Summary
The complement system is an important part of the innate immune defence. It contributes not only to local inflammation, removal and killing of pathogens, but it also assists in shaping of the adaptive immune response. Besides a role in inflammation, complement is also involved in physiological processes such as waste disposal and developmental programmes. The complement system comprises several soluble and membrane‐bound proteins. The bulk of the soluble proteins is produced mainly by the liver. While several complement proteins are produced by a wide variety of cell types, other complement proteins are produced by only a few related cell types. As these data suggest that local production by specific cell types may have specific functions, more detailed studies have been employed recently analysing the local and even intracellular role of these complement proteins. Here we review the current knowledge about extrahepatic production and/or secretion of complement components. More specifically, we address what is known about complement synthesis by cells of the human immune system.
Keywords: cell activation, complement, human
Introduction
The complement system is an ancient cascade of proteins which was first described in the 19th century 1. The complement system has been described to have many different functions, but especially three main functions have been well documented: opsonisation, chemotaxis and lysis.
The complement system is part of the innate immune defence, and functions as a cascade of proteases that activate each other in an enzymatic fashion. Next to a set of soluble proteins, complement also comprises several membrane‐bound complement regulators and receptors. The complement cascade can be activated via three different pathways: the classical pathway (CP), the lectin pathway (LP) and the alternative pathway (AP). These pathways are activated via different recognition molecules. The recognition molecule for the CP is C1q, which upon binding to ligands such as surface‐bound immunoglobulin (Ig)M, hexameric IgG or pentraxins, such as C‐reactive protein and pentraxin‐3, trigger CP activation 2. Several recognition molecules have been described for the LP, including mannose‐binding lectin (MBL), ficolins‐1, 2 and 3 and collectins (CL‐10, CL‐11) 3. The AP is activated spontaneously via a tick‐over activation mechanism, but a role for properdin (FP) has also been proposed 4. Activation of each of these pathways results in the formation of C3 convertases, C4b2a by the CP and the LP and/or C3bBb via the AP. The C3 convertase cleaves C3 into C3a and C3b, where C3a serves as a chemoattractant and C3b serves as an opsonin. C3b becomes bound covalently to its target via its thioester, which binds to amine and carbohydrate groups on the activating surface. When a threshold of activation is reached and another C3b binds to the C3‐convertase, the C5‐convertase is formed. The C5‐convertase will cleave C5 and this initiates the terminal pathway, resulting in the generation of C5a and a multimeric complex called the membrane attack complex (MAC) C5b‐C9, which eventually can cause lysis of cells 5 (Fig. 1).
Figure 1.
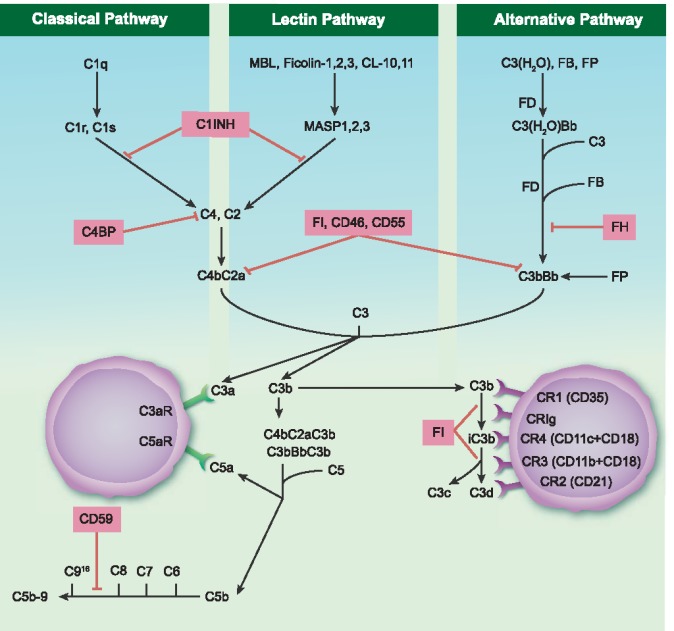
Schematic representation of the complement system. The complement system can be activated via three different pathways: the classical pathway (CP), the lectin pathway (LP) and the alternative pathway (AP). These pathways have their own sequential manner in forming a C3 convertase: C4b2a or C3bBb. These C3 convertases cleave the central component C3 generating two activation fragments, C3a and C3b. The C3a is able to bind its anaphylatoxin receptor the C3aR, whereas C3b can opsonize a target membrane. C3b and its further degradation products, iC3b, C3c and C3d/C3dg are able to bind various complement receptors (CRs). Additionally, C3b can bind to the former C3 convertase which then results in formation of the C5 convertase: C4bC2aC3b or C3bBbC3b. The C5 convertase cleaves C5 in two activation fragments C5a and C5b. C5a can bind to its anaphylatoxin receptors C5aR1 and C5aR2, whereas C5b marks the start of the formation of the membrane attack complex (MAC). In a sequential manner C5b, C6, C7, C8 and up to 16 molecules of C9 bind together to form a MAC. Various inhibitors of this system are marked in pink boxes. MBL = mannose binding lectin; MASP = MBL‐associated serine protease; FB = factor B; FP = factor P; FD = factor D; FH = factor H; C1INH = C1 inhibitor; FI = factor I (FI), C4BP = C4b‐binding protein; CR = complement receptor.
Apart from these traditional activities of the complement system in attacking invading pathogens, it has become clear that its effector functions extend to instruction of the adaptive immune system and to several physiological processes. A large part of these effects are translated into cellular effector functions via a set of complement receptors (CR) specific for proteolytically cleaved complement fragments. CR1 (CD35) is both a complement receptor for C3b, iC3b and C4b and a complement inhibitor, by competing with factor B (FB) for C3b binding and by functioning as a co‐factor for factor I (FI) 6. CR2 (CD21) binds iC3b, and especially C3d/C3dg, CR3 (MAC‐1, CD11b/CD18) binds iC3b and C3d/C3dg, whereas CR4 (gp150/95, CD11c/CD18) binds only iC3b 7, 8, 9, 10, 11, 12. Another complement receptor, complement receptor of the immunoglobulin superfamily (CRIg), binds to C3b and iC3b and also to soluble C3c 13, 14.
Several receptors have also been described for C1q; however, the relative contributions of these receptors and their functions is not yet resolved 15, 16, 17, 18. Next to receptors for the complement opsonins, a set of receptors can also be triggered by the anaphylatoxins, C3a and C5a. One receptor is known for C3a, the C3aR, and for C5a two receptors are identified; C5aR1 (CD88) and C5aR2 (Fig. 1). The different cellular expression profiles of these receptors are outlined below.
