Abstract
Background and objectives
Studies using high-resolution peripheral quantitative computed tomography showed progressive abnormalities in cortical and trabecular microarchitecture and biomechanical competence over the first year after kidney transplantation. However, high-resolution peripheral computed tomography is a research tool lacking wide availability. In contrast, the trabecular bone score is a novel and widely available tool that uses gray-scale variograms of the spine image from dual-energy x-ray absorptiometry to assess trabecular quality. There are no studies assessing whether trabecular bone score characterizes bone quality in kidney transplant recipients.
Design, settings, participants, & measurements
Between 2009 and 2010, we conducted a study to assess changes in peripheral skeletal microarchitecture, measured by high-resolution peripheral computed tomography, during the first year after transplantation in 47 patients managed with early corticosteroid–withdrawal immunosuppression. All adult first-time transplant candidates were eligible. Patients underwent imaging with high-resolution peripheral computed tomography and dual-energy x-ray absorptiometry pretransplantation and 3, 6, and 12 months post-transplantation. We now test if, during the first year after transplantation, trabecular bone score assesses the evolution of bone microarchitecture and biomechanical competence as determined by high-resolution peripheral computed tomography.
Results
At baseline and follow-up, among the 72% and 78%, respectively, of patients having normal bone mineral density by dual-energy x-ray absorptiometry, 53% and 50%, respectively, were classified by trabecular bone score as having high fracture risk. At baseline, trabecular bone score correlated with spine, hip, and ultradistal radius bone mineral density by dual-energy x-ray absorptiometry and cortical area, density, thickness, and porosity; trabecular density, thickness, separation, and heterogeneity; and stiffness and failure load by high-resolution peripheral computed tomography. Longitudinally, each percentage increase in trabecular bone score was associated with increases in trabecular number (0.35%±1.4%); decreases in trabecular thickness (−0.45%±0.15%), separation (−0.40%±0.15%), and network heterogeneity (−0.48%±0.20%); and increases in failure load (0.22%±0.09%) by high-resolution peripheral computed tomography (all P<0.05).
Conclusions
Trabecular bone score may be a useful method to assess and monitor bone quality and strength and classify fracture risk in kidney transplant recipients.
Keywords: renal osteodystrophy; renal transplantation; mineral metabolism; Absorptiometry, Photon; Adrenal Cortex Hormones; Adult; Bone Density; Bone and Bones; Follow-Up Studies; Fractures, Bone; Humans; kidney transplantation; Porosity; Radius; Spine; Tomography
Introduction
Kidney transplantation is characterized by progressive deterioration of cortical and trabecular microarchitecture that results in decreased bone strength and high risk of fractures (1–5). One quarter of recipients fracture within the first 5 years after transplantation (5). Hip fracture risk is 34% higher than for patients on dialysis (4), and hip and spine fracture risks are more than four- and 23-fold higher, respectively, than for the general population (6,7). In large epidemiologic cohorts of kidney transplant recipients, peripheral fractures (e.g., ankle, foot, wrist, and arm) make up the majority of fracture types (5,8,9). Although fracture risk screening in kidney transplant recipients has been controversial, the 2016 updates to the Kidney Disease Improving Global Outcomes Guidelines (out for public review) recommend bone density screening for fracture risk classification. Therefore, studies are needed to help us understand how to apply fracture risk screening tools used in the general population to patients with kidney disease.
The trabecular bone score (TBS) is a novel method developed to quantify trabecular microarchitecture from lumbar spine dual-energy x-ray absorptiometry (DXA) images. TBS is a gray-scale textural analysis of the DXA image, and TBS measurements correlate with direct measures of trabecular microarchitecture both ex vivo as assessed by micro-computed tomography (10,11) and in vivo from iliac crest bone biopsy (12). TBS has also been shown to predict fractures independent of major clinical risk factors or areal bone mineral density (BMD) measured by DXA at the spine and hip (13–15), and it has been reported to increase the accuracy of fracture prediction in patients with BMD above the osteoporotic threshold (13,14,16). TBS is widely available, and its results are incorporated into international fracture classification guidelines (17,18) as well as the World Health Organizations (WHO) Fracture Risk Assessment (FRAX) Tool (19,20). Recently, in a retrospective study of kidney transplant recipients, Naylor et al. (21) reported that TBS values <1.37 predicted future fractures independent of both lumbar spine and femoral neck BMD and clinical risk factors. There are no studies investigating how TBS relates to trabecular microarchitecture in patients with kidney disease. Therefore, we applied TBS to lumbar spine DXA images from our previously described longitudinal cohort study of 47 kidney transplant recipients that was conducted to assess the effect of kidney transplantation on bone density and microarchitecture (1). We hypothesized that spine TBS would be related to trabecular bone parameters assessed by high-resolution peripheral quantitative computed tomography (HR-pQCT) at the ultradistal radius and tibia and that, during the first 12 months after transplantation, changes in TBS would reflect changes in both cortical and trabecular microarchitecture and biomechanical estimates of bone strength.
