyte counts may facilitate progression of renal injury (11,12,32,33).
Lee et al. (34) reported that, as CKD advances, the proportion of proinflammatory CD14+CD16+ monocytes expands, and notably, the expansion of CD14+CD16+ subtypes correlated with measures of impaired vascular health, including brachial-ankle pulse wave velocity. It is unclear whether the expansion of proinflammatory subtypes is the result of impaired kidney function or alternatively, in the pathway leading to deterioration in kidney function (34). In our studies, we noted an association between baseline monocyte counts and risk of incident CKD and progression to ESRD, and although we did not have data on the subtypes of monocytes, the epidemiologic findings that higher monocyte count predicts future risk of development of CKD and its progression lend further plausibility to the hypothesis that higher monocyte count may be a marker that precedes or is in the pathway mediating the development of CKD and its progression. Further studies are needed to determine which monocyte subtype exhibits the strongest association with or best predicts renal outcomes.
Experimental evidence suggests that inflammatory cell infiltrates play a role in the development and progression of kidney disease (11,12). Blockade of chemokine receptor 1 (CCR1)—which plays an important role in monocyte/macrophage adhesion to activated vascular endothelium, transendothelial migration, and activation—can effectively prevent recruitment of monocytes into the renal interstitial compartment. CCR1 blockade abrogates the development of kidney disease in various types of experimental kidney disease, including unilateral ureteral obstruction, lupus-like GN, adriamycin-induced nephrotic syndrome, Alport syndrome, diabetic renal disease, and kidney allograft murine models (11,33,35–38). In experimental models, CCR1 blockade also seems to be effective, even when treatment is initiated after the disease process is established (39). The biology of monocytes, chemokines, and chemokine receptor in renal disease is highly complex, because these moieties are highly promiscuous in that they drive both injury and repair processes, which makes identifying translational therapeutic opportunities challenging (12). Further studies are needed to develop a more comprehensive understanding of the role of chemokines, chemokine receptors, and their genetic variants that promulgate or—conversely—abrogate chronic renal injury. Our findings add to the existing body of knowledge—largely derived from murine experimental models—and provide that, in a large national cohort, monocyte count—a widely available marker—is a significant predictor of kidney disease development and progression. The epidemiologic observations establish a clinical epidemiologic relevance of monocyte in kidney disease, further illuminate our understanding of this important field, and inform the discussion on filling the translational gap between experimental biology and clinical epidemiology—where a bidirectional flow of information from clinical epidemiologic observations informs biologic research (17,21,40). More experimental and clinical investigation is needed to better understand the biology of monocyte in kidney disease and translate the understanding into opportunities and therapeutic avenues to halt kidney disease progression (17,29).
The study has several limitations. There were significant baseline differences in demographic and health characteristics according to monocyte quartiles, in which those in the highest quartile were generally less healthy. Although we included these covariates in the models, the results seen may still represent residual confounding, in that there may be confounders either unmeasured or unknown that may explain the higher risk of renal outcomes seen in those with higher monocyte counts. A more cautious interpretation of the study results is that monocyte count may be a heavily confounded measure that undermines the validity of making causal inferences with renal outcomes and as such, that monocyte count may just be a—valuable clinical—predictor (or surrogate marker) of kidney disease progression. Higher monocyte counts could be present in the context of inflammation or infection, which may confound the described association. To minimize this possibility, we included measures related to burden of illness and cancer as covariates in the models. We used a single value of monocyte as a predictor in the primary analyses, and to account to intraindividual variation, we conducted sensitivity analyses using average monocyte count in participants with more than one monocyte count before cohort entry; these sensitivity analyses are necessarily limited by the selection bias of requiring more than one value. Monocytes are not a homogenous group of cells, because there are functionally and phenotypically distinct subtypes on the basis of CD14 and CD16 positivity; our datasets did not include information on these subtypes of monocytes. In this observational study, cohort participants were mostly older white men; additionally, potential participants had to meet criteria for cohort inclusion, and as such, the results may not be generalizable to a less defined population. The imperfect nature of administrative data and the retrospective design of the study may also lead to sampling bias and inaccurate measurements of the predictor variables. To minimize such measurement bias, we used definitions of comorbid illnesses that are validated for use in the VA administrative data (16,17,41,42). Although AF and PAF are statistically significant, these estimates are small and must be interpreted with caution. Strengths of the described results include the fact that this is a very large cohort of veterans followed for nearly a decade to ascertain renal outcomes, the consistency of the magnitude and direction of the association across multiple renal outcomes tested, and the robustness of study findings to changes in epidemiologic design and specification of statistical models. In sum, our results show a significant association between higher monocyte count and risks of incident CKD and CKD progression to ESRD; the findings provide epidemiologic context to enhance our understanding of the experimental observations of a role for monocyte in the biology of kidney disease and its progression.