Next to activators, the complement system comprises both fluid phase and membrane‐bound regulators to keep complement activation in check. C1‐inhibitor (C1INH) is a circulating complement regulatory protein which can inactivate C1r, C1s, MBL‐associated serine protease (MASP)‐1 and MASP‐2, thereby preventing/limiting complement activation via both the CP and the LP. C4b‐binding protein (C4BP) acts as an inhibitor by accelerating the decay of the C3 convertase and is a co‐factor for FI‐mediated cleavage of C4b and C3b. FH serves as a co‐factor for FI‐mediated cleavage of C3b/iC3b in a similar manner. Next to a role as a co‐factor, FH also functions as a decay accelerator. Besides the full‐length FH, a truncated splice variant FH‐like 1 exists, and other complement FH‐related proteins have been found, of which the functions are just recently being explored 19. For the membrane‐bound regulators of complement, CD46 or membrane co‐factor protein (MCP) serves as a co‐factor for FI; additionally, CD46 can bind the C3 activation fragments. CD55, a decay accelerating factor (DAF), accelerates the decay of C3 convertases. Finally, CD59 or protectin inhibits the binding of C9 to the C5b‐8 complex, thereby preventing the last step needed for MAC formation 20. These‐membrane bound regulators, CD46, CD55 and CD59, are expressed on all circulating cells, including all the cell types addressed in this review 21. It is conceivable that the expression of abundant membrane‐bound complement inhibitors is necessary to protect these immune cells from the high levels of complement in circulation or in the local environment where they reside.
Complement has been considered mainly in the systemic compartment, and serum levels of most components of the complement system, including C3, C4 and MBL, are produced by hepatocytes 22, 23. Other tissues also contain cells capable of complement production; for example, endothelial and epithelial cells are also able to secrete various complement components 24, thereby contributing to local processes (Fig. 2). However, for C1q, FP and factor D (FD) it has been shown that the major/only site of production is outside the liver 24, 25, 26, 27, 28, 29, 30. Additionally, there is a growing body of evidence that local secretion of complement proteins plays an important role in regulating physiological processes even in the absence of further complement activation. C1q also has effector functions that are outside the scope of traditional complement activation. For example, C1q exerts effects during pregnancy (where it is involved in remodelling of the maternal decidua), embryonic development, coagulation processes and neurological synapse function, which has been reviewed by Nayak et al. 31. In the tumour microenvironment, C1q can also serve as a tumour‐promoting factor by favouring cell adhesion, migration and proliferation independently of complement activation 32. Finally, a new dimension has been provided by the observation that there might even be an intracellular role for complement and complement activation. The importance of intracellular processing of C3 by cathepsin‐L (CTSL), where the intracellularly produced C3a was involved in the mTOR phosphorylation pathway in CD4+ cells, was first shown by Liszweski et al. 33. Furthermore, Garbore et al. described intracellular signalling by the C5aR1 in CD4+ cells which was required for NLRP3 assembly 34.
Figure 2.
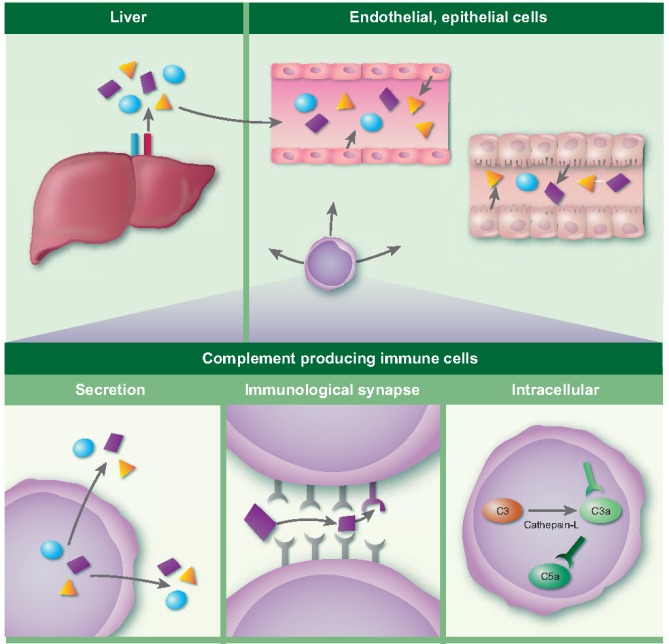
Production of complement proteins occurs in different tissues and by different cell types. Several sites are depicted in the figure, on the top left by hepatocytes in the liver, on the top right by endothelial and epithelial cells. In this review we focus on the production of complement proteins by immune cells, as illustrated in the bottom panel. Immune cells can secrete complement proteins, respond to complement proteins in the immunological synapse and more recently intracellular functions for complement proteins have been proposed.
Altogether, it is clear that it is important to look at complement outside its traditional functions. Complement can have an important role in immune regulation, and immune cells have been identified as an additional source for local complement activation. In this review we focus on the extrahepatic complement production and more specifically complement proteins expressed and/or secreted by various human immune cells and discuss its functional implications. We focus on the proteins for which evidence is available that they are actually produced. Lack of production is interesting, but scientifically difficult to prove, and therefore we refrain from strong statements on the absence of certain complement proteins in specific immune cells.
Polymorphonuclear leucocytes
Polymorphonuclear leucocytes (PMNs) are the most abundant leucocytes in blood and accumulate quickly at sites of infection, and may therefore be important for the production of local inflammatory mediators such as complement proteins. Stimulated PMNs were reported to secrete C3 with an intact thioester and it was speculated that the PMN‐derived proteases, which are also released locally, can subsequently activate this newly secreted C3 35, 36. Neutrophils not only store C3 but also FP, the only complement regulator that enhances complement activation, which can be secreted upon stimulation 30, 35, 37. Besides the release of C3 and FP, FB was also demonstrated to be secreted upon activation of neutrophils 38. Locally released FP, FB and C3 could provide a local platform for further complement activation via the AP. Based on liver and/or bone marrow transplantations, using organs from individuals with differences in the C7 M/N genetic variant, it was found that a substantial part of serum C7 was not derived from the liver 39, 40. PMNs were found to be of importance for the C7 serum levels when comparing the C7 content between PMN and PBMCs 41. Furthermore, it was found that C6, next to C7, also belonging to the terminal pathway of complement activation, was also secreted by PMNs 41. M‐ficolin/ficolin‐1, which is an activator of the LP pathway, was shown to be localized in secretory granules of neutrophils 42. PMNs express a wide array of complement receptors on their cell surface, including CR1, CR3, CR4, C3aR and C5aR 43, 44, 45. The production of complement proteins by each of the immune cells is summarized in Table 1.
Table 1.