Materials and Methods
Patients
Study design and rationale for this post hoc study are described in detail in our previous publications (1,2). In brief, between September of 2009 and May of 2010, 61 consecutive first-time living and deceased donor recipients from Columbia University Medical Center (CUMC) were recruited (Figure 1). Patients ranged between 18 and 75 years old and were managed with early corticosteroid–withdrawal immunosuppression. At transplantation, patients received induction with 4 days of tapering methylprednisolone along with either thymoglobulin or basiliximab; corticosteroids were withdrawn by the end of postoperative day 3. Maintenance immunosuppression was with tacrolimus and mycophenolic acid. Patients were converted to a corticosteroid-based regimen only if they experienced rejection—in our cohort, four patients required conversion. Patients were excluded for history of prior transplantation, malignancy, or taking bisphosphonates, gonadal steroids, aromatase inhibitors, or anticonvulsants that induce hepatic cytochrome P450 enzymes. There were no prevalent or incident fractures. The clinical and research activities are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. The CUMC Institutional Review Board approved this study, and written informed consent and all data were maintained in compliance with the CUMC and Healthcare Insurance Portability and Accountability Act guidelines.
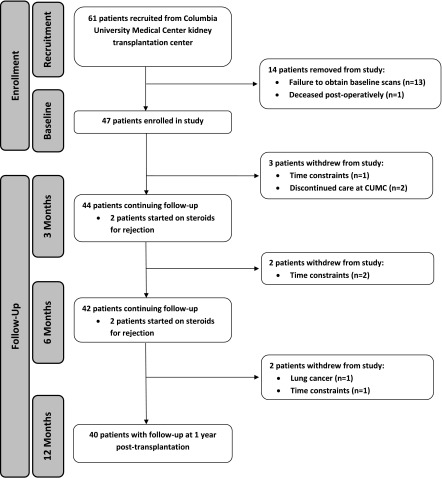
Figure 1.
61 patients were recruited, 47 enrolled, and 44, 42, and 40 patients completed study procedures at 3, 6, and 12 mo respectively. Flowchart of patients who enrolled, dropped out, and completed the study. CUMC, Columbia University Medical Center.
DXA and HR-pQCT Imaging
Patients underwent imaging at baseline and 3, 6, and 12 months (1). Areal BMD by DXA was measured at the total lumbar spine, total hip, femoral neck, and nondominant 1/3 and ultradistal radii using a Hologic QDR 4500 Densitometer (Hologic, Inc., Waltham, MA) in the array (fan beam) mode. Short-term in vivo precision is 0.68% for the spine, 1.36% for the femoral neck, and 0.70% for the radius. HR-pQCT (XtremeCT; nominal resolution of 82 μm3; Scanco Medical, Bruttisellen, Switzerland) and finite element analysis methods have been described (1,2). HR-pQCT images were assessed for quality and motion artifact (22), and scores greater than or equal to four were excluded from analyses.
TBS Analyses
Spine TBS parameters were extracted from the DXA image using TBS iNsight software (version 2.1; Medimaps Group; Plan-Les-Ouates, Geneva, Switzerland). TBS was performed blinded from clinical outcome in the Bone Disease Unit at the University of Lausanne using deidentified spine DXA files from the CUMC. The variogram of the trabecular bone–projected image was calculated as the sum of the squared gray-level differences between pixels at a specific distance and angle. TBS was calculated as the slope of the log-log transform of the variogram (11). The mean value of the individual measurements for L1-L4 represents the lumbar spine TBS (unitless). To ensure comparability with previous TBS studies, calibration of the DXA instrument at the CUMC was performed using a TBS fractal-specific phantom provided by Medimaps. The least significant change in TBS at the CUMC is 3.10%. From the work by Naylor et al. (21), high and normal fracture risks were classified as TBS<1.37 and ≥1.37, respectively.
Laboratory Measurements
Serum creatinine was determined by Jaffe reaction, and serum calcium, phosphorus, and bicarbonate were measured by spectrophotometry. GFR was estimated by Modification of Diet in Renal Disease (23). Bone metabolic markers were measured at the CUMC in a specialized research laboratory. Intact parathyroid hormone (PTH), bone-specific alkaline phosphatase, N-midosteocalcin, procollagen of type 1 N-terminal propeptide, and C-terminal telopeptides of type 1 collagen (CTX) were measured by a Roche Elecsys 2010 Analyzer (Roche Diagnostics, Indianapolis, IN); 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D were measured by HPLC. Intra- and interassay precisions are intact PTH, 1.0% and 4.4%, respectively; bone-specific alkaline phosphatase, 6.0% and 8.0%, respectively; osteocalcin, 0.8% and 2.9%, respectively; procollagen of type 1 N-terminal propeptide, 1.1% and 5.5%, respectively; and CTX, 1.1% and 5.5%, respectively. As in our previous publication, time-averaged levels of calciotropic hormones and bone turnover markers were used in all longitudinal models (1).
