Summary
Splenic macrophages play a key role in immune thrombocytopenia (ITP) pathogenesis by clearing opsonized platelets. Fcγ receptors (FcγR) participate in this phenomenon, but their expression on splenic macrophages and their modulation by treatment have scarcely been studied in human ITP. We aimed to compare the phenotype and function of splenic macrophages between six controls and 24 ITP patients and between ITP patients according to the treatments they received prior to splenectomy. CD86, human leucocyte antigen D‐related (HLA‐DR) and FcγR expression were measured by flow cytometry on splenic macrophages. The major FcγR polymorphisms were determined and splenic macrophage function was assessed by a phagocytosis assay. The expression of the activation markers CD86 and HLA‐DR was higher on splenic macrophages during ITP compared to controls. While the expression of FcγR was not different between ITP and controls, the phagocytic function of splenic macrophages was reduced in ITP patients treated with intravenous immunoglobulin (IVIg) within the 2 weeks prior to splenectomy. The FCGR3A (158V/F) polymorphism, known to increase the affinity of FcγRIII to IgG, was over‐represented in ITP patients. Thus, these are the first results arguing for the fact that the therapeutic use of IVIg during human chronic ITP does not modulate FcγR expression on splenic macrophages but decreases their phagocytic capabilities.
Keywords: autoimmunity, Fc receptors, macrophages, spleen
Introduction
The Fcγ receptor (FcγR) family is composed of several receptors, the ligation of which with immunoglobulin (Ig)G leads to opposite signals. Activating receptors are represented by FcγRI (CD64), FcγRIIa/c (CD32a/c) and FcγRIII (CD16), whereas FcγRIIb (CD32b) gives an inhibitory signal 1. As most of the monocytes/macrophages express both activating and inhibitory FcγR, the activating threshold to the ligation of IgG is tuned by the ratio of activator/inhibitory receptors. Imbalance between activating and inhibitory FcγR has been involved in various other autoimmune diseases than immune thrombocytopenia (ITP), such as systemic lupus erythematosus 2 and rheumatoid arthritis 3, 4. Evidence is also provided by animal models such as CD32B–/– mice that develop lupus‐like disease 1. Conversely, the up‐regulation of CD32b in lupus‐prone mice strains, such as NZB and BXSB, restores tolerance and reduces autoimmune manifestations 5.
ITP is an autoimmune disease responsible for a peripheral immune destruction of platelets 6. In most cases the disease is caused by autoreactive B cells producing autoantibodies targeting glycoproteins (GP) expressed on platelet membrane, such as GPIIb/IIIa, GPIb/IX and/or GPIa/IIa 7, 8. Subsequently, autoantibody‐opsonized platelets are phagocytosed by splenic macrophages in a FcγR‐dependent mechanism 9. Consistent with the role of FcγR in ITP pathogenesis, IVIg, the mechanism of action of which is due partly to interaction with FcγR 10, has been used for more than 30 years to increase platelet count during ITP 11, 12. It has also been shown in a pilot study that syk‐inhibitors, by interfering with the FcγR signalling pathway, can improve ITP 13. Recently, it has been shown on monocyte‐derived macrophages that the effect of IVIg was mediated by the blockade of activating receptors and was independent of IgG sialylation and FcγRIIb expression 14, conversely to what has been observed in murine models 15, 16.
As well as the level of expression and the type of FcγR, polymorphisms of FcγR can also participate in ITP pathogenesis, the FCGR3A–158V/F polymorphism, which leads to a stronger affinity to IgG, is increased in childhood ITP 17, 18, 19, 20; the prevalence of the open reading frame (ORF) of FCGR2C, encoding an activator receptor, is increased during ITP 21 and the FCGR2B–232I/T genotype is observed preferentially in children ITP with a chronic course 22.
Until now, data concerning human splenic macrophage phenotype and function have been scarce, particularly during ITP, in which the spleen is the major place of platelet destruction 23 and the primary site of maintenance of the autoimmune response 9. We thus took advantage of splenectomy as part of the treatment of ITP to study for the first time the expression and polymorphism of FcγR on human splenic macrophages from ITP patients treated or not with IVIg prior to splenectomy compared to post‐traumatic control spleens.