Summary of complement protein synthesis/secretion by various immune cells
| Classical | Lectin | Alternative | Terminal | |||||
|---|---|---|---|---|---|---|---|---|
| PMN | Ficolin‐1 | (42) | C3, FB, FP | (30, 35–38) | C6, C7 | (41) | ||
| Mast cell | C1q | (52) | C3 | (46, 47) | C5 | (46, 47) | ||
| Monocyte | C1q, C1r, C1s, C4, C2 | (26, 28, 57–59) | C3, FB, FD, FP | (24, 59–64) | C5, C6, C7, C8, C9 | (24, 65) | ||
| Macrophage | C1q, C1r, C1s, C4, C2 | (72–77, 79) | C3, FB, FD, FP | (73, 74, 77–79) | C5 | (73, 74) | ||
| Dendritic cell | C1q, C1r, C1s, C4, C2 | (25, 85, 88) | C3, FB, FD, FP | (86‐88) | C5, C7, C8, C9 | (86‐88) | ||
| Natural killer cell | ||||||||
| B lymphocyte | C5 | (99) | ||||||
| T lymphocyte | C3, FB, FD, FP | (101–105) | C5 | (102) | ||||
| Regulators soluble | Regulators membrane‐bound | Receptors | ||||||
| PMN | CD46, CD55, CD59 | (21) | CR1, CR3, CR4, C3aR, C5aR1 | (43–45) | ||||
| Mast cell | CD46, CD55, CD59 | (50) | CR1, CR4, C3aR, C5aR1 | (47, 49–51, 53, 54) | ||||
| Monocyte | C1INH, C4BP, FH, FI | (59, 62, 67) | CD46, CD55, CD59 | (21) | CR1, CR3, CR4, C3aR, C5aR1 | (45, 68–70) | ||
| Macrophage | C1INH, FH, FI | (73, 74, 77) | CD46, CD55, CD59 | (80, 81) | CR1, CR3, CR4, CRIg, C3aR, C5aR1, C5aR2 | (14, 44, 70, 82–84) | ||
| Dendritic cell | C4BP, FH, FI | (86–88) | CD46, CD55, CD59 | (80, 88, 89) | CR1, CR3, CR4, CRIg, C3aR, C5aR1 | (87, 88, 90) | ||
| Natural killer cell | CD46, CD55, CD59 | (21) | CR3, CR4, C3aR, C5aR1, C5aR2 | (91, 92) | ||||
| B lymphocyte | CD46, CD55, CD59 | (21) | CR1, CR2, CR4, C5aR1 | (93–96, 99) | ||||
| T lymphocyte | CD46, CD55, CD59 | (21) | CR1, C3aR, C5aR1 | (100, 109) | ||||
PMN = polymorphonuclear; FB = factor B; FP = factor P; FD = factor D; FH = factor H; C1INH = C1 inhibitor; FI = factor I; C4BP = C4b‐binding protein; CR = complement receptor. We have only indicated the proteins for which evidence is available that they are actually produced. Lack of production is scientifically difficult to prove, and therefore we have not included lack of production in the table.
Mast cells
Mast cells (MC) are known mainly for their role in inflammatory and allergic responses, where they release potent inflammatory mediators upon stimulation through IgE receptors. From mast cells different subsets exist, which are often classified based upon the presence of the serine proteases tryptase and chymase. It was demonstrated that different subsets of MC express C5 and C3 46, 47. Human skin mast cells showed a diffuse cytoplasmic labelling of both C3 and C5. The basal secretion of C3 by MCs can be up‐regulated with various cytokines [tumour necrosis factor (TNF)‐α with interleukin (IL)‐4 or IL‐13] 46. Laufer et al. investigated Crohn's disease and with in‐situ hybridization found C4 mRNA but not C3 mRNA in mast cells in both normal and diseased intestines 48. Both tryptase and chymase are able to cleave C3, and as a consequence the locally secreted C3 can be activated by the MC enzymes and give rise to C3a. C3a is a powerful anaphylatoxin, which can bind to the C3aR present on surrounding cells or exert an autocrine effect on MCs. In this setting, the MC‐derived C3 has a role in the periphery which is independent from the conventional complement pathways 47, 49. Besides the C3aR, skin MC also express C5aR1, whereas MC from lung, uterus or tonsils do not express C5aR 50, 51. Furthermore, Schaarenburg et al. showed recently that functionally active C1q is produced by MCs generated in vitro from CD34+ progenitor cells. Additionally, skin MCs were stained for C1q and the MCs were positive for C1q both in normal and in diseased conditions 52. Human mast cells express CR1 and CR4 50, 53, 54, and murine data suggest that mouse mast cells also express CR2 and CR3 55, 56.
Monocytes
Monocytes are the circulating precursors of tissue macrophages and subsets of dendritic cells (DCs). Monocytes produce a broad range of complement components belonging to several complement pathways, and this has been studied extensively. The whole C1 complex can be assembled locally, as C1q, C1r and C1s are secreted. Additionally, C2 and C4, which are also part of the CP, have been shown to be secreted by monocytes 26, 28, 57, 58, 59. Monocytes also secrete various AP components as C3, FB, FD and FP 24, 59, 60, 61, 62, 63, 64. Moreover, the terminal pathway components C5, C6, C7, C8 and C9 are secreted, which are able to assemble as MAC 24, 65. For the recognition molecules of the LP there is less evidence of local production by monocytes; however, it was shown that monocytes have intracellular M‐ficolin and in synovial fluid of RA patients there was a correlation between M‐ficolin and the total number of blood monocytes 42, 66. Several control proteins are also secreted by cultured monocytes, such as FH, FI, C4BP and C1INH 59, 62, 67. Monocytes express receptors for both C3a and C5a: C3aR and C5aR1, respectively 45, 68, 69. Besides the receptors for these anaphylatoxins, monocytes can also respond to C3b and iC3b via CR1, CR3 and CR4 70. Taken together, monocytes can produce practically the full array of complement proteins. The complement system and monocytes (and the derived macrophages and DCs) are integrated on several levels, making them significant partners that interact with each other to bridge the innate and adaptive immune response.
Macrophages
Macrophages are tissue‐resident phagocytes that have differentiated from monocyte precursors. The complement system and macrophages have close interactions; as part of the traditional functions of the complement system to attract cells by chemotaxis and opsonize pathogenic surface by tagging the surface with C3b, macrophages are capable to respond to the chemotaxis and of clearing pathogens or apoptotic and necrotic cells by phagocytosis 71. Early research has focused upon guinea pig and murine macrophages, where a wide variety of complement components are found to be expressed and/or secreted. However, in these original papers a clear distinction is not always made between monocytes and macrophages, but a wide variety of complement syntheses has been described. For human macrophages, the CP proteins C1, C1q, C1s, C2 and C4 are found for the AP C3, FB, FD and for the terminal pathway C5. Regulators are also secreted, such as FH, FI, FP and C1INH 72, 73, 74, 75, 76. With human mononuclear phagocytes from synovial fluid of RA patients, it was demonstrated that these cells were able to synthesize C2, FH, FI, FD, FP and FB, which were functionally active in a haemolytic assay. C3, C4 and C5 were also detected, but appeared to be inactive 77. For human macrophages, it has been described that they are able to secrete C2, C3 and FB. For C3 it was shown that the biosynthesis was increased by stimulating the macrophages with acetylated low‐density lipoprotein, oxidized low‐density lipoprotein, IgA or IgG immune complexes 78, 79. Macrophages cultured from isolated peripheral blood monocytes express complement regulators as CD46, CD55 and CD59 80, 81. A wide array of complement receptors has been detected on the surface of macrophages, the anaphylatoxin receptors C3aR, C5aR1 and C5aR2 44, 82, 83 and the CR1, CR3 and CR4 70. The opsonin receptor CRIg is expressed on various tissue resident macrophages such as Küppfer cells 14, 84. To what extent different macrophage subsets, proinflammatory versus anti‐inflammatory, differ in their capacity to produce and secrete specific complement proteins remains to be established firmly.