Statistical Methods
Statistical analyses were conducted using SAS (v9.4; SAS Institute, Cary, NC). Categorical data were compared using the chi-squared test. Continuous data were evaluated for normality and outliers before analyses, log-transformed when appropriate, and compared with paired t test or Wilcoxon rank sum. Spearman correlations determined relationships between TBS, DXA, HR-pQCT, and levels of biochemical markers. The DXA T score was used to stratify TBS, because it has been shown to predict fractures after kidney transplantation (24). Predictors of baseline TBS were determined by linear regression. Patients were defined as having a significant decline in TBS at 12 months if the decrease in TBS was greater than or equal to the least significant change (3.10%), and predictors of declining TBS at 12 months were determined in this subcohort by linear regression adjusted for the baseline TBS. To determine predictors of the change in HR-pQCT measure, a mixed model with fixed effect of time and continuous covariates for the initial level of the HR-pQCT measure, the initial value of TBS, and the change in TBS was performed on the repeated measures data. Sixty-one patients were enrolled, 47 patients completed baseline procedures and were followed, and 40 patients had data at both the baseline and 12-month visits (Figure 1). A sensitivity analysis conducted with only these 40 patients showed no material or statistical change in the assessed bone outcomes. Therefore, all 47 patients are included in our report.
Results
Cohort Characteristics
Demographic characteristics of the 47 patients with baseline data are presented in Table 1. Mean±SD age was 50.0±14.0 years old, 70% were men, 48% were on dialysis pretransplantation, and median T scores were in the normal range at all skeletal sites. Before transplantation, levels of PTH, osteocalcin, and CTX were greater than the reference ranges. On average, patients were not vitamin D deficient.
Table 1.
Cohort characteristics
| Variable, n=47 | Data |
|---|---|
| Age at transplantation, mean ±SD yr | 50.0±14.0 |
| Sex, N (%) | |
| Men | 33 (70) |
| Women | 14 (30) |
| Race/ethnicity, N (%) | |
| White | 33 (70) |
| Hispanic | 9 (19) |
| Body mass index mean ±SD | 27.8±5.7 |
| Type of transplantation, N (%) | |
| Living | 38 (81) |
| Deceased | 9 (19) |
| Cause of ESRD, N (%) | |
| DM/HTN | 14 (30) |
| Glomerular | 23 (49) |
| Other | 10 (21) |
| Baseline T score by DXA | |
| Lumbar spine | 0.4 (−0.9–1.2) |
| Total hip | −0.3 (−1.1–0.6) |
| Femoral neck | −0.8 (−1.3–−0.06) |
| 1/3 Radius | 0.08 (−0.6–1.3) |
| Ultradistal radius | −0.3 (−1.1–0.5) |
| Baseline kidney function | |
| eGFR, ml/min per 1.73 m2 | 11 (8.5–15.5) |
| Serum creatinine, mg/dl | 5.5 (4.1–7.6) |
| 12-mo Kidney function and CS exposure | |
| eGFR, ml/min per 1.73 m2 | 45 (39–57) |
| Cumulative CS dose (ECSW regimen), mg | 1188 (1188–1250) |
| Cumulative CS dose (CS-based regimen), mg | 5113 (3157–6196) |
| Biochemistries | |
| Calcium, mg/dl | 9.0 (8.6–9.4) |
| Phosphorus, mg/dl | 3.4 (2.5–4.6) |
| Parathyroid hormone, pg/ml | 232 (137–340) |
| 25-Hydroxyvitamin D, ng/ml | 27 (18–41) |
| 1,25-Dihydroxyvitamin D, pg/ml | 22 (15–28) |
| Bone-specific alkaline phosphatase (reference range =15–65), U/L | 25 (19–34) |
| Osteocalcin (reference range =9.7–35.1), ng/ml | 137.3 (58.5–232.3) |
| P1NP (reference range =20–100), μl/L | 63 (41–92) |
| CTX (reference range =0.162–0.573), U/L | 1.580 (0.822–2.520) |
All values are median (interquartile range) unless otherwise noted. DM, diabetes mellitus; HTN, hypertension; DXA, dual-energy x-ray absorptiometry; CS, corticosteroid; ECSW, early corticosteroid withdrawal; P1NP, procollagen type 1 N-terminal propeptide; CTX, C-terminal telopeptides of type 1 collagen.
