Abstract
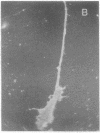
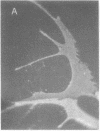
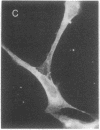
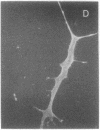
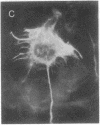
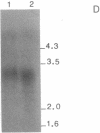
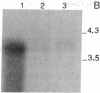
Permanent clonal cell lines from newborn mouse striatum have been established after transfer of the simian virus 40 large tumor oncogene by means of a retroviral vector. Some of the lines obtained displayed properties of bipotential and plastic glio-neuronal precursors. Depending on the culture conditions, these cells express either the glial fibrillary acidic protein or neurofilaments. In addition, the cells can display adrenergic, D1 and D2 dopaminergic, muscarinic, and 5-hydroxytryptamine type 2 serotoninergic receptors, which are coupled either to the adenylate cyclase or to the phosphatidylinositol signaling pathways. The panel of receptors for neurotransmitters exhibited by these lines closely resembles that of primary striatal neurons. Results suggest that plastic common precursors of astrocytes and neurons persist in the striatum at a late developmental stage. As these permanent cell lines constitute an unlimited source of homogenous cell material, we suggest that they should be useful for molecular and pharmacological studies on the mechanisms and regulation of signal transduction as well as the commitment, plasticity, and differentiation of neural cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alliot F., Pessac B. Astrocytic cell clones derived from established cultures of 8-day postnatal mouse cerebella. Brain Res. 1984 Jul 23;306(1-2):283–291. doi: 10.1016/0006-8993(84)90377-9. [DOI] [PubMed] [Google Scholar]
- Bartlett P. F., Reid H. H., Bailey K. A., Bernard O. Immortalization of mouse neural precursor cells by the c-myc oncogene. Proc Natl Acad Sci U S A. 1988 May;85(9):3255–3259. doi: 10.1073/pnas.85.9.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Grob P., Akert K. Papain-derived fragment IIc of tetanus toxin: its binding to isolated synaptic membranes and retrograde axonal transport. Brain Res. 1981 Apr 6;210(1-2):291–299. doi: 10.1016/0006-8993(81)90902-1. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnoni A. T., Macklin W. B. Cellular and molecular aspects of myelin protein gene expression. Mol Neurobiol. 1988 Spring;2(1):41–89. doi: 10.1007/BF02935632. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Dautigny A., Pham-Dinh D., Roussel C., Felix J. M., Nussbaum J. L., Jollès P. The large neurofilament subunit (NF-H) of the rat: cDNA cloning and in situ detection. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1099–1106. doi: 10.1016/0006-291x(88)90254-9. [DOI] [PubMed] [Google Scholar]
- De Vitry F. Growth and differentiation of a primitive nervous cell line after in vivo transplantation into syngeneic mice. Nature. 1977 May 5;267(5606):48–50. doi: 10.1038/267048a0. [DOI] [PubMed] [Google Scholar]
- Denis-Donini S., Glowinski J., Prochiantz A. Glial heterogeneity may define the three-dimensional shape of mouse mesencephalic dopaminergic neurones. Nature. 1984 Feb 16;307(5952):641–643. doi: 10.1038/307641a0. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard C., Galiana E., Rouget P. Establishment of 'normal' nervous cell lines after transfer of polyoma virus and adenovirus early genes into murine brain cells. EMBO J. 1986 Dec 1;5(12):3157–3162. doi: 10.1002/j.1460-2075.1986.tb04623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard C., Galiana E., Rouget P. Immortalization of bipotential glial progenitors and generation of permanent "blue" cell lines. J Neurosci Res. 1988 Sep;21(1):80–87. doi: 10.1002/jnr.490210112. [DOI] [PubMed] [Google Scholar]
- Frederiksen K., Jat P. S., Valtz N., Levy D., McKay R. Immortalization of precursor cells from the mammalian CNS. Neuron. 1988 Aug;1(6):439–448. doi: 10.1016/0896-6273(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Geller H. M., Dubois-Dalcq M. Antigenic and functional characterization of a rat central nervous system-derived cell line immortalized by a retroviral vector. J Cell Biol. 1988 Nov;107(5):1977–1986. doi: 10.1083/jcb.107.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengrass P., Bremner R. Binding characteristics of 3H-prazosin to rat brain alpha-adrenergic receptors. Eur J Pharmacol. 1979 May 1;55(3):323–326. doi: 10.1016/0014-2999(79)90202-4. [DOI] [PubMed] [Google Scholar]
- Hallermayer K., Harmening C., Hamprecht B. Cellular localization and regulation of glutamine synthetase in primary cultures of brain cells from newborn mice. J Neurochem. 1981 Jul;37(1):43–52. doi: 10.1111/j.1471-4159.1981.tb05289.x. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P. S., Cepko C. L., Mulligan R. C., Sharp P. A. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986 Apr;6(4):1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien J. P., Tretjakoff I., Beaudet L., Peterson A. Expression and assembly of a human neurofilament protein in transgenic mice provide a novel neuronal marking system. Genes Dev. 1987 Dec;1(10):1085–1095. doi: 10.1101/gad.1.10.1085. [DOI] [PubMed] [Google Scholar]