Materials and methods
Patients
ITP patients, admitted to the University Hospital of Dijon, France, were enrolled into the study after giving written informed consent in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board and the Research Ethic Review Committee of the University Hospital of Dijon. The main inclusion criterion was primary immune thrombocytopenia, i.e. a platelet count below 100 G/l with exclusion of familial, viral, drug‐induced or systemic autoimmune disease‐related thrombocytopenia. Treatments were initiated when platelet count was below 30 G/l with steroids for 3–4 weeks and, if necessary, with intravenous Ig (IVIg) 26 as first‐line therapy. The spleens of 24 ITP patients (Table 1) were available for flow cytometry (FCM), phagocytosis assay and multiplex ligation‐dependent probe amplification (MLPA). Post‐traumatic spleens (n = 6) were used as controls.
Table 1.
Characteristics of splenectomized patients
| Controls | ITP | |||
|---|---|---|---|---|
| (n = 6) | Total (n = 24) | Responders (n = 19) | Refractory (n = 5) | |
| Age, years | 52 ± 24 | 46·7 ± 5 | 41·2 ± 5 | 67·8 ± 11 |
| Sex ratio (F/M) | 2/4 | 17/7 | 14/5 | 3/2 |
| Lowest platelet count during the 3 months prior to splenectomy, G/l | 19·4 ± 2 | 21·4 ± 2 | 13·8 ± 2 | |
| Platelet count at the time of splenectomy, G/l | 209 ± 43 | 195·5 ± 26 | 206·3 ± 32 | 156·4 ± 39 |
| Disease duration, months | – | 29 ± 5 | 30·6 ± 6 | 25·7 ± 15 |
| Previous treatments, n (%) | ||||
| Steroids | – | 24 (100) | 18 (100) | 5 (100) |
| IVIg | – | 19 (79·1) | 14 (77·8) | 5 (100) |
| Dapsone | – | 13 (54·1) | 10 (55·5) | 3 (60) |
| Rituximab | – | 9 (37·5) | 5 (27·8) | 4 (80) |
| TPO‐RA | – | 2 (8·3) | 1 (5·6) | 1 (20) |
| Treatment within the 2 weeks prior to splenectomy, n (%) | ||||
| IVIg | – | 16 (66·7) | 11 (57·9) | 5 (100) |
| Steroids | – | 6 (25) | 6 (31·5) | – |
| TPO‐RA | – | 1 (4·2) | 1 (5·3) | – |
| None | – | 1 (4·2) | 1 (5·3) | – |
IVIg = intravenous immunoglobulins; TPO‐RA = thrombopoietin receptor agonists.
Spleen preparation
Splenocytes were obtained as described previously 27 and stored in liquid nitrogen until needed. Cells were thawed rapidly and washed before use.
Flow cytometry
The following antibodies were used: anti‐CD14 allophycocyanin (APC)‐Hilite7, anti‐CD16 Brilliant Violet 500, CD206 APC (BD Biosciences, San Jose, CA, USA), anti‐CD32a fluorescein isothiocyanate (FITC) (clone IV.3; Stemcell Technologies, Vancouver, Canada), anti‐CD32b Alexa Fluor 480 (clone 2B6; MacroGenics, Rockville, MD, USA), anti‐CD64 phycoerytrin (PE) (Dako, Carpenteria, CA, USA), anti‐human leucocyte antigen D‐related (HLA‐DR) Pacific Blue and anti‐CD163 peridinin chlorophyll protein complex (PerCP)‐cyanin5.5 (Biolegend, San Diego, CA, USA). Importantly, anti‐CD32a clone IV.3 antibody, has been shown to bind specifically to CD32a but not to CD32b 28, 29. Similarly, anti‐CD32b clone 2B6 antibody does not bind to CD32a, but to CD32b, as shown by enzyme‐linked immunosorbent assay and fluorescence activated cell sorter (FACS) staining of specific cell lines and CD32B‐transfected cells 29. However, it has been shown recently to bind to CD32c, the expression of which is due to a single nucleotide polymorphism in exon 3 (FCGR2C–ORF) 30. Cells were suspended in phosphate‐buffered saline (PBS) supplemented with bovine serum albumin (BSA) (0·1%) and incubated for 20 min in V‐bottomed plates with the appropriate antibodies or isotype controls. Data were acquired on a BD Biosciences LSRIITM cytometer and analyzed with FlowJoTM (TreeStar Inc., Ashland, OR, USA) software. MFI refers to as the median fluorescence intensity.
