Abstract
2,5-hexanedione (HD) is the ultimate neurotoxic metabolite of hexane, causing the progression of nerve diseases in human. It was reported that HD induced apoptosis and oxidative stress. Taurine has been shown to be a potent antioxidant. In the present study, we investigated the protection of taurine against HD-induced apoptosis in PC12 cells and the underlying mechanism. Our results showed the decreased viability and increased apoptosis in HD-exposed PC12 cells. HD also induced the disturbance of Bax and Bcl-2 expression, the loss of MMP, the release of mitochondrial cytochrome c and caspase-3 activation in PC12 cells. Moreover, HD resulted in an increase in reactive oxygen species (ROS) level and a decline in the activities of superoxidedismutase and catalase in PC12 cells. However, taurine pretreatment ameliorated the increased apoptosis and the alterations in key regulators of mitochondria-dependent pathway in PC12 exposed to HD. The increased ROS level and the decreased activities of the antioxidant enzymes in HD group were attenuated by taurine. These results indicate that pretreatment of taurine may, at least partly, prevent HD-induced apoptosis via inhibiting mitochondria-dependent pathway. It is also suggested that the potential of taurine against HD-induced apoptosis may benefit from its anti-oxidative property.
Keywords: Taurine; 2,5-hexanedione; Oxidative stress; Apoptosis; Mitochondrial pathway
Introduction
N-hexane, one of organic solvents which are prominent environmental pollutants, has been shown to cause neurotoxicity in vivo and in vitro. Central-peripheral neuropathy induced by n-hexane exposure has been a major health concern and occupational health hazard. In living beings, 2,5-hexanedione (HD) is the ultimate neurotoxic metabolite of n-hexane and mediates the neurotoxicity of the parent compound1, 2). Some studies suggest that HD induces apoptosis in neurons and neuron-like cell lines, and the increased apoptosis is being the major cause for the pathophysiological changes induced by HD3, 4, 5, 6). It is well known that apoptosis could be mediated by several different pathways and the mitochondrial pathway is a major signaling leading to apoptosis. It has been documented that mitochondria-mediated pathway includes the disturbed expression of Bax and Bcl-2, loss of mitochondrial transmembrane potential (MMP), release of mitochondrial cytochrome c (Cyt C), activation of caspase-3 and ultimately triggers apoptosis. It was reported that HD up-regulated Bax expression and caspase-3 activity in rat nerve tissue5). Mishra et al.7) showed the loss of MMP in HD-exposed spermatogenic cells. In previous studies, we also found that HD altered the key regulators of mitochondria-dependent apoptosis8, 9). These studies indicate that the mitochondria-dependent pathway may involve in HD-induced apoptosis.
Environmental toxicants induce the production of reactive oxygen species (ROS). ROS elicit oxidative stress causing a state of imbalance between the antioxidant defense system levels and the production of free radicals. It is known that ROS plays a key role in cell signaling to affect many pathological and physiological processes10). Especially, it has been reported that oxidative stress, as a trigger for cell death, is involved in the apoptotic signaling mechanism. Chang et al.11) showed that Cd-induced ROS resulted in pancreatic β apoptosis. Lu et al.10) reported that arsenic significantly induced ROS and apoptosis in Neuro-2a cells. Pretreatment with the antioxidant N-acetyl cysteine effectively reversed the arsenic-induced responses. These results indicated that ROS leads to apoptosis in various toxicant-exposed cells and tissues. Rashid et al.12) found that ROS triggered the loss of MMP and the release of mitochondrial Cyt C after alloxan treatment. It was also reported that the increased ROS promoted several characteristics of mitochondria-dependent apoptotic events, including loss of MMP and alteration of Bax and Bcl-2 expression, in the testis of arsenic-treated rat13). Kim et al.14) reported that exposure to HD significantly increased ROS level in murine neural progenitor cells and in the hippocampus of ICR mice. McDermott et al.15) also showed a concentration-dependent increase in ROS formation in Jurkat T-cell exposed to n-hexane, the parent of HD. These studies implied that HD might exert the pro-apoptotic action via ROS-regulated mitochondrial-dependent pathway.
Taurine, the major free amino acid, is normally present at high concentrations in nerve tissue and has no side-effects even if administered at high doses16). It has been reported that taurine is an antioxidant and protects against oxidative stress-induced pathology in nervous system17). Taurine also possesses anti-apoptotic properties in neurons and neuron-like cells18, 19, 20). In recent, Chang et al.21) reported that taurine effectively suppressed the disturbance of Bax and Bcl-2 in human proximal tubular epithelial cells exposed to oxidized LDL. Taurine treatment also significantly inhibited endosulfan-induced the disturbance of MMP and caspase-3 activation in rat testis22). These results indicate that taurine may have protective effect on the important regulators of mitochondria-dependent apoptosis altered by toxicants. Therefore, we are interested in whether taurine prevent HD-induced apoptosis via inhibiting mitochondria-dependent pathway.
In the present study, the viability and apoptosis of HD-exposed PC12 cells were observed with or without taurine pretreatment. Moreover, we also investigated the expression of Bax and Bcl-2, MMP, Cyt C translocation and caspase-3 activity. To assess the effect of taurine on the oxidative stress and the activities of major antioxidases in HD-exposed PC12 cells, the ROS level and activities of superoxidedismutase (SOD) and catalase (CAT) were further examined. This study aimed at investigating the prevention of taurine against HD-induced apoptosis in PC12 cells and its underlying mechanism. Output of this study may offer an interesting therapeutic approach in HD-induced neurotoxicity.
Materials and Methods
Cell culture
PC12 cells, a rat pheochromocytoma cell line, possess properties of neurons and exhibit cellular responses to toxicants that are similar to those found in human primary cultures. It has been used extensively as a model for neurotoxicity studies23). In this study, PC12 cells were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM with 10% fetal bovine serum, 100 U/mlpenicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2.