Dendritic Cells
DCs are antigen‐presenting cells (APC) which function at the interface of innate and adaptive immunity. DCs are present in various tissues, where they reside as immature DCs with a high phagocytic capacity. Once they receive maturation signals they migrate to the draining lymph node and gain the ability to activate T cells. Most research concerning complement production by DCs is performed in vitro. DCs are generated from isolated monocytes from the blood and are then cultured towards DC phenotype (moDC). In their immature state DCs are able to produce functionally active C1q; however, upon stimulation [TNF‐α, lipopolysaccharide (LPS) or CD40L], which results in the maturation of these DCs, they lose their ability to produce C1q 25. However, there are conflicting data regarding whether the C1q production remains intact after maturation of moDCs 85. DCs present in human tissue stain positive for C1q 25, 85. DCs are also able to synthesize C3, FI, FB, C4BP, C7 and C8 86, 87. Moreover, it was shown that moDCs expressed C1q, C1s, C1r, C2, C3, C4, C5, C8, C9, FB, FD, FI, FH and FP at the mRNA level, and protein expression was confirmed for all except FI by using flow cytometry, Western blot and/or enzyme‐linked immunosorbent assay (ELISA) 88. moDCs have been shown to produce various functional complement components, and they additionally express complement regulators CD46, CD55 and CD59 [80,88]. Importantly, transient down‐regulation of CD55 during APC–T cell interaction has been identified as an important mechanism to allow local complement activation 89. Other membrane‐bound proteins found on the moDCs are several complement receptors, namely CR1, CR3, CR4, CRIg, C3aR and C5aR1 [87,88]. Differences in CR expression have been observed depending on the maturation state of the DC. Immature DCs have low expression of CR1 which disappears upon maturation, whereas CR2 is not found on either immature or mature DCs 90. Follicular DCs (FDCs) express high levels of CR2. However, although these cells play an important role in the adaptive immune response, particularly by presenting intact antigens to B cells, these cells are not derived from monocytes and are not ‘conventional’ DCs.
Natural killer (NK) cells
NK cells are cytotoxic innate immune cells that respond mainly to virally infected cells and tumour cells. Regarding the NK cell–complement interaction, it is known that there is extensive cross‐talk via several complement receptors and complement fragments. NK cells express several complement receptors as C3aR, C5aR, C5aR2, CR3 and CR4. The expression of these receptors was based on both gene expression and protein expression as analysed by flow cytometry. C5aR1 and C5aR2 protein expression has been reported so far only in CD56+CD3– cells upon permeabilization 91, 92. Therefore, NK cells can act upon complement factors such as C3a, C5a and C3b and its further degradation products iC3b and C3d. However, to our knowledge it has not been studied whether complement factors such as C3 or C5 can be generated and secreted by the NK cell itself.
B cells
The B cells produce antibodies as part of the adaptive immune response. The secreted IgG and IgM antibodies have, upon binding to their target, the capacity to activate the classical pathway by binding C1q 2. While it is still unclear how many complement proteins are produced by B cells, it is well known that C3‐activation products interact via CRs on B cells. Opsonization of an antigen by (i)C3b/C3d results, via CR2, in stimulation of the B cells, which results subsequently in a lowered threshold to produce antibodies 5, 93, 94. Besides the well‐studied CR2 and C3d interaction, B cells also express CR1 and CR4 which recognize C3b and its degradation product iC3b 95, 96. Synthesis of the separate complement components by B cells has been less well studied. Several B cell lines are positive for C5 when analysing cell lysates and for some B cell lines secreted C5 was also detectable in culture supernatant 97. Raji cells are able to synthesize FH and FI 98. Tonsillar B cells were shown to express C5aR1 in part of the memory and naive B cells while germinal B cells were found to be negative 99. Additionally, both mRNA and protein C5 expression was found in naive, memory and germinal centre B cells 99.
T cells
T cells are an important subset of lymphocytes which are required for proper functioning of the adaptive immune response. Complement has been considered more recently to be involved in both the homeostasis and the effector functions of the T cell. In cognate T cell and APC interactions the locally produced C3a and C5a are able to bind to their respective receptor on the T cell membrane, which subsequently stimulates the effector functions and maintains the viability of the T cell 100. The T cell itself can produce complement components upon activation via T cell receptor (TCR) and CD28, which results in induced protein expression of C3, C5, FB and FD 101, 102, 103, 104. Moreover, FP from the AP is expressed by various T cell lines and T cells from peripheral blood 105. Furthermore, it was demonstrated that T helper type 1 (Th1) induction, and not Th2, depended upon T cell‐produced C3 cleavage fragments, as was shown by using T cells from C3–/– donors and the earlier observation that not serum‐derived C3 but T cell‐derived C3 was needed for CD46 activation 106, 107. In T cells, CD46 is not solely a co‐factor for complement regulation; it also functions as a co‐stimulatory molecule for CD3 and is able to induce proliferation to levels that are comparable to CD28 stimulation 108. Next to CD46, CR1 is also expressed by T cells 109. CD4+ cells not only secrete C3 but also seem to contain intracellular C3 and C3a, where the C3 is cleaved by CTSL in a tonic manner. This intracellular C3a is subsequently able to bind the intracellular C3aR and signals via mTOR 33. Besides intracellular C3aR, a role was assigned more recently to intracellular C5aR1 cells in CD4+ cells by Arbore et al. 34.
Intracellular complement activation
The paradigm that complement solely affects the extracellular space has been challenged quite recently. It was demonstrated by Liszewski et al. that CD4+ T cell have intracellular stores of C3 and C3a, but also that C3a is generated intracellularly by CTSL. Subsequently, the newly generated C3a is able to bind to the intracellular C3aR, where it is linked towards a survival mechanism mediated by mTOR 33. It is suggested that the intracellular C3 phenomenon that was observed is not exclusive to the CD4+ T cells, but C3/C3a stores were also found in both other immune cells and non‐immune cells. Furthermore, it was reported recently that FH can be internalized by apoptotic cells (Jurkat T cells), where it did not become degraded but instead could bind directly to CTSL. The FH was then able to function intracellularly as a co‐factor for CTSL‐mediated cleavage of C3. Therefore, the authors hypothesized that this could be a consequence of FH binding to both CTSL and C3, hence bringing them into proximity with each other 110. Besides a role for intracellular C3 and C3aR, it was reported that there is also a role for intracellular C5 and C5aR1 in human T cells. It was hypothesized that the NLRP3 inflammasome in T cells receives signals via intracellular engagement of C5aR1, which increases the expression of IL1B and induces the production of reactive oxygen species (ROS), thereby activating the inflammasome 34. It therefore appears that more complement components are involved in intracellular processes. This new concept for intracellular complement has been referred to by Kolev et al. as the ‘Complosome’ 111. These newly discovered functions for complement components open up exiting new opportunities to endeavour.