Phagocytosis assay
The isolation of splenic macrophages was performed with CD14 microbeads (Miltenyi Biotec, San Diego, CA, USA) on AutoMACS® following the manufacturer's instructions (Miltenyi Biotec). Macrophages (5·105/ml) were suspended in RPMI supplemented with fetal bovine serum (FBS) (10%) and incubated with streptavidin FITC‐fluorospheres (2 µM diameter fluorescent microspheres (Fluoresbrite®); Polysciences, Warrington, PA, USA) at a ratio of 1 : 10 for 1 h at either 4°C or 37°C in flat‐bottomed 96‐well plates. Fluorospheres were used uncoated or coated with human biotinylated IgG. Cells were collected by washing with cold PBS. In some experiments, FcγR on macrophages were blocked using FcγR blocking agent (Miltenyi Biotec) for 10 min before adding fluorospheres. Phagocytosis was checked on a fluorescence microscope (EVOS®). The phagocytosis index was defined as the ratio of stained macrophages at 37°C compared to that at 4°C. Data were acquired on a BD Biosciences LSRIITM cytometer and analysed with FlowJoTM software.
Multiplex ligation‐dependent probe amplification
MLPA was used to analyse single nucleotide polymorphisms (SNP) in FCGR2A (131H/R), FCGR2B (232I/T), promoter of FCGR2B and FCGR2C (−386G/C), FCGR3A (158V/F) and FCGR3B (HNA1a/1b/1c), as described previously 21. As the size of the spleen control group was too small to allow reliable comparison, 200 controls from a previously described cohort 21 were included in the analysis.
Statistics
Student's or paired t‐tests were used to compare quantitative variables as appropriate. Fisher's exact test or Pearson's χ2 test were used to compare qualitative data as appropriate. Analysis of variance with a Bonferroni correction was performed when more than two variables were compared. Results were considered statistically significant when P < 0·05. Results are given by mean ± standard error of the mean. Analyses were performed on GraphPad Prism®, San Diego, CA, USA.
Results
Patient's characteristics
The mean age of patients was 46.7 ± 5 years at splenectomy (Table 1). Seventy per cent were female (17 of 24). Patients had an active disease with a lowest platelet count of 19·4 ± 2 G/l within the 3 months prior to splenectomy. The mean disease duration at splenectomy was 29 ± 5 months. All patients were first treated with steroids, followed by dapsone (54·1%), rituximab (37·5%) and thrombopoietin receptor agonist (TPO‐RA, 8·3%). IVIg were used as rescue therapy in 79·1%. The response rate of splenectomy was 79% (19 of 24). Refractory patients were older than responders (67·8 ± 11 versus 41·2 ± 5, P = 0·02), and tended to have a lower platelet count than responders (13·8 ± 2·5 versus 21·4 ± 1·8, P = 0·04). Disease duration at splenectomy was not different between responders and refractory patients. A platelet count above 50 G/l was required to perform splenectomy: 14 patients received IVIg within the 2 weeks prior to splenectomy, whereas eight patients received either steroids (n = 6), TPO‐RA (n = 1) or no treatment (n = 1; Table 1). The mean platelet count before splenectomy was 195 ± 26 G/l. The use of either steroids or IVIg was determined by the physician managing the patient. The patient treated with TPO‐RA was refractory to both steroids and IVIg. The patient who did not receive any treatment had a spontaneous platelet count above 50 G/l, but splenectomy was indicated because of a suspect splenic nodule that was finally consistent with a primary angiomyolipoma.
FCGR3A (158V/F) polymorphism is over‐represented in ITP
Because some polymorphisms of FcγR can modulate their activity or expression, the most common SNPs of the different FCGR were analysed. The allele frequency of FCGR2A‐131H/R, FCGR2B‐232I/T and FCGR3B‐HNA1a/1b/1c were not significantly different between patients and controls (Table 2). Concerning FCGR3A, an over‐representation of FCGR3A‐158V during ITP (50% versus 30.6%, P = 0·016) was observed (Table 2).
Table 2.