MTT assay
To establish the exposure dose and time of HD necessary for maximum damage in PC12 cells, cells were treated with five different doses of HD (0, 2.5, 5, 10 and 20 mM) and each dose experiments were carried out at for 6 h, 12 h and 24 h, respectively. To determine the optimal dose of taurine, PC12 cells were treated with six different doses of taurine (0, 6.25, 12.5, 25, 50 and 100 mM) followed by HD intoxication (20 mM). Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. 24 h after the final dose of HD intoxication, all cells were incubated with 0.5 mg/ml MTT (Sigma, USA) for 4 h. The medium was then removed and replaced with 750 μL DMSO. The plate was shaken for 10 min and the absorbance was measured at 570 nm using a microplate reader. The effect of HD and taurine on cell viability was expressed as percentage to the control.
Cell treatment
Cells were divided into four groups: control group, cell received neither taurine nor HD; taurine group, cells received 100 mM taurine for 0.5 h; HD group, cells received 20 mM HD for 24 h; taurine+HD group, cells received 100 mM taurine for 0.5 h, then removed and replaced with HD for 24 h .
ROS quantization
The formation of ROS was estimated with dichlorohydrofluoresce indiacetate (DCFH-DA, Beyotime, China). Cells were washed with PBS and suspended in DMEM loaded with DCFH-DA for 20 min at 37°C. Fluorescence dye was measured with a fluorescence plate reader (TECAN, Austria) at excitation and emission wave lengths of 485 and 530 nm, respectively.
SOD assay
Cells were lysed with RIPA buffer (Beyotime, China) with 1% tyrosine phosphatase inhibitor and 1% phenylmethylsulfonyl fluoride. Cells were centrifuged at 8,000 g for 15 min at 4°C. SOD activity in the proteins was determined by using the SOD analysis kit (Beyotime, China).
CAT activity assay
Cells were lysed as above and centrifuged at 13,000 rpm for 20 min at 4°C. The activity of CAT in the proteins was determined by using the CAT analysis kit (Beyotime, China).
Flow cytometry with AnnexinV/PI double staining
After treatment as described above, the cells were gently digested with trypsin without EDTA, washed thrice with PBS and collected 4×106 cells. Then were suspended in 500 μL annexin V Binding buffer and incubated with PI and annexin V (KeyGEN, China) at room temperature in dark for 30 min. Fluorescence analysis was carried out using a flow cytometer (BD FACS Calibur, USA).
MMP measurement
JC-1 is a sensitive and relatively mitochondrion-specific lipophilic cationic probe fluorochrome. JC-1 accumulates and emits red fluorescence in the mitochondria of higher membrane potentials, yet dissociates into monomers and emits green fluorescence in those that lose cross-membrane electrochemical gradient. The ratio of green to red fluorescence intensity (MFI) therefore provides a reliable estimate of impairment of MMP. For this assay, PC12 cells were washed with PBS, then incubated with JC-1 (5 μmol/L) in DMEM at 37°C for 20 min and analyzed with confocal microscope (TCS SP5, Leica, Mannheim, Germany) and Image-Pro Plus 6.0 (Labsystems, MA, USA) to determine the ratio of green (excitation/emission wavelength=485/538 nm) to red (excitation/emission wavelength=485/590 nm) fluorescence, both normalized to baseline value.
Western blot
Cells were homogenized in ice-cold RIPA Tissue Protein Extraction Reagent (Beyotime, China) supplemented with 1% proteinase inhibitor mix. The proteins were separated by SDS-PAGE and then electrotransferred to Hybond-P PVDF membrane (Millipore, France). The membrane was incubated with appropriate primary antibodies overnight at 4°C. Antibodies used were Bcl-2 and Bax (1:500, Cell Signaling Technology, USA), Cyt C and β-actin (1:500, Beyotime, China), VDAC (1:1,000, Cell Signaling Technology, USA). Immunoreactivity was visualized by second horseradish peroxidase-conjugated antibody (1:5,000, Sigma, USA) and enhanced chemoluminescence (Beyotime, China). Quantified densitometric analysis was using with UVP BioSpectrum Multispectral Imaging System (Ultra-Violet Products Ltd. USA).
Caspase-3 activity detection
Caspase-3 activities were determined with a caspase-3 activity assay kit (Beyotime, China), which detects the production of the chromophore p-nitroanilide after its cleavage from the peptide substrate DEVD-p-nitroanilide and LEHD-p-nitroanilide. Colorimetric reaction was developed and measured at 405 nm in a BioRad plate reader.
Statistical analysis
Data were presented as mean±standard deviation (SD). All data were analyzed with SPSS 11.0 for windows. Difference in mean values between groups was tested with the one-way ANOVA and LSD test. P values less than 0.05 were considered significant.
Results
Dose and time dependent effect of HD intoxication
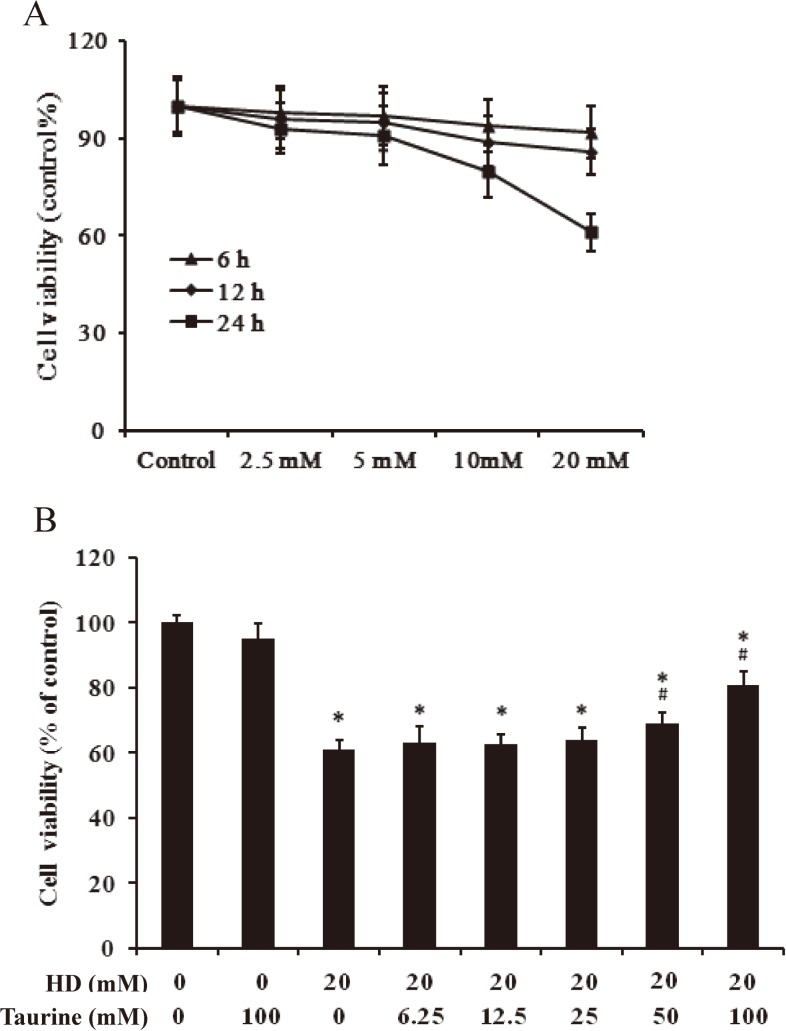
To determining the dose and time necessary for HD to induce maximum cellular damage, we carried out the dose and time dependent assays by MTT. As evidenced from Fig. 1A, HD intoxication decreased the cell viability linearly up to a dose of 20 mM for 24 h in a dose- and time-dependent. This dose and time were, therefore, chosen as the optimum dose and time of HD throughout the study.