Discussion
The complement system has long been thought to be a system that solely attacks invading pathogens. We now know that the complement system comprises more functions than the conventional chemotaxis, opsonization and lysis. It interacts on many levels with various cell types, and various complement proteins exert functions that are independent from activation of the complement cascade. For example, the hierarchical association of deficiencies of the classical pathway with the risk to develop systemic lupus erythematosus (SLE) is striking. C1q deficiency provides the largest risk, while in C1r/s deficiency the risk is somewhat lower. In C4 and C2 deficiency the risk is even less, and C3 deficiency is not a very strong risk factor for SLE 112, 113. If all effects of C1q could be mediated via activation of the CP, then all deficiencies should have the same risk. Clearly, different proteins have different functions that are partially independent from their role in the complement pathways.
The bulk of the complement proteins that are present in serum are produced and secreted by the liver, in particular by hepatocytes. However, serum does not reach all sites in the body where complement activation is needed. Therefore, there are also cells that produce complement proteins locally at those serum‐restricted sites. Local complement production and activation play a role in the initiation phase of the immune response. This activation impacts upon the permeability of the local vasculature that subsequently will allow more systemic plasma proteins to leave the vessel and contribute to, or even take over, the initial local response. In this review, we have now addressed the local production of complement by the different cells of the immune system (Table 1).
From several cell types, such as the monocytes, macrophages and the DC, their complement secretion is well studied and these cells seem to possess the capacity to produce locally all proteins needed to form fully functioning complement pathways. Conversely, for other cell types the repertoire of complement proteins that is produced is less well documented. Secretion of the recognition molecules of the LP by immune cells is hardly addressed. The same holds true for the NK cells, where the focus seems to have been on expression of complement receptors. Other innate immune cells such as eosinophils and basophils have also not been studied elaborately regarding complement secretion.
Local complement production not only adds to the total pool of complement proteins that circulates, but influences other local processes via paracrine or autocrine interactions. An important example is the production, targeted secretion and local activation of complement in the T cell–DC synapse 89. Another exciting example is the production and intracellular activation of C3 and C5 as recently reported to be operational in human T cells 33, 34.
Taken together, it seems that various immune cells have the capacity to form fully functioning complement pathways in their own environment. This is especially of importance for sites where the access to serum complement is initially restricted. Because of the existence of additional C3/C5 cleaving enzymes, local secretion of C3 and C5 and the expression of the anaphylatoxin receptors, various cells are capable of creating an environment that is needed for autocrine stimulation with complement proteins which acts independently of the traditional complement cascade.
Now that complement targeting therapies are becoming available for use in the clinic it will be interesting to see how such drugs impact upon systemic versus local complement activation. These drugs, often applied intravenously, will target mainly the circulating pool of complement. While animal models using complement‐deficient mice may have indicated an essential role for complement in pathogenesis, it may now emerge that the intracellular/autocrine/paracrine complement activation is not targetable easily by complement drugs that are administered intravenously. Exciting work lies ahead, where the relative importance of locally produced complement versus systemically delivered complement will be unravelled further.
Disclosure
The authors declare no conflicts of interest for this paper.
Acknowledgements
The authors wish to acknowledge the financial support from the IMI JU‐funded project BeTheCure, contract no. 115142‐2 and the Dutch Kidney Foundation (COMBAT consortium no. 13OCA27). L.A.T. is supported by a NWO ZON‐MW Vidi grant.
References
- 1. Pillemer L. Recent advances in the chemistry of complement. Chem Rev 1943; 33:1–26. [Google Scholar]
- 2. Beurskens FJ, van Schaarenburg RA, Trouw LA. C1q, antibodies and anti‐C1q autoantibodies. Mol Immunol 2015; 68:6–13. [DOI] [PubMed] [Google Scholar]
- 3. Garred P, Genster N, Pilely K et al A journey through the lectin pathway of complement‐MBL and beyond. Immunol Rev 2016; 274:74–97. [DOI] [PubMed] [Google Scholar]
- 4. Cortes C, Ohtola JA, Saggu G, Ferreira VP. Local release of properdin in the cellular microenvironment: role in pattern recognition and amplification of the alternative pathway of complement. Front Immunol 2012; 3:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 2010; 11:785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khera R, Das N. Complement receptor 1: disease associations and therapeutic implications. Mol Immunol 2009; 46:761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol 2009; 9:729–40. [DOI] [PubMed] [Google Scholar]
- 8. Ricklin D, Reis ES, Mastellos DC, Gros P, Lambris JD. Complement component C3 – the ‘Swiss Army Knife’ of innate immunity and host defense. Immunol Rev 2016; 274:33–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bajic G, Yatime L, Sim RB, Vorup‐Jensen T, Andersen GR. Structural insight on the recognition of surface‐bound opsonins by the integrin I domain of complement receptor 3. Proc Natl Acad Sci USA 2013; 110:16426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen X, Yu Y, Mi LZ, Walz T, Springer TA. Molecular basis for complement recognition by integrin alphaXbeta2. Proc Natl Acad Sci USA 2012; 109:4586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin Z, Schmidt CQ, Koutsogiannaki S et al Complement C3dg‐mediated erythrophagocytosis: implications for paroxysmal nocturnal hemoglobinuria. Blood 2015; 126:891–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van den Elsen JM, Isenman DE. A crystal structure of the complex between human complement receptor 2 and its ligand C3d. Science 2011; 332:608–11. [DOI] [PubMed] [Google Scholar]
- 13. Wiesmann C, Katschke KJ, Yin J et al Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature 2006; 444:217–20. [DOI] [PubMed] [Google Scholar]