Allele frequency for the different FCGR assessed by MLPA
| Controls | ITP | ||||
|---|---|---|---|---|---|
| n | (%) | n | (%) | P‐value | |
| FCGR2A | |||||
| 131R | 98 | (45·4) | 15 | (31·3) | n.s. |
| 131H | 118 | (54·6) | 33 | (68·8) | |
| FCGR2B | |||||
| 232I | 190 | (87·9) | 44 | (91·7) | n.s. |
| 232T | 26 | (12) | 4 | (8·3) | |
| FCGR3A | |||||
| 158F | 152 | (69·4) | 23 | (50) | 0·02 |
| 158V | 67 | (30·6) | 23 | (50) | |
| FCGR3B | |||||
| HNA1a | 79 | (35·4) | 16 | (34·8) | n.s. |
| HNA1b | 140 | (62·8) | 27 | (58·7) | |
| HNA1c | 4 | (1·8) | 3 | (6·5) | |
| FCGR2C exon 3 | |||||
| STOP | 200 | (88·1) | 34 | (73·9) | n.s. |
| ORF | 27 | (11·9) | 9 | (19·6) | |
| Non‐classical ORF | 0 | 0 | 3 | (6·5) | |
| Promoter −386 of FCGR2B and FCGR2C | |||||
| GG | 104 | (78·2) | 76 | (80·9) | n.s. |
| GC | 27 | (20·3) | 18 | (19·1) | |
| CC | 1 | (0·8) | 8 | (8·5) | |
MPLA = multiplex ligation‐dependent probe amplification; ORF = open reading frame.
The ORF of the FCGR2C is due to a SNP in exon 3 that leads to the expression of an activating receptor, CD32c, excepted when associated with a second mutation in exon 7 (non‐classical ORF), resulting in a STOP codon and the absence of expression of CD32c 30. There was no significant difference in allele frequency between patients and controls.
Splenic macrophages of ITP patients express higher CD86 and HLA‐DR than controls
The expression of the different FcγR (CD16/FcγRIII, CD32a/FcγRIIa, CD32b/FcγRIIb and CD64/FcγRI) on human splenic macrophages was first assessed by flow cytometry (Fig. 1a). Overall, CD16, CD32a and CD64 were expressed by more than 90% of splenic macrophages, when CD32b was expressed by an average of only 30%. Splenic macrophages also expressed CD86 and HLA‐DR (95·2 and 98·9%, respectively). The scavenger receptor CD163 was expressed by an average of 61%, whereas the mannose receptor (CD206) was expressed by only 13·4% of splenic macrophages.
Figure 1.
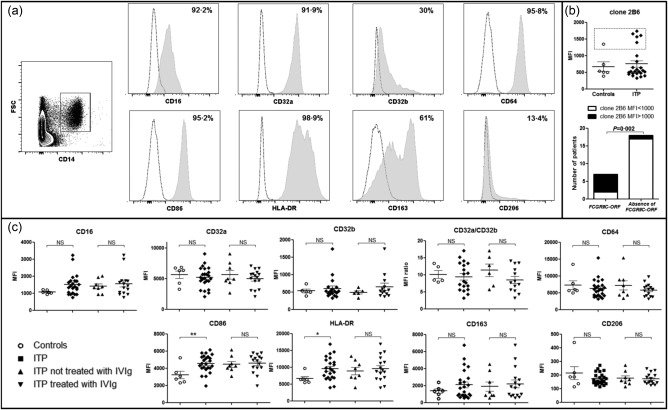
Splenic macrophage phenotype between controls and immune thrombocytopenia (ITP) patients. (a) Macrophage phenotype was determined by flow cytometry. Splenocytes were first gated on the expression of CD14. The expression of the different FcγR (CD16, CD32A, CD32B, CD64), of activation markers [CD86, human leucocyte antigen D‐related (HLA‐DR)], of scavenger receptor (CD163) and of mannose receptor (CD206) were measured. Results of one representative ITP patient are depicted in grey shaded histograms. Dashed lines represent control isotype staining. The mean percentage of positive macrophages for each staining in overall patients is given. (b) Expression of CD32b was determined using clone 2B6. Five patients and one control (dashed square) had high median fluorescence intensity (MFI) compared to others that was associated with the presence of the FCGR2C–ORF polymorphism. (c) The expression of the different FcγR, CD86, HLA‐DR, CD163 and CD206 measured as the median fluorescence intensity (MFI) was compared between controls (n = 6) and ITP patients (n = 24), with exclusion of FCGR2C–ORF patients for CD32b and CD32a/CD32b expression. Results are summarized in dot‐plots. The horizontal bar represents the mean with the standard error of the mean. P‐value derived by Student's t‐test; n.s. = non‐significant; *P < 0·05; **P < 0·01.