Fig. 1.
The viability of PC12 cells received HD alone or with taurine. A. Dose- and time-dependent effect of HD on cell viability. PC12 cells were exposed to different concentrations (5, 10, 20, 40 mM) of HD for 0, 6, 12 and 24 h; B. PC12 cells were treated with different concentrations of taurine (0–100 mM) for 30 min, followed by 20 mM HD for 24 h. Cell viability was assessed by MTT assay. Each column represents mean±SD, n=6. *p<0.05, compared with control group; #p<0.05, compared with HD group.
Taurine protects against hd-induced toxicity in a dose-dependent manner
To determine the optimal levels of taurine exposure, PC12 cells were preincubated with serial concentrations of taurine (0, 6.25, 12.5, 25 and 100 mM) for 0.5 h before being exposed to 20 mM HD for 24 h. Figure 1B indicates that taurine protected against HD-induced neurotoxicity in a dose-dependent manner. Preincubation with 100 mM taurine resulted in optimal recovery from neuronal injury (Fig. 1B, lane 8). This optimal concentration of 100 mM taurine was used in the later experiments.
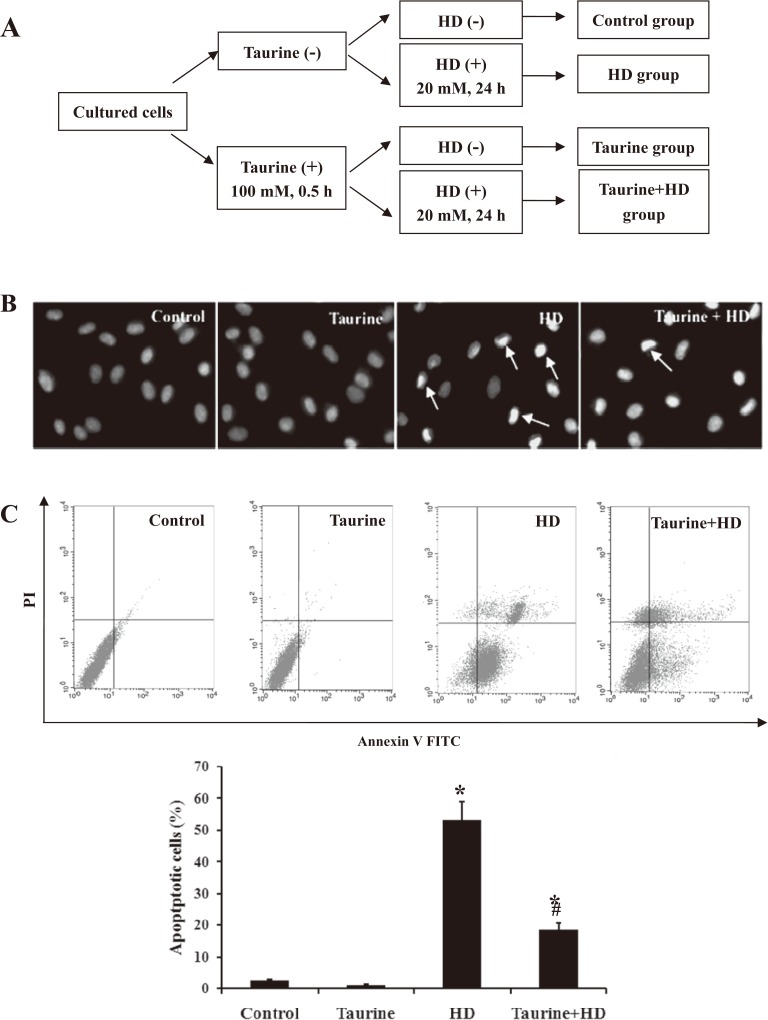
Taurine attenuates HD-induced apoptosis in PC12 cells
PC12 cells were treated with 100 mM taurine for 0.5 h, followed by exposed to 20 mM HD for 24 h (Fig. 2A). To determine the anti-apoptotic efficacy of taurine, Hochest 33342 staining was used to characterize the morphological changes of HD-intoxicated-PC12 cells with or without taurine. As shown in Fig. 2B, the nuclei of control cells were of a rounded shape with homogeneous intensity. However, the HD-exposed PC12 cells showed crescent-shaped nuclei and fragmentation with heterogeneous intensity in the nuclei, suggesting that these cells underwent gross morphological change indicative of apoptosis. Interestingly, compared with HD group, taurine pretreatment markedly decreased the number of cells with condensation and fragmentation in HD-intoxicated cells, indicating that taurine ameliorated apoptotic morphological changes induced by HD. To further determine the number of apoptotic cells, the cells were stained with annexin V and propidium iodide and were then analyzed via flow cytometry. We observed a significant increase in apoptotic cell numbers in the HD-treated cells (Fig. 2C). The pretreatment of cells with taurine, however, the apoptosis rate in HD-exposed PC12 cells was reduced from about 55% to 18%, suggesting taurine attenuated HD-induced apoptosis in PC12 cells.