- 14. Helmy KY, Katschke KJ Jr, Gorgani NN et al CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 2006; 124:915–27. [DOI] [PubMed] [Google Scholar]
- 15. Eggleton P, Tenner AJ, Reid KB. C1q receptors. Clin Exp Immunol 2000; 120:406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghebrehiwet B, Kaplan AP, Joseph K, Peerschke EI. The complement and contact activation systems: partnership in pathogenesis beyond angioedema. Immunol Rev 2016; 274:281–9. [DOI] [PubMed] [Google Scholar]
- 17. McGreal EP, Ikewaki N, Akatsu H, Morgan BP, Gasque P. Human C1qRp is identical with CD93 and the mNI‐11 antigen but does not bind C1q. J Immunol 2002; 168:5222–32. [DOI] [PubMed] [Google Scholar]
- 18. Steinberger P, Szekeres A, Wille S et al Identification of human CD93 as the phagocytic C1q receptor (C1qRp) by expression cloning. J Leukoc Biol 2002; 71:133–40. [PubMed] [Google Scholar]
- 19. Pouw RB, Vredevoogd DW, Kuijpers TW, Wouters D. Of mice and men: the factor H protein family and complement regulation. Mol Immunol 2015; 67:12–20. [DOI] [PubMed] [Google Scholar]
- 20. Alegretti AP, Schneider L, Piccoli AK, Xavier RM. The role of complement regulatory proteins in peripheral blood cells of patients with systemic lupus erythematosus: review. Cell Immunol 2012; 277:1–7. [DOI] [PubMed] [Google Scholar]
- 21. Christmas SE, de la Mata Espinosa CT, Halliday D, Buxton CA, Cummerson JA, Johnson PM. Levels of expression of complement regulatory proteins CD46, CD55 and CD59 on resting and activated human peripheral blood leucocytes. Immunology 2006; 119:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alper CA, Johnson AM, Birtch AG, Moore FD. Human C'3: evidence for the liver as the primary site of synthesis. Science 1969; 163:286–8. [DOI] [PubMed] [Google Scholar]
- 23. Morris KM, Aden DP, Knowles BB, Colten HR. Complement biosynthesis by the human hepatoma‐derived cell line HepG2. J Clin Invest 1982; 70:906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morgan BP, Gasque P. Extrahepatic complement biosynthesis: where, when and why? Clin Exp Immunol 1997; 107:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castellano G, Woltman AM, Nauta AJ et al Maturation of dendritic cells abrogates C1q production in vivo and in vitro . Blood 2004; 103:3813–20. [DOI] [PubMed] [Google Scholar]
- 26. Gulati P, Lemercier C, Guc D, Lappin D, Whaley K. Regulation of the synthesis of C1 subcomponents and C1‐inhibitor. Behring Institute Mitteilungen 1993; 196–203. [PubMed] [Google Scholar]
- 27. Maves KK, Weiler JM. Properdin: approaching four decades of research. Immunol Res 1993; 12:233–43. [DOI] [PubMed] [Google Scholar]
- 28. Tenner AJ, Volkin DB. Complement subcomponent C1q secreted by cultured human monocytes has subunit structure identical with that of serum C1q. Biochem J 1986; 233:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. White RT, Damm D, Hancock N et al Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem 1992; 267:9210–3. [PubMed] [Google Scholar]
- 30. Wirthmueller U, Dewald B, Thelen M et al Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J Immunol 1997; 158:4444–51. [PubMed] [Google Scholar]
- 31. Nayak A, Ferluga J, Tsolaki AG, Kishore U. The non‐classical functions of the classical complement pathway recognition subcomponent C1q. Immunol Lett 2010; 131:139–50. [DOI] [PubMed] [Google Scholar]
- 32. Bulla R, Tripodo C, Rami D et al C1q acts in the tumour microenvironment as a cancer‐promoting factor independently of complement activation. Nat Commun 2016; 7:10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liszewski MK, Kolev M, Le Friec G et al Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 2013; 39:1143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arbore G, West EE, Spolski R et al T helper 1 immunity requires complement‐driven NLRP3 inflammasome activity in CD4(+) T cells. Science 2016; 352:aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Botto M, Lissandrini D, Sorio C, Walport MJ. Biosynthesis and secretion of complement component (C3) by activated human polymorphonuclear leukocytes. J Immunol 1992; 149:1348–55. [PubMed] [Google Scholar]
- 36. Faried HF, Tachibana T, Okuda T. The secretion of the third component of complement (C3) by human polymorphonuclear leucocytes from both normal and systemic lupus erythematosus cases. Scand J Immunol 1993; 37:19–28. [DOI] [PubMed] [Google Scholar]
- 37. Camous L, Roumenina L, Bigot S et al Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood 2011; 117:1340–9. [DOI] [PubMed] [Google Scholar]
- 38. Yuen J, Pluthero FG, Douda DN et al NETosing neutrophils activate complement both on their own NETs and bacteria via alternative and non‐alternative pathways. Front Immunol 2016; 7:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wurzner R, Joysey VC, Lachmann PJ. Complement component C7. Assessment of in vivo synthesis after liver transplantation reveals that hepatocytes do not synthesize the majority of human C7. J Immunol 1994; 152:4624. [PubMed] [Google Scholar]
- 40. Naughton MA, Walport MJ, Wurzner R et al Organ‐specific contribution to circulating C7 levels by the bone marrow and liver in humans. Eur J Immunol 1996; 26:2108–12. [DOI] [PubMed] [Google Scholar]
- 41. Hogasen AK, Wurzner R, Abrahamsen TG, Dierich MP. Human polymorphonuclear leukocytes store large amounts of terminal complement components C7 and C6, which may be released on stimulation. J Immunol 1995; 154:4734–40. [PubMed] [Google Scholar]
- 42. Liu Y, Endo Y, Iwaki D et al Human M‐ficolin is a secretory protein that activates the lectin complement pathway. J Immunol 2005; 175:3150–6. [DOI] [PubMed] [Google Scholar]
- 43. Sengelov H. Complement receptors in neutrophils. Crit Rev Immunol 1995; 15:107–31. [PubMed] [Google Scholar]
- 44. Wetsel RA. Structure, function and cellular expression of complement anaphylatoxin receptors. Curr Opin Immunol 1995; 7:48–53. [DOI] [PubMed] [Google Scholar]
- 45. Martin U, Bock D, Arseniev L et al The human C3a receptor is expressed on neutrophils and monocytes, but not on B or T lymphocytes. J Exp Med 1997; 186:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fukuoka Y, Hite MR, Dellinger AL, Schwartz LB. Human skin mast cells express complement factors C3 and C5. J Immunol 2013; 191:1827–34. [DOI] [PubMed] [Google Scholar]
- 47. Lipitsa T, Naukkarinen A, Laitala J, Harvima IT. Complement C3 is expressed by mast cells in cutaneous vasculitis and is degraded by chymase. Arch Dermatol Res 2016; 308:575–84. [DOI] [PubMed] [Google Scholar]