The expression rate of the different markers was compared using median fluorescence intensity (MFI). Because of the extracellular domain homology between CD32b and CD32c, they are both recognized by clone 2B6 30. Thus, the presence of FCGR2C–ORF, leading to CD32c expression, was associated with a high MFI of CD32b/c (> 1000) in one control and six patients (Fig. 1b). Considering that CD32c is an activating receptor, analyses of the expression of CD32b and the balance between activating and inhibitory FcγR represented by the CD32a/CD32b ratio were performed with exclusion of FCGR2C–ORF patients. No difference in the expression of FcγRs on splenic macrophages was observed between ITP patients and controls (Fig. 1c). The balance between activating and inhibitory FcγR represented by the CD32a : CD32b ratio was similar between the two groups. On the contrary, HLA‐DR expression was higher during ITP compared to controls (9634 ± 694 versus 6718 ± 628, P = 0·04). Similarly, the activation molecule CD86 was expressed more highly during ITP compared to controls (4553 ± 206 versus 3232 ± 440, P = 0·009). The expression of CD163 and CD206 was not different between the two groups.
Splenic macrophage phenotype was also compared between ITP patients, depending on the response to splenectomy. Nineteen (79%) patients who were in remission after splenectomy were compared to five refractory patients. No difference in the expression of the different markers on splenic macrophages was observed (Supporting information, Fig. S1).
The use of IVIg prior to splenectomy does not affect the expression of FcγR and activation markers on human splenic macrophages
To determine whether IVIg could affect the expression of the different FcγR and activation markers on splenic macrophages in vivo, their phenotype was compared between ITP patients, depending on the use or not of IVIg prior to splenectomy. No difference was observed between the two groups regarding the expression of the different FcγR or the CD32a/CD32b ratio. The expression of HLA‐DR, CD86, CD163 and CD206 was also similar between groups (Fig. 1c).
Phagocytosis by splenic macrophages is decreased after IVIg treatment during ITP
In addition to macrophage phenotype, their functionality was determined by comparing their phagocytic capacity (Fig. 2a, b) and compared between three controls and six ITP patients treated with IVIg prior to splenectomy. A significant decrease in the phagocytosis index between controls and ITP patients was observed when using unopsonized fluorescent beads (2·6 ± 0·5 versus 1·4 ± 0·2, respectively; P = 0·04; Fig. 2c). The phagocytosis index also tended to be lower when human IgG‐coated fluorospheres were used (3·2 ± 1 versus 2·3 ± 0·3; P = 0·3; Fig. 2c). Overall, the phagocytosis index was higher when IgG‐coated fluorospheres were used, compared to uncoated beads (1·5 ± 0·4 versus 2·7 ± 0·6, P = 0·02; Fig. 2c). This increase was abrogated by FcγR blockade (2·7 ± 0·6 versus 1·5 ± 0·3, P = 0·02; Fig· 2c), thus confirming the role of FcγR during the phagocytosis process.
Figure 2.
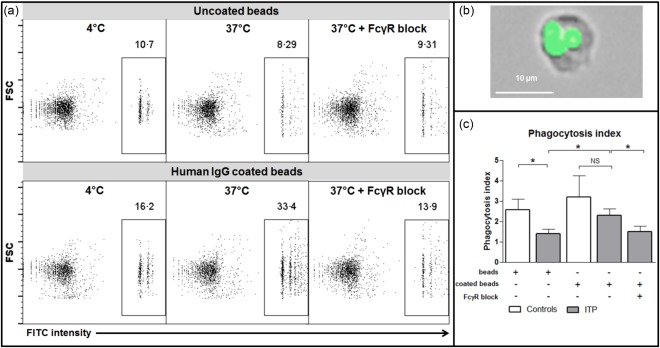
Splenic macrophage phagocytic functions. To assess their phagocytic capability, macrophages isolated from the spleen of three controls and six immune thrombocytopenia (ITP) patients were incubated with fluorospheres either uncoated or coated with human immunoglobulin (Ig)G at 4°C and 37°C. For some experiments, a FcγR blocking agent was added. (a) The percentage of phagocytosis was determined by flow cytometry. (b) The localization of fluorospheres within macrophages was confirmed by fluorescence microscopy. (c) Phagocytosis index measured is summarized by histograms (mean with standard error of the mean). P‐value derived by Student's t‐test or paired t‐test, as appropriate; n.s. = non‐significant; *P < 0·05. [Colour figure can be viewed at wileyonlinelibrary.com].