Fig. 2.
Effect of taurine on apoptosis induced by HD in PC12 cells. A. Hoechst 33342 staining was used to detect the morphological changes (400×); B. Flow cytometric analysis of effects of taurine on PC12 cells apoptosis and necrosis induced by HD. Representatively original data of flow cytometry and the apoptotic rate in different groups. Data were presented as mean±SD, n=6. *p<0.05, compared with control group; #p<0.05, compared with HD group.
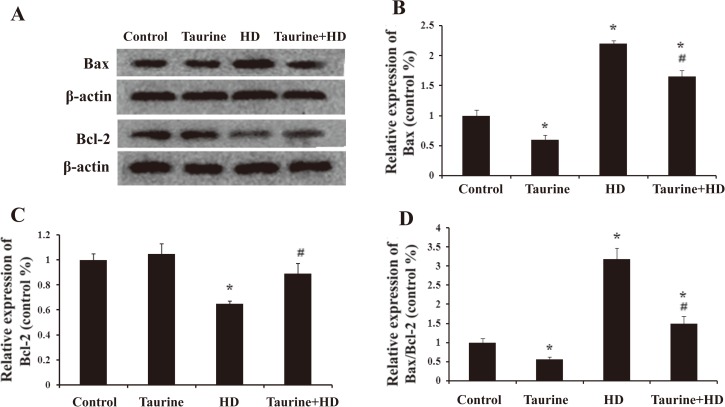
Taurine inhibited the disruption of Bax and Bcl-2 expression in HD-treated PC12 cells
As taurine suppressed HD-induced apoptosis, we assessed the effects of taurine on the expression levels of Bax and Bcl-2 proteins. As shown in Fig. 3A-C, compared with control group, HD intoxication increased the pro-apoptotic protein expression of Bax in PC12 cells, which was significantly attenuated once HD-intoxicated cells were pretreated with taurine. On the contrary, HD intoxication decreased the anti-apoptotic protein expression of Bcl2 in PC12 cells, which was markedly inhibited once HD-intoxicated cells were pretreated with taurine. It was shown that the ratio of Bax to Bcl-2 (Bax/Bcl-2) determines the susceptibility of a cell to apoptosis5). Bax/Bcl-2 ratio was also assessed in this study. As seen in Fig. 3D, consistent with Bax protein expression, taurine significantly mitigated the elevation of Bax/Bcl-2 ratio induced by HD, indicating that taurine inhibited the disruption of Bax and Bcl-2 expression in HD-treated PC12 cells.
Fig. 3.
Effect of taurine on the expression of Bax and Bcl-2 in HD-exposed PC12 cells. Western blot analysis was used to detect Bax and Bcl-2 (A-C) protein levels. The ratio of Bax/Bcl-2 was analyzed (D). Representatively WB images were shown and quantified data were presented as mean±SD. *p<0.05, compared with control group; #p<0.05, compared with HD group.
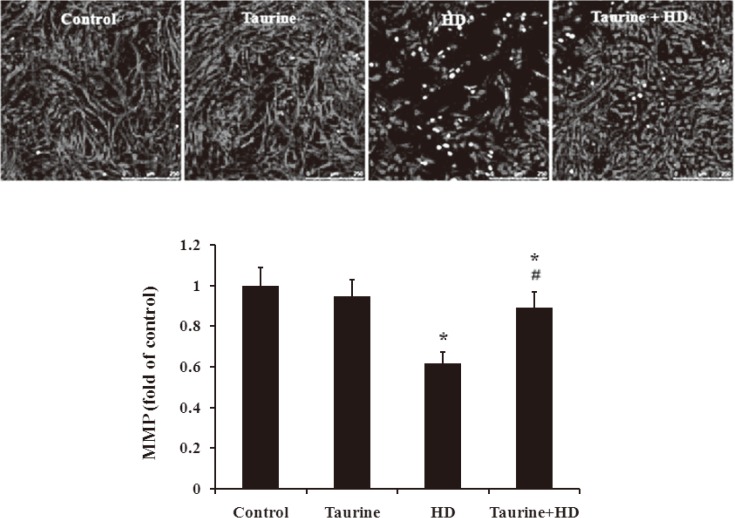
Taurine blocked the collapse of MMP in HD-intoxicated PC12 cells
Increasing of Bax protein but decreasing of Bcl-2 protein would trigger the dissipation of MMP in mitochondria-mediated pathway8, 9). As shown in Fig. 4, compared with the control, HD exposure markedly reduced MMP in PC12 cells. In contrast, in the presence of taurine, HD failed to interfere with MMP in PC12 cells, indicating that taurine inhibite blocked HD-induced collapse of MMP in PC12 cells.
Fig. 4.
Effect of taurine on MMP in HD-exposed PC12 cells. *p<0.05, compared with control group; #p<0.05, compared with HD group.
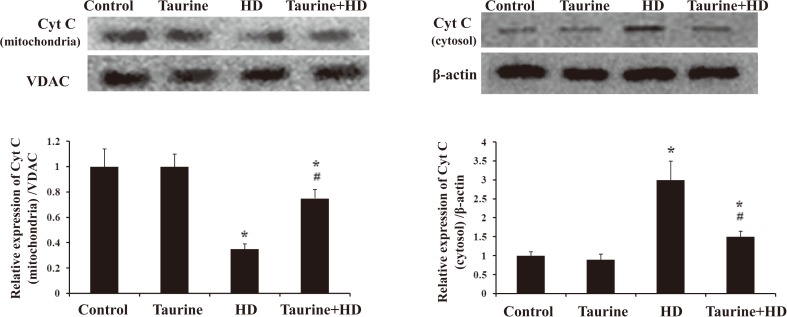
Taurine suppressed the Cyt c release from mitochondria in HD-treated PC12 cells
Disruption of MMP is one of the earliest intracellular events that occur following the onset of apoptosis, which leads to the subsequent release of Cyt C from mitochondria and consequently triggers other apoptotic factors8, 9). Our results showed that compared with vehicle controls, the protein level of Cyt C in mitochondria was significantly decreased (Fig. 5A), while markedly increased in cytosolic fraction in HD-intoxicated PC12 cells (Fig. 5B). Interestingly, taurine markedly suppressed HD-induced Cyt C release by showing elevated expression of mitochondrial Cyt C and reduced expression of cytosol Cyt C in taurine/HD-treated cells compared with HD alone group (Fig. 5).