- Kellermann O., Kelly F. Immortalization of early embryonic cell derivatives after the transfer of the early region of simian virus 40 into F9 teratocarcinoma cells. Differentiation. 1986;32(1):74–81. doi: 10.1111/j.1432-0436.1986.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: thirty-five years later. EMBO J. 1987 May;6(5):1145–1154. doi: 10.1002/j.1460-2075.1987.tb02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Balcarek J. M., Krek V., Shelanski M., Cowan N. J. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984 May;81(9):2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leysen J. E., Gommeren W., Van Gompel P., Wynants J., Janssen P. F., Laduron P. M. Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist. Mol Pharmacol. 1985 Jun;27(6):600–611. [PubMed] [Google Scholar]
- Liem R. K., Hutchison S. B. Purification of individual components of the neurofilament triplet: filament assembly from the 70 000-dalton subunit. Biochemistry. 1982 Jun 22;21(13):3221–3226. doi: 10.1021/bi00256a029. [DOI] [PubMed] [Google Scholar]
- Mallat M., Moura Neto V., Gros F., Glowinski J., Prochiantz A. Two simian virus 40 (SV40)-transformed cell lines from the mouse striatum and mesencephalon presenting astrocytic characters. II. Interactions with mesencephalic neurons. Brain Res. 1986 Apr;391(1):23–31. doi: 10.1016/0165-3806(86)90004-0. [DOI] [PubMed] [Google Scholar]
- Omlin F. X., Waldmeyer J. Differentiation of neuron-like cells in cultured rat optic nerves: a neuron or common neuron-glia progenitor? Dev Biol. 1989 May;133(1):247–253. doi: 10.1016/0012-1606(89)90315-1. [DOI] [PubMed] [Google Scholar]
- Pearce B., Morrow C., Murphy S. Receptor-mediated inositol phospholipid hydrolysis in astrocytes. Eur J Pharmacol. 1986 Feb 18;121(2):231–243. doi: 10.1016/0014-2999(86)90494-2. [DOI] [PubMed] [Google Scholar]
- Pollerberg E. G., Sadoul R., Goridis C., Schachner M. Selective expression of the 180-kD component of the neural cell adhesion molecule N-CAM during development. J Cell Biol. 1985 Nov;101(5 Pt 1):1921–1929. doi: 10.1083/jcb.101.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont J., Daguet-de Montety M. C., Herbet A., Glowinski J., Bockaert J., Prochiantz A. Biogenic amines and adenosine-sensitive adenylate cyclases in primary cultures of striatal neurons. Brain Res. 1983 Jul;285(1):53–61. doi: 10.1016/0165-3806(83)90108-6. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Glial cell diversification in the rat optic nerve. Science. 1989 Mar 17;243(4897):1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Nicolas J. F., Jacob F. Construction of a retrovirus capable of transducing and expressing genes in multipotential embryonic cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7137–7140. doi: 10.1073/pnas.81.22.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Saneto R. P., Low K. G., Melner M. H., de Vellis J. Insulin/insulin-like growth factor I and other epigenetic modulators of myelin basic protein expression in isolated oligodendrocyte progenitor cells. J Neurosci Res. 1988 Oct-Dec;21(2-4):210–219. doi: 10.1002/jnr.490210213. [DOI] [PubMed] [Google Scholar]
- Saneto R. P., de Vellis J. Characterization of cultured rat oligodendrocytes proliferating in a serum-free, chemically defined medium. Proc Natl Acad Sci U S A. 1985 May;82(10):3509–3513. doi: 10.1073/pnas.82.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B. H., Weiss S., Sebben M., Kemp D. E., Bockaert J., Sladeczek F. Dual action of excitatory amino acids on the metabolism of inositol phosphates in striatal neurons. Mol Pharmacol. 1987 Sep;32(3):364–368. [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Edgar D. Neurotrophic factors. Science. 1985 Jul 19;229(4710):238–242. doi: 10.1126/science.2409599. [DOI] [PubMed] [Google Scholar]
- Turner D. L., Cepko C. L. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987 Jul 9;328(6126):131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Weiss S., Sebben M., Garcia-Sainz J. A., Bockaert J. D2-dopamine receptor-mediated inhibition of cyclic AMP formation in striatal neurons in primary culture. Mol Pharmacol. 1985 Jun;27(6):595–599. [PubMed] [Google Scholar]
- el-Etr M., Cordier J., Glowinski J., Premont J. A neuroglial cooperativity is required for the potentiation by 2-chloroadenosine of the muscarinic-sensitive phospholipase C in the striatum. J Neurosci. 1989 May;9(5):1473–1480. doi: 10.1523/JNEUROSCI.09-05-01473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]