Discussion
An increase in CD64 expression and in the CD32a/CD32b ratio has been described on circulating monocytes during ITP 31. As thrombocytopenia is mediated mainly by splenic macrophages during ITP, our aim was to investigate their phagocyte function and FcγR expression. To date, whether IVIg modulate CD32b expression on macrophages in humans in vivo is not known, and has been extrapolated from mouse models. Moreover, the results concerning the modulation of CD32b by IVIg are debated, as the first reports showed that the effects of IVIg rely on CD32b expression 32, which was increased after IVIg infusion 16, whereas others demonstrated that IVIg efficacy did not depend on CD32b 33.
During ITP, CD32b expression by splenic human macrophages has been assessed in only one study 34, which showed a decrease in CD32b expression determined by immunochemistry. We do not confirm this result by measuring CD32b expression using flow cytometry, which is a more sensitive technique. Moreover, our results are in line with more recent publications that reported the absence of variation of CD32b expression on circulating monocytes in children ITP following IVIg 35, and that the inhibition of FcγR mediated phagocytosis by monocyte‐derived macrophages was independent of CD32b in vitro 14. However, as the expression of FcγR on human splenic macrophages could not be assessed before and after IVIg treatment in vivo, patients were compared according to the treatment they received prior to splenectomy. The expression of FcγR was not different between the two groups, suggesting that IVIg do not modulate FcγR expression in vivo in humans. However, it cannot be excluded that this absence of difference in FcγR expression could be due to similar effects of the treatments used in these patients, as steroids and TPO‐RA have been shown to shift the balance of FcγR towards inhibitory CD32b on circulating monocytes during ITP 31, 36. We also show here for the first time, to our knowledge, that splenic macrophages from ITP patients have a decreased phagocytic function in vivo, due probably to IVIg used prior to surgery. Taken together, our results show that, in vivo, IVIg do not seem to modulate FcγR expression, notably CD32b, but induce a decrease in phagocytic capability of splenic macrophages.
Because not only the expression level but also the affinity between antibody and FcγR could play a role in antibody‐mediated diseases, we investigated the presence of different SNPs of FcγR. In this cohort, we observed an over‐representation of FCGR3A−158V, as reported previously in children with ITP 17, 20, 21, a polymorphism known to increase the affinity of the receptor CD16 to IgG 37.
Interestingly, human splenic macrophages in ITP showed an increase in HLA‐DR and CD86 expression compared to controls, consistent with a higher activation state. Such an increase in the co‐stimulatory molecule CD86 has already been observed in monocyte‐derived dendritic cells, and was associated with an increase in CD4+ T cell proliferation 38. The higher activation status on splenic macrophages of ITP patients probably participates in CD4+ T cell activation and to the maintenance of the autoimmune response, as macrophages are the main T cell activators in the spleen compared to dendritic cells 9. However, this activated status was not correlated with the response to splenectomy.
In conclusion, we showed here that the expression of the different FcγR on splenic macrophages is not different between ITP patients and controls, whereas these cells display a higher expression of activation markers such as CD86 and HLA‐DR in accordance with their major role of antigen‐presenting cells in ITP. Furthermore, we also demonstrated that IVIg used prior to splenectomy during ITP leads to a decrease in phagocytic function of splenic macrophages.
Disclosure
The authors have no competing interests to disclose.