Fig. 5.
Effect of taurine on the release of cytochrome c in HD-exposed PC12 cells. Western blot was used to detect cytochrome c levels in mitochondrial fraction (A) and cytosolic fraction (B). Data were presented as mean±SD. *p<0.05, compared with control group; #p<0.05, compared with HD group.
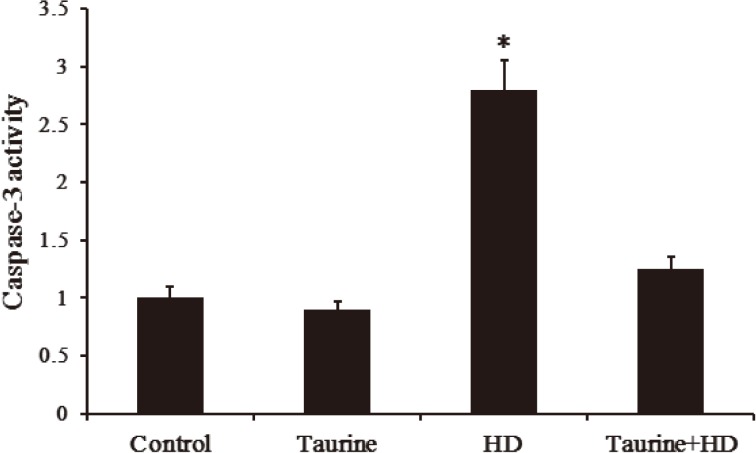
Taurine prevented HD-induced activation of caspase-3 in PC12 cells
Caspase-3 is the effector of mitochondria-mediated apoptosis pathway. In the present study, the effect of taurine on the activity of caspase-3 protein in PC12 cells was shown in Fig. 6. As expected, compared with control group, a significant increase in caspase-3 activity was observed in HD-intoxicated PC12 cells, which was significantly repressed once HD-intoxicated cells were pretreated with taurine, suggesting that HD-induced caspase-3 activation was prevented by taurine.
Fig. 6.
Effect of taurine on caspase-3 activity in HD-exposed PC12 cells. Data were presented as mean±SD, n=6. *p<0.05, compared with control group; #p<0.05, compared with HD group.
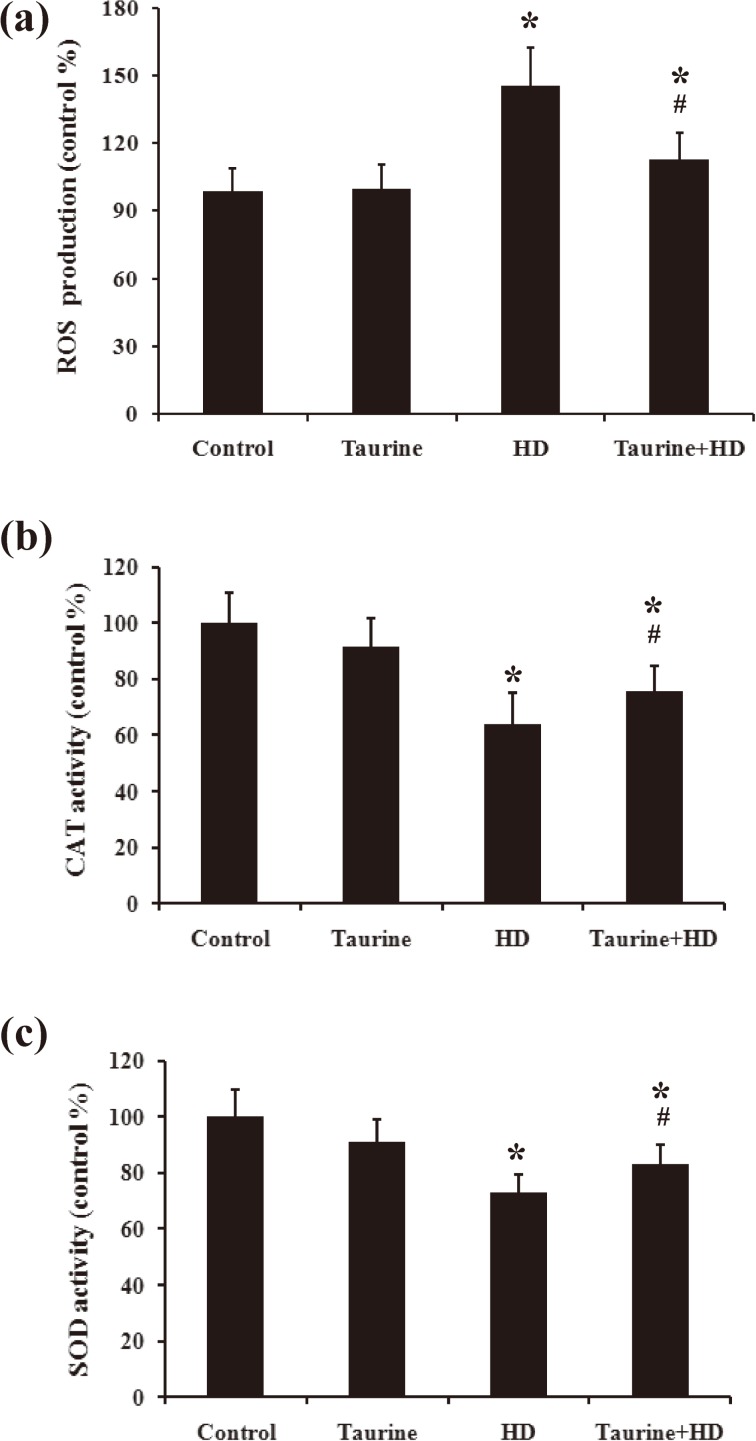
Taurine lowered ROS production in HD-treated PC12 cells
Oxidative stress seems to responsible for the activation of mitochondria-mediated pathway 12). ROS is assumed to be the one of the important parameters of oxidative stress, therefore, we determined the ROS production in taurine/HD-treated PC12 cells. As seen in Fig. 7A, HD treatment significantly increased the production of ROS, indicating an oxidative stress to cells. However, such effect was reversed when HD-intoxicated cell was pretreated with taurine.