- 48. Laufer J, Oren R, Goldberg I et al Cellular localization of complement C3 and C4 transcripts in intestinal specimens from patients with Crohn's disease. Clin Exp Immunol 2000; 120:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwartz LB, Kawahara MS, Hugli TE, Vik D, Fearon DT, Austen KF. Generation of C3a anaphylatoxin from human C3 by human mast cell tryptase. J Immunol 1983; 130:1891–5. [PubMed] [Google Scholar]
- 50. Fureder W, Agis H, Willheim M et al Differential expression of complement receptors on human basophils and mast cells. Evidence for mast cell heterogeneity and CD88/C5aR expression on skin mast cells. J Immunol 1995; 155:3152–60. [PubMed] [Google Scholar]
- 51. Nilsson G, Johnell M, Hammer CH et al C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin‐sensitive signal transduction pathway. J Immunol 1996; 157:1693–8. [PubMed] [Google Scholar]
- 52. van Schaarenburg RA, Suurmond J, Habets KL et al The production and secretion of complement component C1q by human mast cells. Mol Immunol 2016; 78:164–70. [DOI] [PubMed] [Google Scholar]
- 53. Nunez‐Lopez R, Escribano L, Schernthaner GH et al Overexpression of complement receptors and related antigens on the surface of bone marrow mast cells in patients with systemic mastocytosis. Br J Haematol 2003; 120:257–65. [DOI] [PubMed] [Google Scholar]
- 54. Fearon DT. Human complement receptors for C3b (CR1) and C3d (CR2). J Invest Dermatol 1985; 85:53s–7s. [DOI] [PubMed] [Google Scholar]
- 55. Rosenkranz AR, Coxon A, Maurer M et al Impaired mast cell development and innate immunity in Mac‐1 (CD11b/CD18, CR3)‐deficient mice. J Immunol 1998; 161:6463–7. [PubMed] [Google Scholar]
- 56. Andrasfalvy M, Prechl J, Hardy T, Erdei A, Bajtay Z. Mucosal type mast cells express complement receptor type 2 (CD21). Immunol Lett 2002; 82:29–34. [DOI] [PubMed] [Google Scholar]
- 57. Bensa JC, Reboul A, Colomb MG. Biosynthesis in vitro of complement subcomponents C1q, C1s and C1 inhibitor by resting and stimulated human monocytes. Biochem J 1983; 216:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Whaley K. Biosynthesis of the complement components and the regulatory proteins of the alternative complement pathway by human peripheral blood monocytes. J Exp Med 1980; 151:501–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lappin DF, Guc D, Hill A, McShane T, Whaley K. Effect of interferon‐gamma on complement gene expression in different cell types. Biochem J 1992; 281:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maves KK, Weiler JM. Detection of properdin mRNA in human peripheral blood monocytes and spleen. J Lab Clin Med 1992; 120:762–6. [PubMed] [Google Scholar]
- 61. Einstein LP, Hansen PJ, Ballow M et al Biosynthesis of the third component of complement (C3) in vitro by monocytes from both normal and homozygous C3‐deficient humans. J Clin Invest 1977; 60:963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lappin DF, Whaley K. Interferon‐induced transcriptional and post‐transcriptional modulation of factor H and C4 binding‐protein synthesis in human monocytes. Biochem J 1990; 271:767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Naughton MA, Botto M, Carter MJ, Alexander GJ, Goldman JM, Walport MJ. Extrahepatic secreted complement C3 contributes to circulating C3 levels in humans. J Immunol 1996; 156:3051–6. [PubMed] [Google Scholar]
- 64. Schwaeble W, Huemer HP, Most J et al Expression of properdin in human monocytes. Eur J Biochem 1994; 219:759–64. [DOI] [PubMed] [Google Scholar]
- 65. Hetland G, Johnson E, Falk RJ, Eskeland T. Synthesis of complement components C5, C6, C7, C8 and C9 in vitro by human monocytes and assembly of the terminal complement complex. Scand J Immunol 1986; 24:421–8. [DOI] [PubMed] [Google Scholar]
- 66. Ammitzboll CG, Thiel S, Ellingsen T et al Levels of lectin pathway proteins in plasma and synovial fluid of rheumatoid arthritis and osteoarthritis. Rheumatol Int 2012; 32:1457–63. [DOI] [PubMed] [Google Scholar]
- 67. Yeung Laiwah AC, Jones L, Hamilton AO, Whaley K. Complement‐subcomponent‐C1‐inhibitor synthesis by human monocytes. Biochem J 1985; 226:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zwirner J, Gotze O, Begemann G, Kapp A, Kirchhoff K, Werfel T. Evaluation of C3a receptor expression on human leucocytes by the use of novel monoclonal antibodies. Immunology 1999; 97:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Okusawa S, Yancey KB, van der Meer JW et al C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro. Comparison with secretion of interleukin 1 beta and interleukin 1 alpha. J Exp Med 1988; 168:443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Merle NS, Church SE, Fremeaux‐Bacchi V, Roumenina LT. Complement system part I – molecular mechanisms of activation and regulation. Front Immunol 2015; 6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bohlson SS, O'Conner SD, Hulsebus HJ, Ho MM, Fraser DA. Complement, C1q, and C1q‐related molecules regulate macrophage polarization. Front Immunol 2014; 5:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cole FS, Matthews WJ Jr, Marino JT, Gash DJ, Colten HR. Control of complement synthesis and secretion in bronchoalveolar and peritoneal macrophages. J Immunol 1980; 125:1120–4. [PubMed] [Google Scholar]
- 73. Hartung HP, Hadding U. Synthesis of complement by macrophages and modulation of their functions through complement activation. Springer Semin Immunopathol 1983; 6:283–326. [DOI] [PubMed] [Google Scholar]
- 74. de Ceulaer C, Papazoglou S, Whaley K. Increased biosynthesis of complement components by cultured monocytes, synovial fluid macrophages and synovial membrane cells from patients with rheumatoid arthritis. Immunology 1980; 41:37–43. [PMC free article] [PubMed] [Google Scholar]
- 75. Rabs U, Martin H, Hitschold T, Golan MD, Heinz HP, Loos M. Isolation and characterization of macrophage‐derived C1q and its similarities to serum C1q. Eur J Immunol 1986; 16:1183–6. [DOI] [PubMed] [Google Scholar]
- 76. Muller W, Hanauske‐Abel H, Loos M. Biosynthesis of the first component of complement by human and guinea pig peritoneal macrophages: evidence for an independent production of the C1 subunits. J Immunol 1978; 121:1578–84. [PubMed] [Google Scholar]
- 77. Strunk RC, Cole FS, Perlmutter DH, Colten HR. Gamma‐interferon increases expression of class III complement genes C2 and factor B in human monocytes and in murine fibroblasts transfected with human C2 and factor B genes. J Biol Chem 1985; 260:15280–5. [PubMed] [Google Scholar]
- 78. Mogilenko DA, Kudriavtsev IV, Trulioff AS et al Modified low density lipoprotein stimulates complement C3 expression and secretion via liver X receptor and Toll‐like receptor 4 activation in human macrophages. J Biol Chem 2012; 287:5954–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Laufer J, Boichis H, Farzam N, Passwell JH. IgA and IgG immune complexes increase human macrophage C3 biosynthesis. Immunology 1995; 84:207–12.] [PMC free article] [PubMed] [Google Scholar]