TBS and Lumbar Spine T Score by DXA
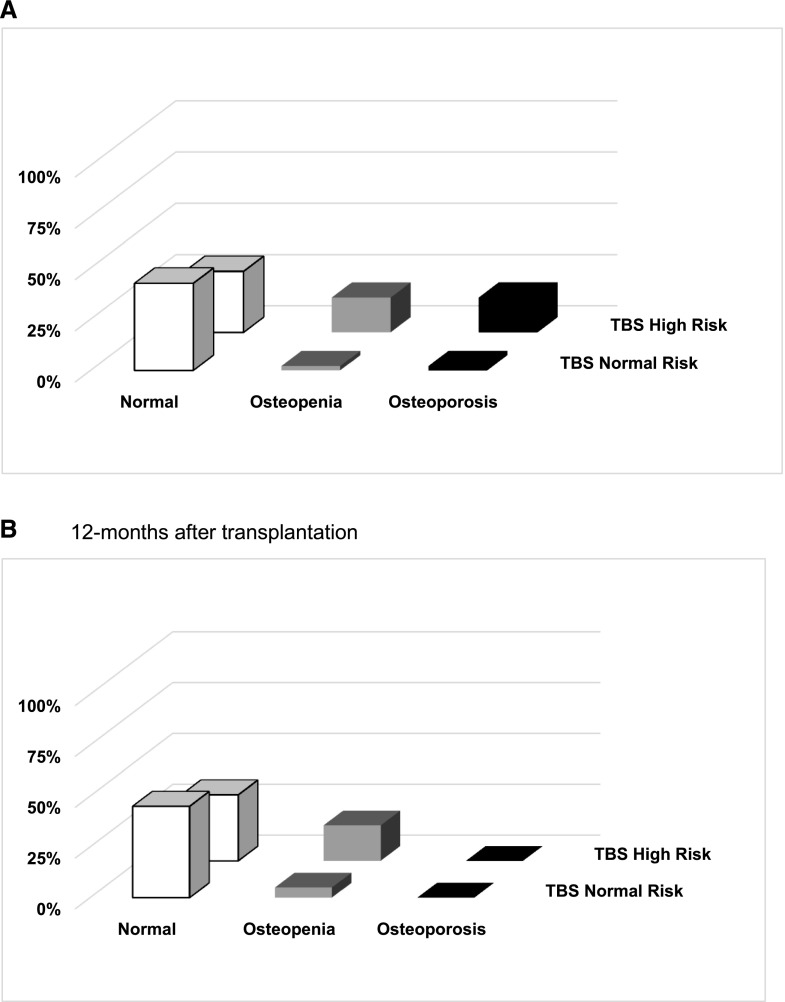
Baseline mean±SD TBS was 1.37±0.12. The majority of patients had normal BMD (T score >−1.0) at the spine both before and after transplantation (72% and 78%, respectively), and no patients had osteoporosis at 12 months. In contrast, by TBS, 53% and 50% of patients, respectively, had TBS that corresponded with high fracture risk before and after transplantation. At 12 months after transplantation, 42% of patients classified as having normal BMD at the spine were classified by TBS as having high risk of fracture (Figure 2), and among patients having a lumber spine T score in the nonosteoporotic range (T score >−2.5), those with TBS<1.37 compared with ≥1.37 had significant impairment in trabecular and cortical bone (Figure 3).
Figure 2.
Bone mineral density stratified by TBS risk score. Both before and after transplantation, TBS classified patients with bone mineral density in the nonosteoporotic range as being at high fracture risk. Prevalence of patients with osteoporosis, osteopenia, or normal lumber spine T score and normal (TBS≥1.37) or high risk (TBS<1.37) of fracture on the basis of TBS at the spine (A) before and (B) 12 months after kidney transplantation. TBS, trabecular bone score.
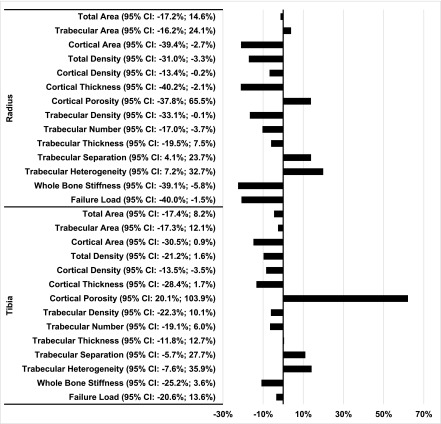
Figure 3.
Despite having bone mineral density in the nonosteoporotic range (T Score > −2.5), patients with a high risk TBS measure had cortical and trabecular microarchitectural features that are associated with increased risk of fracture, and had lower estimates of bone strength by finite element analysis. Percentage difference in trabecular and cortical geometry, density, microarchitecture, and strength at the radius and tibia by high-resolution peripheral computed tomography (HR-pQCT) in patients with lumbar spine T scores >−2.5 stratified by trabecular bone score (TBS) classified as high (TBS<1.37) or normal (≥1.37) fracture risk 12 months after kidney transplantation. Percentage difference = ((HR-pQCT measure at TBS<1.37)−(HR-pQCT measure at TBS≥1.37)/(HR-pQCT measure at TBS≥1.37))×100. 95% CI, 95% confidence interval.