Author contributions
S. A., K. S., A. G. L. and M. S. performed the research; S. A., K. S., B. B. and T. R. designed the research study; O. F. and P. O.‐D. performed splenectomy; S. A., K. S., A. G. L., B. B. and T. R. analysed the data; S. A., K. S., G. V., M. S., N. J., P. S., B. B. and T. R. wrote the paper.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Splenic macrophage phenotype between controls and immune thrombocytopenia (ITP) patients. (a) Macrophage phenotype was determined by flow cytometry. Splenocytes were first gated on the expression of CD14. The expression of the different FcγR (CD16, CD32A, CD32B, CD64), of activation markers [CD86, human leucocyte antigen D‐related (HLA‐DR)], of scavenger receptor (CD163) and of mannose receptor (CD206) were measured. Results of one representative ITP patient are depicted in grey shaded histograms. Dashed lines represent control isotype staining. The mean percentage of positive macrophages for each staining in overall patients is given. (b) Expression of CD32b was determined using clone 2B6. Five patients and one control (dashed square) had high median fluorescence intensity (MFI) compared to others that was associated with the presence of the FCGR2C–ORF polymorphism. (c) The expression of the different FcγR, CD86, HLA‐DR, CD163 and CD206 measured as the median fluorescence intensity (MFI) was compared between controls (n = 6) and ITP patients (n = 24), with exclusion of FCGR2C–ORF patients for CD32b and CD32a/CD32b expression. Results are summarized in dot‐plots. The horizontal bar represents the mean with the standard error of the mean. P‐value derived by Student's t‐test; n.s. = non‐significant; *P < 0·05; **P < 0·01.
Acknowledgements
S. A. was supported by a research grant from the Foundation for the Development of Internal Medicine in Europe (FDIME). We thank MacroGenics for providing us with the 2B6 antibody directed against FcγRIIb and Dr Suzan Rooyakkers from the department of microbiology, University Medical Center Utrecht, for kindly providing biotinylated IgG. We thank Saidy Conception for her involvement in the MLPA experiments. We are grateful to Taco W. Kuijpers for sharing data concerning the MLPA control group.
References
- 1. Nimmerjahn F, Ravetch JV. Antibody‐mediated modulation of immune responses. Immunol Rev 2010; 236:265–75. [DOI] [PubMed] [Google Scholar]
- 2. Niederer HA, Clatworthy MR, Willcocks LC, Smith KG. FcgammaRIIB, FcgammaRIIIB, and systemic lupus erythematosus. Ann NY Acad Sci 2010; 1183:69–88. [DOI] [PubMed] [Google Scholar]
- 3. Radstake TR, Blom AB, Sloetjes AW et al Increased FcgammaRII expression and aberrant tumour necrosis factor alpha production by mature dendritic cells from patients with active rheumatoid arthritis. Ann Rheum Dis 2004; 63:1556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wenink MH, Santegoets KC, Roelofs MF et al The inhibitory Fc gamma IIb receptor dampens TLR4‐mediated immune responses and is selectively up‐regulated on dendritic cells from rheumatoid arthritis patients with quiescent disease. J Immunol 2009; 183:4509–20. [DOI] [PubMed] [Google Scholar]
- 5. McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science 2005; 307:590–3. [DOI] [PubMed] [Google Scholar]
- 6. Stasi R, Evangelista ML, Stipa E, Buccisano F, Venditti A, Amadori S. Idiopathic thrombocytopenic purpura: current concepts in pathophysiology and management. Thromb Haemost 2008; 99:4–13. [DOI] [PubMed] [Google Scholar]
- 7. He R, Reid DM, Jones CE, Shulman NR. Spectrum of Ig classes, specificities, and titers of serum antiglycoproteins in chronic idiopathic thrombocytopenic purpura. Blood 1994; 83:1024–32. [PubMed] [Google Scholar]
- 8. van Leeuwen EF, van der Ven JT, Engelfriet CP, von dem Borne AE. Specificity of autoantibodies in autoimmune thrombocytopenia. Blood 1982; 59:23–6. [PubMed] [Google Scholar]
- 9. Kuwana M, Okazaki Y, Ikeda Y. Splenic macrophages maintain the anti‐platelet autoimmune response via uptake of opsonized platelets in patients with immune thrombocytopenic purpura. J Thromb Haemost 2009; 7:322–9. [DOI] [PubMed] [Google Scholar]
- 10. Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol 2013; 13:176–89. [DOI] [PubMed] [Google Scholar]