Disclosures
None.
Supplementary Material
Acknowledgments
The data reported here have been supplied by the US Renal Data System (USRDS).
This work was funded by a grant from the US Department of Veterans Affairs (VA; to Z.A.-A.). Support for the VA/Centers for USRDS Data is provided by VA, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center Project/Data Use Agreement Al-Aly-01.
The contents do not represent the views of the VA or the US Government. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09710916/-/DCSupplemental.
References
- 1.Ghattas A, Griffiths HR, Devitt A, Lip GY, Shantsila E: Monocytes in coronary artery disease and atherosclerosis: Where are we now? J Am Coll Cardiol 62: 1541–1551, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Swaminathan S, Shah SV: Novel inflammatory mechanisms of accelerated atherosclerosis in kidney disease. Kidney Int 80: 453–463, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Adamsson Eryd S, Smith JG, Melander O, Hedblad B, Engström G: Incidence of coronary events and case fatality rate in relation to blood lymphocyte and neutrophil counts. Arterioscler Thromb Vasc Biol 32: 533–539, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Johnsen SH, Fosse E, Joakimsen O, Mathiesen EB, Stensland-Bugge E, Njølstad I, Arnesen E: Monocyte count is a predictor of novel plaque formation: A 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromsø Study. Stroke 36: 715–719, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Nasir K, Guallar E, Navas-Acien A, Criqui MH, Lima JA: Relationship of monocyte count and peripheral arterial disease: Results from the National Health and Nutrition Examination Survey 1999-2002. Arterioscler Thromb Vasc Biol 25: 1966–1971, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Cortina MG, Campello AR, Conde JJ, Ois A, Voustianiouk A, Téllez MJ, Cuadrado E, Roquer J: Monocyte count is an underlying marker of lacunar subtype of hypertensive small vessel disease. Eur J Neurol 15: 671–676, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Ganda A, Magnusson M, Yvan-Charvet L, Hedblad B, Engström G, Ai D, Wang TJ, Gerszten RE, Melander O, Tall AR: Mild renal dysfunction and metabolites tied to low HDL cholesterol are associated with monocytosis and atherosclerosis. Circulation 127: 988–996, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evangelopoulos AA, Vallianou NG, Bountziouka V, Katsagoni C, Bathrellou E, Vogiatzakis ED, Bonou MS, Barbetseas J, Avgerinos PC, Panagiotakos DB: Association between serum cystatin C, monocytes and other inflammatory markers. Intern Med J 42: 517–522, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Ricardo SD, van Goor H, Eddy AA: Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lech M, Gröbmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, Susanti HE, Kobayashi KS, Flavell RA, Anders HJ: Macrophage phenotype controls long-term AKI outcomes--kidney regeneration versus atrophy. J Am Soc Nephrol 25: 292–304, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anders HJ, Ninichuk V, Schlöndorff D: Progression of kidney disease: Blocking leukocyte recruitment with chemokine receptor CCR1 antagonists. Kidney Int 69: 29–32, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Chung AC, Lan HY: Chemokines in renal injury. J Am Soc Nephrol 22: 802–809, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, Tischendorf JJ, Luedde T, Weiskirchen R, Trautwein C, Tacke F: Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One 5: e11049, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju C, Tacke F: Hepatic macrophages in homeostasis and liver diseases: From pathogenesis to novel therapeutic strategies. Cell Mol Immunol 13: 316–327, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie Y, Bowe B, Xian H, Balasubramanian S, Al-Aly Z: Renal function trajectories in patients with prior improved eGFR slopes and risk of death. PLoS One 11: e0149283, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z: Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol 27: 3153–3163, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowe B, Xie Y, Xian H, Balasubramanian S, Al-Aly Z: Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int 89: 886–896, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS; CKD Prognosis Consortium : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Aly Z: Prediction of renal end points in chronic kidney disease. Kidney Int 83: 189–191, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Bowe B, Xie Y, Xian H, Balasubramanian S, A Zayed M, Al-Aly Z: High density lipoprotein cholesterol and the risk of all-cause mortality among U.S. veterans [published online ahead of print August 11, 2016]. Clin J Am Soc Nephrol doi:10.2215/CJN.00730116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinbaum DG, Klein M: Survival Analysis: A Self-Learning Text, New York, Springer, 2005 [Google Scholar]