Relationships between TBS, DXA, and Peripheral Bone Microarchitecture and Strength
Correlations between TBS, DXA, and HR-pQCT at baseline are in Table 2. Lower TBS reflected lower BMD by DXA at the spine, hip, and ultradistal radius; thinner, less dense, and more porous cortices; lower trabecular density and greater severity of trabecular disconnectivity by HR-pQCT; and lower bone strength by finite element analysis. Correlations at 12 months were similar to those at baseline (data not shown).
Table 2.
Spearman correlations between trabecular bone score and measures of dual-energy x-ray absorptiometry and high-resolution peripheral computed tomography at the baseline visit
| Imaging Method | Correlation Coefficient |
|---|---|
| DXA | |
| Lumbar spine | 0.50a |
| Total hip | 0.29b |
| Femoral neck | 0.14 |
| 1/3 Radius | 0.18 |
| Ultradistal radius | 0.39c |
| Radius HR-pQCT | |
| Cortical area | 0.40c |
| Cortical porosity | −0.05 |
| Cortical density | 0.26 |
| Cortical thickness | 0.40c |
| Trabecular area | −0.06 |
| Trabecular density | 0.37c |
| Trabecular no. | 0.28 |
| Trabecular thickness | 0.40c |
| Trabecular separation | −0.33a |
| Trabecular heterogeneity | −0.34a |
| Whole-bone stiffness | 0.62a |
| Failure load | 0.47a |
| Tibia HR-pQCT | |
| Cotrical area | 0.32b |
| Cortical porosity | −0.37b |
| Cortical density | 0.37b |
| Cortical thickness | 0.27 |
| Trabecular area | 0.06 |
| Trabecular density | 0.27 |
| Trabecular no. | 0.20 |
| Trabecular thickness | 0.21 |
| Trabecular separation | −0.21 |
| Trabecular heterogeneity | −0.20 |
| Whole-bone stiffness | 0.32 |
| Failure load | 0.25 |
DXA, dual-energy x-ray absorptiometry; HR-pQCT, high-resolution peripheral computed tomography.
P<0.001.
P<0.05.
P<0.01.
By linear regression, predictors of lower TBS at baseline included older age (−0.04±0.01 per 10 years; P<0.01) and pretransplantation dialysis (−0.07±0.03 dialysis versus no dialysis; P<0.05). At 12 months, 15 patients experienced a significant decline in TBS (mean±SD change of −7.3%±3.5%), and predictors of declining TBS included men (−3.7%±1.3% men versus women; P<0.05), diabetes (−3.9%±1.5% diabetic versus nondiabetic; P<0.05), and higher levels of the resorption marker CTX (2.5%±1.1% per 0.05-ng/ml increase; P<0.05).
By mixed models, we determined whether longitudinal changes in TBS predicted changes in trabecular and cortical microarchitecture and bone strength at the peripheral skeleton. At the radius, each percentage increase in TBS predicted increases in trabecular area (0.04%±0.02%; P<0.05) and number (0.35%±0.14%; P<0.05) and decreases in trabecular thickness (−0.45%±0.15%; P<0.01), separation (−0.40%±0.15%; P<0.01), and network heterogeneity (−0.48%±0.20%; P<0.05). At the tibia, each percentage increase in TBS predicted increases in trabecular area (0.02%±0.01%; P<0.01) and failure load (0.22%±0.09%; P<0.05).
Discussion
This is the first study to describe cross-sectional and longitudinal relationships between TBS and trabecular and cortical parameters measured by HR-pQCT in kidney transplant recipients. We found that, even when bone density by DXA was in the nonosteoporotic range, TBS assigned patients with microarchitectural impairment to a high-fracture risk category. TBS correlated with trabecular and cortical density and microarchitecture, and changes in TBS reflected the evolution of trabecular microarchitecture and failure load at the peripheral skeleton. Our previous studies using HR-pQCT in this same cohort reported that there was significant deterioration of trabecular and cortical bone at the radius and tibia over the first year of kidney transplantation (1,2). However, although HR-pQCT has greatly informed our understanding of the skeletal effects of kidney disease and transplantation, HR-pQCT lacks wide availability, is a research tool, and cannot be used for general population fracture risk classification. In contrast, TBS, an indirect measure of trabecular microarchitecture, is approved by the US Food and Drug Administration for fracture risk screening and now incorporated into the WHO’s FRAX Tool (19,20). Thus, in light of studies reporting that, after transplantation, the burden of fractures is highest at the peripheral skeleton (5,8,9) and that TBS predicts fracture (21), our data provide mechanistic insight into meaning of TBS.