Fig. 7.
Effect of taurine on ROS production (A), SOD activity (B) and CAT activity (C) in HD-exposed PC12 cells. Data were presented as mean±SD, n=6. *p<0.05, compared with control group; #p< 0.05, compared with HD group.
Taurine ameliorated the decreased activities of antioxidant enzymes induced by HD
The depletion of the activities of antioxidant enzymes would result in ROS overproduction. The activities of CAT and SOD were tested in PC12 cells exposed to HD in the presence of taurine or not. As shown in Fig. 7B and C, HD-induced decrease of CAT and SOD activities in HD-intoxicated cells was significantly ameliorated in the presence of taurine.
Discussion
Apoptosis is a phenomenon of programmed cell death and plays an important role during neuronal development and in the homeostasis of the adult nervous system. The disruption of this process can lead to abnormal neuronal apoptosis and the increased apoptosis may contribute to the pathophysiology of nervous system disorders24, 25). Several studies have demonstrated that an abnormal increase in apoptosis is the main form of cell death caused by HD, and the increased apoptosis of neurons directly involved in HD-induced neurotoxicity3, 4, 5, 6). These findings indicate that apoptosis may be a potential therapeutic target in neuropathy induced by HD. Taurine possesses anti-apoptotic properties in neurons and neuron-like cells18, 19, 20). Therefore, our study focuses mainly on the protection of taurine against HD-induced apoptosis and its underlying mechanism.
In the present study, the viability and apoptosiswere observed in PC12 cells received HD alone or with taurine. The results showed that HD significantly decreased the viability of PC12 cells and increased the number of apoptotic cells. However, the decreased the viability and the increased apoptosis in HD-exposed PC12 cells were significantly ameliorated in the presence of taurine. Das et al.13) reported that the increased apoptosis in primary cardiomyocytes exposed to doxorubicin was reduced by taurine administration. Rashid et al.12) also reported that taurine reduced the increased apoptosis in the hepatic tissue of diabetic rats, supporting our results. These results indicate that taurine pretreatment can prevent HD-induced apoptosis in PC12 cells.
Mitochondrial pathway is the major signaling leading to apoptosis. Bcl-2 family plays critical roles in the regulation of mitochondria-mediated apoptosis. Bax and Bcl-2 are representative members of the Bcl-2 family. The former is pro-apoptotic molecule and the latter is anti-apoptotic molecule. Bax induces the permeabilization of mitochondrial outer membrane, causes the efflux of Cyt C from mitochondria to cytosol and leads to caspase-3 activation. Bcl-2 plays a role in controlling the integrity of the mitochondrial membrane and forms heterodimers with Bax to prevent the mitochondria dysfunction and the activation of caspase-3. Therefore, a shift in the balance between anti- and pro-apoptotic Bcl-2 family proteins could lead to mitochondria-dependent caspase-3 activation and apoptotic cell death. In the present study, the results showed that HD down-regulated Bcl-2 expression, up-regulated Bax expression, promoted the disruption of MMP and mitochondrial release of Cyt C and increased the activity of caspase-3 in PC12 cells, indicating that HD induced dysregulation of Bax and Bcl-2 and the activation of mitochondria-dependent apoptosis pathway in PC12 cells. However, pretreatment with taurine significantly reversed the activated mitochondria-dependent pathway in HD-exposed PC12 cells. Chang et al.11) reported that taurine effectively suppressed the disturbance of Bax and Bcl-2 as well as the enhancement of MMP in human proximal tubular epithelial cells exposed to oxidized LDL. Aly and Khafagy22) showed that taurine pretreatment prevented the increased activity of caspase-3 in adult rat testis exposed to endosulfan. These studies and our results indicate that taurine represses mitochondrial apoptosis pathway and the inhibited mitochondria-dependent pathway may be involved in the prevention of taurine against HD-induced apoptosis in PC12 cells. In addition, whether there is any extra mitochondrial pathway regulating HD-induced apoptosis has to be studied further.
Studies indicate that oxidative stress involved in the apoptotic signaling mechanism. ROS elicit oxidative stress causing an imbalance between pro-oxidant and anti-oxidant mechanisms14). The present study showed that HD exposure induced a significant decline in the activities of SOD and CAT and a significant increase in ROS production in PC12 cells. However, such changes were notably blocked by taurine pretreatment. Incubation with taurine and other toxins showed the similar results12, 13). SOD and CAT are the key antioxidant enzymes to scavenge ROS and defense the oxidative stress. Antioxidant activity of SOD is mediated by dismutation reaction where SOD scavenges highly reactive superoxide radical and converts it to oxygen molecule and less reactive H2O2 molecule. CAT then catalyses the decomposition of this H2O2 to water and O2. Therefore, inhibition of these antioxidant enzymes or decrease in their activities would result in an excessive production of ROS and pathological damage. The over-production of ROS in HD-treated PC12 cells may result from the inhibited activities of SOD and CAT. Meanwhile, taurine pretreatment protected the efficiency of the antioxidant enzymes, and may also quench and detoxify ROS intermediates which all contributed to its protection against ROS-induced toxicity in HD-exposed PC12 cells.