- 24.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z: Long term kidney outcomes among proton pump inhibitors users without intervening acute kidney injury. Kidney Int, 2016, in press [DOI] [PubMed] [Google Scholar]
- 25.Laaksonen MA, Virtala E, Knekt P, Oja H, Härkänen T: SAS Macros for calculation of population attributable fraction in a cohort study design. J Stat Softw 43: 1–25, 2011 [Google Scholar]
- 26.Anonymous: Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl (2011) 3: 63–72, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Aly Z, Balasubramanian S, McDonald JR, Scherrer JF, O’Hare AM: Greater variability in kidney function is associated with an increased risk of death. Kidney Int 82: 1208–1214, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Al-Aly Z, Zeringue A, Fu J, Rauchman MI, McDonald JR, El-Achkar TM, Balasubramanian S, Nurutdinova D, Xian H, Stroupe K, Abbott KC, Eisen S: Rate of kidney function decline associates with mortality. J Am Soc Nephrol 21: 1961–1969, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowe BXY, Xian H, Lian M, Al-Aly Z: Geographic variation and US county characteristics associated with rapid kidney function decline. Kidney Int Rep 2: 5–17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddan DN, Klassen PS, Szczech LA, Coladonato JA, O’Shea S, Owen WF Jr., Lowrie EG: White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant 18: 1167–1173, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anders HJ, Belemezova E, Eis V, Segerer S, Vielhauer V, Perez de Lema G, Kretzler M, Cohen CD, Frink M, Horuk R, Hudkins KL, Alpers CE, Mampaso F, Schlöndorff D: Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRL-Fas(lpr) mice. J Am Soc Nephrol 15: 1504–1513, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Anders HJ, Vielhauer V, Frink M, Linde Y, Cohen CD, Blattner SM, Kretzler M, Strutz F, Mack M, Gröne HJ, Onuffer J, Horuk R, Nelson PJ, Schlöndorff D: A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest 109: 251–259, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JW, Cho E, Kim MG, Jo SK, Cho WY, Kim HK: Proinflammatory CD14(+)CD16(+) monocytes are associated with vascular stiffness in predialysis patients with chronic kidney disease. Kidney Res Clin Pract 32: 147–152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao W, Topham PS, King JA, Smiley ST, Csizmadia V, Lu B, Gerard CJ, Hancock WW: Targeting of the chemokine receptor CCR1 suppresses development of acute and chronic cardiac allograft rejection. J Clin Invest 105: 35–44, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ninichuk V, Gross O, Reichel C, Khandoga A, Pawar RD, Ciubar R, Segerer S, Belemezova E, Radomska E, Luckow B, Perez de Lema G, Murphy PM, Gao JL, Henger A, Kretzler M, Horuk R, Weber M, Krombach F, Schlöndorff D, Anders HJ: Delayed chemokine receptor 1 blockade prolongs survival in collagen 4A3-deficient mice with Alport disease. J Am Soc Nephrol 16: 977–985, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Rodgers KD, Rao V, Meehan DT, Fager N, Gotwals P, Ryan ST, Koteliansky V, Nemori R, Cosgrove D: Monocytes may promote myofibroblast accumulation and apoptosis in Alport renal fibrosis. Kidney Int 63: 1338–1355, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Awad AS, Kinsey GR, Khutsishvili K, Gao T, Bolton WK, Okusa MD: Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury. Am J Physiol Renal Physiol 301: F1358–F1366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Aly Z: Phosphate, oxidative stress, and nuclear factor-κB activation in vascular calcification. Kidney Int 79: 1044–1047, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Marincola FM: In support of descriptive studies; relevance to translational research. J Transl Med 5: 21, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie Y, Bowe B, Xian H, Balasubramanian S, Al-Aly Z: Rate of kidney function decline and risk of hospitalizations in stage 3A CKD. Clin J Am Soc Nephrol 10: 1946–1955, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Y, Bowe B, Xian H, Balasubramanian S, Al-Aly Z: Estimated GFR trajectories of people entering CKD stage 4 and subsequent kidney disease outcomes and mortality. Am J Kidney Dis 68: 219–228, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.