TBS is derived from variograms of the gray-scale DXA image and has been validated against cadaver ex vivo samples of bone (11,25). Winzenrieth et al. (26) determined three-dimensional trabecular bone microarchitecture from micro-computed tomography images of cadaver spine and showed moderate correlations with two-dimensional projection-based images (i.e., TBS) of bone volume fraction, trabecular number, intertrabecular space, and mean bone thickness at low image resolution. These findings were confirmed by Hans et al. (11) for both GEHC-Lunar and Hologic DXA machines. Roux et al. (10) further determined that, independent of bone mass, TBS was directly related to trabecular bone volume (r=0.58; P=0.02) and stiffness (r=0.64; P<0.01) and inversely related to the structure model index (r=−0.62; P=0.01) in cadaveric vertebrae. In a cohort of 80 women and 43 men with idiopathic osteoporosis and low trauma fractures, Muschitz et al. (12) reported that TBS predicted bone volume, structure model index, and trabecular number and separation determined by iliac crest biopsy. In patients with primary hyperparathyroidism, Silva et al. (15) assessed relationships between TBS and trabecular and cortical microarchitecture at the ultradistal radius and tibia by HR-pQCT. Surprisingly, in addition to significant relationships between TBS and trabecular microarchitecture, TBS was also related to cortical parameters and whole bone rather than trabecular stiffness. In contrast, in a cohort of patients with osteogenesis imperfecta, Kocijan et al. (27) reported that TBS was related to trabecular but not cortical parameters. In our cross-sectional analyses, we found that, in patients with kidney disease before transplantation, TBS was related to trabecular microarchitecture and whole-bone stiffness and failure load. Similar to Silva et al. (15), we found that, in cross-sectional analyses, TBS was directly related to cortical density and thickness, and for the first time, we report that TBS was inversely related to cortical porosity. Our findings confirm those of the work by Silva et al. (15) and suggest that TBS may be helpful in indirectly characterizing cortical bone deficits in metabolic disorders driven by high levels of PTH. Our study is unique because of its longitudinal design; we report that decreases in TBS predicted worsening trabecular microarchitecture and failure load, and we did not find that changes in TBS predicted changes to cortical bone.
In contrast to the general population (13,14,16,28), there are few data that inform the role of TBS in assessing fracture risk in patients with kidney disease. Recently, in a cohort of 327 kidney transplant recipients and 981 matched controls from Manitoba, Canada, Naylor et al. (21) reported that TBS predicted fractures (area under the curve=0.64; 95% confidence interval, 0.53 to 0.74) independent of both BMD at the spine and hip and the FRAX tool and that TBS<1.37 was associated with high risk. Our findings add to these data: patients classified as high fracture risk by TBS had abnormal cortical and trabecular microarchitecture and lower bone strength, despite having a spine T score in the nonosteoporotic range. This paradox between TBS and spine DXA may be due to vascular calcifications that are prevalent in patients with CKD and affect the DXA but not the TBS measurement. Finally, longitudinal models showed that increases in TBS predicted changes in HR-pQCT trabecular parameters that correlate with improved connectivity, including increased trabecular number and decreased trabecular spacing and heterogeneity. These findings need to be validated in large cohorts of recipients with fracture outcomes.
We found that older age and pretransplantation dialysis were associated with lower TBS at baseline and that men, pretransplant diabetes, and elevated levels of the bone resorption marker CTX were risk factors for declines in TBS. Epidemiologic investigations have reported that pretransplantation diabetes is a risk factor for fractures (5,9,29), that kidney transplant recipients who are men may be at higher risk of bone loss than those who are women (30), and that TBS (but not BMD) was lower in patients with diabetes versus controls (31,32). In our previous publication with this cohort, we reported that bone loss occurred at the peripheral but not the central skeleton and that, at the radius and tibia, deterioration of cortical and trabecular microarchitecture was predicted by older age, women, greater exposure to glucocorticoids, and higher levels of PTH and markers of bone formation and resorption (1,2). Differences in risk factors for bone loss between these investigations likely reflect the dissimilar skeletal sites that were analyzed and the technologies used to assess bone. TBS and HR-pQCT measurements are obtained at different skeletal sites, and the effects of uremia, hyperparathyroidism, diabetes, sex, and immunosuppressive medications at the central and peripheral skeleton may differ. In addition, TBS is derived from the DXA image and only an indirect measure of trabecular microarchitecture.