ROS are involved in the activation of mitochondrial pathway, which is mediated by various signaling. The present study showed that HD induced oxidative stress and apoptosis in PC12 cells, which was accompanied by the characteristic changes of mitochondrial pathway. Das et al.25) reported that ROS overproduction induced the disturbance of Bax and Bcl-2 during oxidative death in nerve cells. Rashid et al.12) found that oxidative stress induced apoptosis via mitochondria-dependent pathway. These results indicate that HD-induced oxidative stress lead to the apoptosis of PC12 cells via activating mitochondria-dependent pathway. On the other hand, pretreatment with taurine significantly reversed the induced ROS and the increased apoptosis as well as the activated apoptotic pathway in HD-exposed PC12 cells. Our results indicate that taurine prevents oxidative stress-promoted apoptosis in HD-exposed PC12 cells via inhibiting mitochondria-dependent pathway. Moreover, the attenuated oxidative stress may be mainly responsible for the protection of taurine. Although protective mechanisms of taurine against oxidative stress have not been clearly elucidated, several mechanisms may play a role in taurine-mediated reduction in oxidative stress. Taurine could quench and detoxify several ROS, like hypochlorous acid, nitric oxide, H2O2 and hydroxyl radical26, 27, 28, 29). It also could upregulate the activities of antioxidant enzymes. Among the antioxidant enzymes, SOD and CAT defend cells against the toxic effect of oxygen metabolism by eliminating ROS, which attributed to the protection of taurine against HD toxicity in this study. Moreover, it could enhance the level of the non-enzymatic radical scavenger glutathione (GSH) by directing cysteine into the GSH synthesis pathway. GSH scavenges residual free radicals escaping decomposition by the antioxidant enzymes, as the second line of defense. Sulfhydryl groups of GSH easily react with free radicals, which protects the cell against oxidative attack by chemicals28).
Our results showed that pretreatment with taurine significantly ameliorated the increased apoptosis and the alteration in major regulators of mitochondria-dependent pathway in HD-exposed PC12. However, these abnormal changes in HD group could not completely be reversed by taurine pretreatment. These results indicate that taurine pretreatment may partly prevent HD-induced apoptosis in PC12 cells via mitochondria-dependent pathway, and the partial protection of taurine may be associated with that it could not completely inhibited HD-induced ROS, as shown in Fig 8. In addition, apoptotic cell death is mainly regulated by two signaling pathways: mitochondria-dependent pathway and FasL/Fas pathway. It was reported that HD induced germ cell apoptosis via FasL pathway30). Therefore, the mechanism of apoptosis via FasL pathway may affect the protection of taurine against HD-induced apoptosis.
In conclusion, the present study showed the decreased viability and the increased apoptosis in the PC12 cells exposed to HD. HD also induced the disturbance of Bax and Bcl-2 expression, the loss of MMP, Cyt C release and caspase-3 activation in PC12 cells. Moreover, HD resulted in an increase in ROS level and a decline in the activities of SOD and CAT. It indicates that HD elicits oxidative stress and mitochondrial-dependent apoptosis in PC12 cells. However, taurine pretreatment ameliorated the increased apoptosis and the alterations in key regulators of mitochondria-dependent pathway in PC12 exposed to HD. The increased ROS level and the decreased activities of the antioxidant enzymes in HD group were attenuated by taurine pretreatment. These results indicate that pretreatment with taurine may at least partly prevent HD-induced apoptosis from HD-induced apoptosis via inhibiting mitochondria-dependent pathway. It is also suggested that the potential of taurine against HD-induced apoptosis may benefit from its anti-oxidative property. Although the anti-apoptotic effect of taurine in HD-exposed PC12 cells has been linked to the inhibition of oxidative stress, the direct mechanisms involved have remained largely elusive. As for the more precise mechanism, further studies are required.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81273038, No. 81102160), China Postdoctoral Science Foundation funded project (No. 2015M581338) and Dalian Municipal Science and Technology Plan Project (No. 2013E15SF163).
References
- 1.Couri D, Abdel-Rahman MS, Hetland LB (1978) Biotransformation of n-hexane and methyl n-butyl ketone in guinea pigs and mice. Am Ind Hyg Assoc J 39, 295–300. [DOI] [PubMed] [Google Scholar]
- 2.Spencer PS, Schaumburg HH (1975) Experimental neuropathy produced by 2,5-hexanedione--a major metabolite of the neurotoxic industrial solvent methyl n-butyl ketone. J Neurol Neurosurg Psychiatry 38, 771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strange P, Møller A, Ladefoged O, Lam HR, Larsen JJ, Arlien-Søborg P (1991) Total number and mean cell volume of neocortical neurons in rats exposed to 2,5-hexanedione with and without acetone. Neurotoxicol Teratol 13, 401–6. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa Y, Shimizu H, Kim SU (1996) 2,5-Hexanedione induced apoptosis in cultured mouse DRG neurons. Int Arch Occup Environ Health 68, 495–7. [DOI] [PubMed] [Google Scholar]
- 5.Cui N, Li S, Zhao X, Zhang T, Zhang C, Yu L, Zhu Z, Xie K (2007) Expression of Bcl-2, Bax and Caspase-3 in nerve tissues of rats chronically exposed to 2,5-hexanedione. Neurochem Res 32, 1566–72. [DOI] [PubMed] [Google Scholar]
- 6.Coleman MD, Zilz TR, Griffiths HR, Woehrling EK (2008) A comparison of the apoptotic and cytotoxic effects of hexanedione derivatives on human non-neuronal lines and the neuroblastoma line SH-SY5Y. Basic Clin Pharmacol Toxicol 102, 25–9. [DOI] [PubMed] [Google Scholar]