- 80. van Kooten C, Fiore N, Trouw LA et al Complement production and regulation by dendritic cells: molecular switches between tolerance and immunity. Mol Immunol 2008; 45:4064–72. [DOI] [PubMed] [Google Scholar]
- 81. Kurita‐Taniguchi M, Hazeki K, Murabayashi N et al Molecular assembly of CD46 with CD9, alpha3‐beta1 integrin and protein tyrosine phosphatase SHP‐1 in human macrophages through differentiation by GM‐CSF. Mol Immunol 2002; 38:689–700. [DOI] [PubMed] [Google Scholar]
- 82. Gasque P, Singhrao SK, Neal JW et al The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J Immunol 1998; 160:3543–54. [PubMed] [Google Scholar]
- 83. Vijayan S, Asare Y, Grommes J et al High expression of C5L2 correlates with high proinflammatory cytokine expression in advanced human atherosclerotic plaques. Am J Pathol 2014; 184:2123–33. [DOI] [PubMed] [Google Scholar]
- 84. He JQ, Wiesmann C, van Lookeren Campagne M. A role of macrophage complement receptor CRIg in immune clearance and inflammation. Mol Immunol 2008; 45:4041–7. [DOI] [PubMed] [Google Scholar]
- 85. Cao W, Bobryshev YV, Lord RS, Oakley RE, Lee SH, Lu J. Dendritic cells in the arterial wall express C1q: potential significance in atherogenesis. Cardiovasc Res 2003; 60:175–86. [DOI] [PubMed] [Google Scholar]
- 86. Reis ES, Barbuto JA, Isaac L. Human monocyte‐derived dendritic cells are a source of several complement proteins. Inflamm Res 2006; 55:179–84. [DOI] [PubMed] [Google Scholar]
- 87. Reis ES, Barbuto JA, Isaac L. Complement components, regulators and receptors are produced by human monocyte‐derived dendritic cells. Immunobiology 2007; 212:151–7. [DOI] [PubMed] [Google Scholar]
- 88. Li K, Fazekasova H, Wang N et al Expression of complement components, receptors and regulators by human dendritic cells. Mol Immunol 2011; 48:1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kwan WH, van der Touw W, Heeger PS. Complement regulation of T cell immunity. Immunol Res 2012; 54:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sandor N, Kristof K, Parej K, Pap D, Erdei A, Bajtay Z. CR3 is the dominant phagocytotic complement receptor on human dendritic cells. Immunobiology 2013; 218:652–63. [DOI] [PubMed] [Google Scholar]
- 91. Min X, Liu C, Wei Y et al Expression and regulation of complement receptors by human natural killer cells. Immunobiology 2014; 219:671. [DOI] [PubMed] [Google Scholar]
- 92. Ross GD, Vetvicka V. CR3 (CD11b, CD18): a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin Exp Immunol 1993; 92:181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nielsen CH, Fischer EM, Leslie RGQ. The role of complement in the acquired immune response. Immunology 2000; 100:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 1996; 271:348–50. [DOI] [PubMed] [Google Scholar]
- 95. Kremlitzka M, Polgar A, Fulop L, Kiss E, Poor G, Erdei A. Complement receptor type 1 (CR1, CD35) is a potent inhibitor of B‐cell functions in rheumatoid arthritis patients. Int Immunol 2013; 25:25–33. [DOI] [PubMed] [Google Scholar]
- 96. Uotila LM, Aatonen M, Gahmberg CG. Integrin CD11c/CD18 α‐chain phosphorylation is functionally important. J Biol Chem 2013; 288:33494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reed W, Roubey RA, Dalzell JG et al Synthesis of complement component C5 by human B and T lymphoblastoid cell lines. Immunogenetics 1990; 31:145–51. [DOI] [PubMed] [Google Scholar]
- 98. Vetvicka V, Reed W, Hoover ML, Ross GD. Complement factors H and I synthesized by B cell lines function to generate a growth factor activity from C3. J Immunol 1993; 150:4052–60. [PubMed] [Google Scholar]
- 99. Ottonello L, Corcione A, Tortolina G et al rC5a directs the in vitro migration of human memory and naive tonsillar B lymphocytes: implications for B cell trafficking in secondary lymphoid tissues. J Immunol 1999; 162:6510–7. [PubMed] [Google Scholar]
- 100. Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, Heeger PS. Immune cell‐derived C3a and C5a costimulate human T cell alloimmunity. Am J Transplant 2013; 13:2530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pavlov V, Raedler H, Yuan S et al Donor deficiency of decay‐accelerating factor accelerates murine T cell‐mediated cardiac allograft rejection. J Immunol 2008; 181:4580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Strainic MG, Liu J, Huang D et al Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 2008; 28:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kolev M, Le Friec G, Kemper C. The role of complement in CD4(+) T cell homeostasis and effector functions. Semin Immunol 2013; 25:12–9. [DOI] [PubMed] [Google Scholar]
- 104. Heeger PS, Lalli PN, Lin F et al Decay‐accelerating factor modulates induction of T cell immunity. J Exp Med 2005; 201:1523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Schwaeble W, Dippold WG, Schafer MK et al Properdin, a positive regulator of complement activation, is expressed in human T cell lines and peripheral blood T cells. J Immunol 1993; 151:2521–8. [PubMed] [Google Scholar]
- 106. Ghannam A, Fauquert JL, Thomas C, Kemper C, Drouet C. Human complement C3 deficiency: Th1 induction requires T cell‐derived complement C3a and CD46 activation. Mol Immunol 2014; 58:98–107. [DOI] [PubMed] [Google Scholar]
- 107. Cardone J, Le Friec G, Vantourout P et al Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol 2010; 11:862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Astier A, Trescol‐Biemont MC, Azocar O, Lamouille B, Rabourdin‐Combe C. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J Immunol 2000; 164:6091–5. [DOI] [PubMed] [Google Scholar]
- 109. Torok K, Dezso B, Bencsik A, Uzonyi B, Erdei A. Complement receptor type 1 (CR1/CD35) expressed on activated human CD4+ T cells contributes to generation of regulatory T cells. Immunol Lett 2015; 164:117–24. [DOI] [PubMed] [Google Scholar]
- 110. Martin M, Leffler J, Smolag KI et al Factor H uptake regulates intracellular C3 activation during apoptosis and decreases the inflammatory potential of nucleosomes. Cell Death Differ 2016; 23:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kolev M, Le Friec G, Kemper C. Complement‐tapping into new sites and effector systems. Nat Rev Immunol 2014; 14:811–20. [DOI] [PubMed] [Google Scholar]
- 112. Macedo AC, Isaac L. Systemic lupus erythematosus and deficiencies of early components of the complement classical pathway. Front Immunol 2016; 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bryan AR, Wu EY. Complement deficiencies in systemic lupus erythematosus. Curr Allergy Asthma Rep 2014; 14:448. [DOI] [PubMed] [Google Scholar]