This study has limitations. Our sample size limited the number of predictors included in multivariate models. The majority of our cohort was on an early corticosteroid–withdrawal regimen; if patients had been managed with corticosteroids, we may have detected more dramatic changes in spine TBS and robust associations between TBS declines and cumulative glucocorticoid dose. We compared TBS at the spine with microarchitecture at the peripheral skeleton; more robust associations may have been detected if we used central quantitative computed tomography at the spine. However, compared with HR-pQCT, the lower resolution of central quantitative computed tomography limits the analysis of the thin vertebral cortical shell and is subject to greater partial volume effect, and because the majority of fractures in kidney transplant recipients are peripheral (5,8,9), our findings are particularly relevant. Resolution of HR-pQCT (82 μm3) is at the limit of trabecular width and subject to partial volume effects; this may have accounted for findings at the radius that seem inconsistent (i.e., increased trabecular number with decreased trabecular thickness). Our TBS cutpoint for high fracture risk was derived from a single study. Also, our cohort was small, and its characteristics regarding sex, donor type, and exposure to pretransplantation dialysis were homogenous; therefore, generalizability of our findings and an appropriate TBS cutpoint need to be tested in large heterogeneous kidney transplant populations before TBS can be widely used. Finally, bone biopsy studies are needed to determine how TBS reflects cortical defects and whether TBS is related to defects in turnover and mineralization that are common in CKD.
In conclusion, TBS was associated with trabecular and cortical microarchitecture and computational measures of bone strength at the peripheral skeleton in patients with kidney disease. TBS was superior to spine DXA in classifying patients with microarchitectural impairments that correlate with high fracture risk. In longitudinal analyses, changes in TBS reflected the evolution of trabecular microarchitecture and failure load at the radius and tibia over the first year after transplantation. These data suggest that TBS, a widely available and inexpensive tool, may be a useful method to assess and monitor bone quality and strength in kidney transplant recipients. Studies are needed in heterogeneous kidney transplant populations to determine if TBS can be used as a diagnostic tool to predict fractures and monitor responses to antifracture strategies.
Disclosures
D.H. is the inventor of trabecular bone score and owner of MediMaps (Geneva, Switzerland).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Noninvasive Imaging of Bone Microarchitecture in Patients Receiving Renal Transplant: Can it Replace Histology?,” on pages 562–564.
References
- 1.Iyer SP, Nikkel LE, Nishiyama KK, Dworakowski E, Cremers S, Zhang C, McMahon DJ, Boutroy S, Liu XS, Ratner LE, Cohen DJ, Guo XE, Shane E, Nickolas TL: Kidney transplantation with early corticosteroid withdrawal: Paradoxical effects at the central and peripheral skeleton. J Am Soc Nephrol 25: 1331–1341, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishiyama KK, Pauchard Y, Nikkel LE, Iyer S, Zhang C, McMahon DJ, Cohen D, Boyd SK, Shane E, Nickolas TL: Longitudinal HR-pQCT and image registration detects endocortical bone loss in kidney transplantation patients. J Bone Miner Res 30: 554–561, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD: Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med 325: 544–550, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, Kestenbaum BR, Stehman-Breen C: Risk of hip fracture among dialysis and renal transplant recipients. JAMA 288: 3014–3018, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Nikkel LE, Hollenbeak CS, Fox EJ, Uemura T, Ghahramani N: Risk of fractures after renal transplantation in the United States. Transplantation 87: 1846–1851, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Abbott KC, Oglesby RJ, Hypolite IO, Kirk AD, Ko CW, Welch PG, Agodoa LY, Duncan WE: Hospitalizations for fractures after renal transplantation in the United States. Ann Epidemiol 11: 450–457, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Vautour LM, Melton LJ 3rd, Clarke BL, Achenbach SJ, Oberg AL, McCarthy JT: Long-term fracture risk following renal transplantation: A population-based study. Osteoporos Int 15: 160–167, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Nikkel LE, Mohan S, Zhang A, McMahon DJ, Boutroy S, Dube G, Tanriover B, Cohen D, Ratner L, Hollenbeak CS, Leonard MB, Shane E, Nickolas TL: Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am J Transplant 12: 649–659, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikkel LE, Iyer SP, Mohan S, Zhang A, McMahon DJ, Tanriover B, Cohen DJ, Ratner L, Hollenbeak CS, Rubin MR, Shane E, Nickolas TL; CURE Group (The Columbia University Renal Epidemiology Group) : Pancreas-kidney transplantation is associated with reduced fracture risk compared with kidney-alone transplantation in men with type 1 diabetes. Kidney Int 83: 471–478, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roux JP, Wegrzyn J, Boutroy S, Bouxsein ML, Hans D, Chapurlat R: The predictive value of trabecular bone score (TBS) on whole lumbar vertebrae mechanics: An ex vivo study. Osteoporos Int 