- 7.Mishra DP, Pal R, Shaha C (2006) Changes in cytisikuc Ca2+ levels regulated Bcl-xL expression in spermatogenic cells during apoptotic death. J Biol Chen 281, 2133–43. [DOI] [PubMed] [Google Scholar]
- 8.Qi Y, Li S, Piao F, Wang Z, Chen R, Liu S, Shen J (2015) 2,5-Hexanedione induces apoptosis via a mitochondriamediated pathway in PC12 cells. Mol Cell Toxicol 11, 79–87. [Google Scholar]
- 9.Chen R, Liu S, Piao F, Wang Z, Qi Y, Li S, Zhang D, Shen J (2015) 2,5-hexanedione induced apoptosis in mesenchymal stem cells from rat bone marrow via mitochondria-dependent caspase-3 pathway. Ind Health 53, 222–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu TH, Tseng TJ, Su CC, Tang FC, Yen CC, Liu YY, Yang CY, Wu CC, Chen KL, Hung DZ, Chen YW (2014) Arsenic induces reactive oxygen species-caused neuronal cell apoptosis through JNK/ERK-mediated mitochondria-dependent and GRP 78/CHOP-regulated pathways. Toxicol Lett 224, 130–40. [DOI] [PubMed] [Google Scholar]
- 11.Chang KC, Hsu CC, Liu SH, Su CC, Yen CC, Lee MJ, Chen KL, Ho TJ, Hung DZ, Wu CC, Lu TH, Su YC, Chen YW, Huang CF (2013) Cadmium induces apoptosis in pancreatic β-cells through a mitochondria-dependent pathway: the role of oxidative stress-mediated c-Jun N-terminal kinase activation. PLoS One 8, e54374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rashid K, Das J, Sil PC (2013) Taurine ameliorate alloxan induced oxidative stress and intrinsic apoptotic pathway in the hepatic tissue of diabetic rats. Food Chem Toxicol 51, 317–29. [DOI] [PubMed] [Google Scholar]
- 13.Das J, Ghosh J, Manna P, Sinha M, Sil PC (2009) Taurine protects rat testes against NaAsO2-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol Lett 187, 201–10. [DOI] [PubMed] [Google Scholar]
- 14.Kim MS, Park HR, Park M, Kim SJ, Kwon M, Yu BP, Chung HY, Kim HS, Kwack SJ, Kang TS, Kim SH, Lee J (2009) Neurotoxic effect of 2,5-hexanedione on neural progenitor cells and hippocampal neurogenesis. Toxicology 260, 97–103. [DOI] [PubMed] [Google Scholar]
- 15.McDermott C, O’Donoghue MH, Heffron JJ (2008) n-Hexane toxicity in Jurkat T-cells is mediated by reactive oxygen species. Arch Toxicol 82, 165–71. [DOI] [PubMed] [Google Scholar]
- 16.Beyranvand MR, Khalafi MK, Roshan VD, Choobineh S, Parsa SA, Piranfar MA (2011) Effect of taurine supplementation on exercise capacity of patients with heart failure. J Cardiol 57, 333–7. [DOI] [PubMed] [Google Scholar]
- 17.Rosemberg DB, da Rocha RF, Rico EP, Zanotto-Filho A, Dias RD, Bogo MR, Bonan CD, Moreira JC, Klamt F, Souza DO (2010) Taurine prevents enhancement of acetylcholinesterase activity induced by acute ethanol exposure and decreases the level of markers of oxidative stress in zebrafish brain. Neuroscience 171, 683–92. [DOI] [PubMed] [Google Scholar]
- 18.Gharibani PM, Modi J, Pan C, Menzie J, Ma Z, Chen PC, Tao R, Prentice H, Wu JY (2013) The mechanism of taurine protection against endoplasmic reticulum stress in an animal stroke model of cerebral artery occlusion and stroke-related conditions in primary neuronal cell culture. Adv Exp Med Biol 776, 241–58. [DOI] [PubMed] [Google Scholar]
- 19.Leon R, Wu H, Jin Y, Wei J, Buddhala C, Prentice H, Wu JY (2009) Protective function of taurine in glutamate-induced apoptosis in cultured neurons. J Neurosci Res 87, 1185–94. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Hu Z, Chen B, Bu Q, Lu W, Deng Y, Zhu R, Shao X, Hou J, Zhao J, Li H, Zhang B, Huang Y, Lv L, Zhao Y, Cen X (2012) Taurine attenuates methamphetamine-induced autophagy and apoptosis in PC12 cells through mTOR signaling pathway. Toxicol Lett 215, 1–7. [DOI] [PubMed] [Google Scholar]
- 21.Chang CY, Shen CY, Kang CK, Sher YP, Sheu WH, Chang CC, Lee TH (2014) Taurine protects HK-2 cells from oxidized LDL-induced cytotoxicity via the ROS-mediated mitochondrial and p53-related apoptotic pathways. Toxicol Appl Pharmacol 279, 351–63. [DOI] [PubMed] [Google Scholar]
- 22.Aly HA, Khafagy RM (2014) Taurine reverses endosulfan-induced oxidative stress and apoptosis in adult rat testis. Food Chem Toxicol 64, 1–9. [DOI] [PubMed] [Google Scholar]
- 23.Qian JJ, Cheng YB, Yang YP, Mao CJ, Qin ZH, Li K, Liu CF (2008) Differential effects of overexpression of wild-type and mutant human alpha-synuclein on MPP+-induced neurotoxicity in PC12 cells. Neurosci Lett 435, 142–6. [DOI] [PubMed] [Google Scholar]
- 24.Thompson CB. (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456–62. [DOI] [PubMed] [Google Scholar]
- 25.Das J, Ghosh J, Manna P, Sil PC (2012) Taurine protects rat testes against doxorubicin-induced oxidative stress as well as p53, Fas and caspase 12-mediated apoptosis. Amino Acids 42, 1839–55. [DOI] [PubMed] [Google Scholar]
- 26.Aruoma OI, Halliwell B, Hoey BM, Butler J (1988) The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem J 256, 251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das J, Ghosh J, Manna P, Sil PC (2008) Taurine provides antioxidant defense against NaF-induced cytotoxicity in murine hepatocytes. Pathophysiology 15, 181–90. [DOI] [PubMed] [Google Scholar]
- 28.Cozzi R, Ricordy R, Bartolini F, Ramadori L, Perticone P, De Salvia R (1995) Taurine and ellagic acid: two differently-acting natural antioxidants. Environ Mol Mutagen 26, 248–54. [DOI] [PubMed] [Google Scholar]
- 29.Redmond HP, Wang JH, Bouchier-Hayes D (1996) Taurine attenuates nitric oxide- and reactive oxygen intermediate-dependent hepatocyte injury. Arch Surg 131, 1280–7, discussion 1287–8. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Richburg JH, Younkin SC, Boekelheide K (1997) The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 138, 2081–8. [DOI] [PubMed] [Google Scholar]