24: 2455–2460, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA: Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: An experimental study on human cadaver vertebrae. J Clin Densitom 14: 302–312, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Muschitz C, Kocijan R, Haschka J, Pahr D, Kaider A, Pietschmann P, Hans D, Muschitz GK, Fahrleitner-Pammer A, Resch H: TBS reflects trabecular microarchitecture in premenopausal women and men with idiopathic osteoporosis and low-traumatic fractures. Bone 79: 259–266, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Hans D, Goertzen AL, Krieg MA, Leslie WD: Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: The Manitoba study. J Bone Miner Res 26: 2762–2769, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R: Trabecular bone score improves fracture risk prediction in non-osteoporotic women: The OFELY study. Osteoporos Int 24: 77–85, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Silva BC, Boutroy S, Zhang C, McMahon DJ, Zhou B, Wang J, Udesky J, Cremers S, Sarquis M, Guo X-DE, Hans D, Bilezikian JP: Trabecular bone score (TBS)--a novel method to evaluate bone microarchitectural texture in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 98: 1963–1970, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCloskey EV, Oden A, Harvey NC, Leslie WD, Hans D, Johansson H, Barkmann R, Boutroy S, Brown J, Chapurlat R, Elders PJ, Fujita Y, Gluer CC, Goltzman D, Iki M, Karlsson M, Kindmark A, Kotowicz M, Kurumatani N, Kwok T, Lamy O, Leung J, Lippuner K, Ljunggren O, Lorentzon M, Mellstrom D, Merlijn T, Oei L, Ohlsson C, Pasco JA, Rivadeneira F, Rosengren B, Sornay-Rendu E, Szulc P, Tamaki J, Kanis JA: A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 31: 940–948 2016 [DOI] [PubMed] [Google Scholar]
- 17.Harvey NC, Glüer CC, Binkley N, McCloskey EV, Brandi ML, Cooper C, Kendler D, Lamy O, Laslop A, Camargos BM, Reginster JY, Rizzoli R, Kanis JA: Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 78: 216–224, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD: Fracture risk prediction by non-BMD DXA measures: The 2015 ISCD official positions part 2: Trabecular bone score. J Clin Densitom 18: 309–330, 2015 [DOI] [PubMed] [Google Scholar]
- 19.McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, Kanis JA: Adjusting fracture probability by trabecular bone score. Calcif Tissue Int 96: 500–509, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Leslie WD, Johansson H, Kanis JA, Lamy O, Oden A, McCloskey EV, Hans D: Lumbar spine texture enhances 10-year fracture probability assessment. Osteoporos Int 25: 2271–2277, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Naylor KL, Lix LM, Hans D, Garg AX, Rush DN, Hodsman AB, Leslie WD: Trabecular bone score in kidney transplant recipients. Osteoporos Int 27: 1115–1121, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S: Visual grading of motion induced image degradation in high resolution peripheral computed tomography: Impact of image quality on measures of bone density and micro-architecture. Bone 50: 111–118, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Akaberi S, Simonsen O, Lindergård B, Nyberg G: Can DXA predict fractures in renal transplant patients? Am J Transplant 8: 2647–2651, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Pothuaud L, Carceller P, Hans D: Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: Applications in the study of human trabecular bone microarchitecture. Bone 42: 775–787, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Winzenrieth R, Michelet F, Hans D: Three-dimensional (3D) microarchitecture correlations with 2D projection image gray-level variations assessed by trabecular bone score using high-resolution computed tomographic acquisitions: Effects of resolution and noise. J Clin Densitom 16: 287–296, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Kocijan R, Muschitz C, Haschka J, Hans D, Nia A, Geroldinger A, Ardelt M, Wakolbinger R, Resch H: Bone structure assessed by HR-pQCT, TBS and DXL in adult patients with different types of osteogenesis imperfecta. Osteoporos Int 26: 2431–2440, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Johansson H, Kanis JA, Oden A, McCloskey E, Chapurlat RD, Christiansen C, Cummings SR, Diez-Perez A, Eisman JA, Fujiwara S, Gluer CC, Goltzman D, Hans D, Khaw KT, Krieg MA, Kroger H, Lacroix AZ, Lau E, Leslie WD, Mellstrom D, Melton LJ 3rd, O’Neill TW, Pasco JA, Prior JC, Reid DM, Rivadeneira F, van Staa T, Yoshimura N, Zillikens MC: A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 29: 223–233, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Nikkel LE, Mohan S, Zhang A, McMahon DJ, Boutroy S, Dube G, Tanriover B, Cohen D, Ratner L, Hollenbeak CS, Leonard MB, Shane E, Nickolas TL: Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am J Transplant 12: 649–659, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horber FF, Casez JP, Steiger U, Czerniak A, Montandon A, Jaeger P: Changes in bone mass early after kidney transplantation. J Bone Miner Res 9: 1–9, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM: Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int 25: 1969–1973, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Leslie WD, Aubry-Rozier B, Lamy O, Hans D; Manitoba Bone Density Program : TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 98: 602–609, 2013 [DOI] [PubMed] [Google Scholar]