- 11. Imbach P, Barandun S, Baumgartner C, Hirt A, Hofer F, Wagner HP. High‐dose intravenous gammaglobulin therapy of refractory, in particular idiopathic thrombocytopenia in childhood. Helv Paediatr Acta 1981; 36:81–6. [PubMed] [Google Scholar]
- 12. Imbach P, Barandun S, Hirt A, Wagner HP. Intravenous immunoglobulin for idiopathic thrombocytopenic purpura (ITP) in childhood. Am J Pediatr Hematol Oncol 1984; 6:171–4. [DOI] [PubMed] [Google Scholar]
- 13. Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB. Of mice and men: an open‐label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood 2009; 113:3154–60. [DOI] [PubMed] [Google Scholar]
- 14. Nagelkerke SQ, Dekkers G, Kustiawan I et al Inhibition of FcgammaR‐mediated phagocytosis by IVIg is independent of IgG‐Fc sialylation and FcgammaRIIb in human macrophages. Blood 2014; 124:3709–18. [DOI] [PubMed] [Google Scholar]
- 15. Kaneko Y, Nimmerjahn F, Ravetch JV. Anti‐inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006; 313:670–3. [DOI] [PubMed] [Google Scholar]
- 16. Samuelsson A, Towers TL, Ravetch JV. Anti‐inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 2001; 291:484–6. [DOI] [PubMed] [Google Scholar]
- 17. Carcao MD, Blanchette VS, Wakefield CD et al Fcgamma receptor IIa and IIIa polymorphisms in childhood immune thrombocytopenic purpura. Br J Haematol 2003; 120:135–41. [DOI] [PubMed] [Google Scholar]
- 18. Amorim DM, Silveira Vda S, Scrideli CA, Queiroz RG, Tone LG. Fcgamma receptor gene polymorphisms in childhood immune thrombocytopenic purpura. J Pediatr Hematol Oncol 2012; 34:349–52. [DOI] [PubMed] [Google Scholar]
- 19. Foster CB, Zhu S, Erichsen HC et al Polymorphisms in inflammatory cytokines and Fcgamma receptors in childhood chronic immune thrombocytopenic purpura: a pilot study. Br J Haematol 2001; 113:596–9. [DOI] [PubMed] [Google Scholar]
- 20. Papagianni A, Economou M, Tragiannidis A et al FcgammaRIIa and FcgammaRIIIa polymorphisms in childhood primary immune thrombocytopenia: implications for disease pathogenesis and outcome. Blood Coagul Fibrinolysis 2013; 24:35–9. [DOI] [PubMed] [Google Scholar]
- 21. Breunis WB, van Mirre E, Bruin M et al Copy number variation of the activating FCGR2C gene predisposes to idiopathic thrombocytopenic purpura. Blood 2008; 111:1029–38. [DOI] [PubMed] [Google Scholar]
- 22. Bruin M, Bierings M, Uiterwaal C et al Platelet count, previous infection and FCGR2B genotype predict development of chronic disease in newly diagnosed idiopathic thrombocytopenia in childhood: results of a prospective study. Br J Haematol 2004; 127:561–7. [DOI] [PubMed] [Google Scholar]
- 23. Najean Y, Rain JD, Billotey C. The site of destruction of autologous 111In‐labelled platelets and the efficiency of splenectomy in children and adults with idiopathic thrombocytopenic purpura: a study of 578 patients with 268 splenectomies. Br J Haematol 1997; 97:547–50. [DOI] [PubMed] [Google Scholar]
- 24. Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood 2011; 117:4190–207. [DOI] [PubMed] [Google Scholar]
- 25. Provan D, Stasi R, Newland AC et al International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010; 115:168–86. [DOI] [PubMed] [Google Scholar]
- 26. Khellaf M, Michel M, Schaeffer A, Bierling P, Godeau B. Assessment of a therapeutic strategy for adults with severe autoimmune thrombocytopenic purpura based on a bleeding score rather than platelet count. Haematologica 2005; 90:829–32. [PubMed] [Google Scholar]
- 27. Audia S, Samson M, Guy J et al Immunologic effects of rituximab on the human spleen in immune thrombocytopenia. Blood 2011; 118:4394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest 2005; 115:2914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Veri MC, Gorlatov S, Li H et al Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma‐receptor IIB (CD32B) from the activating Fcgamma‐receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology 2007; 121:392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Heijden J, Breunis WB, Geissler J, de Boer M, van den Berg TK, Kuijpers TW. Phenotypic variation in IgG receptors by nonclassical FCGR2C alleles. J Immunol 2012; 188:1318–24. [DOI] [PubMed] [Google Scholar]
- 31. Liu XG, Ma SH, Sun JZ et al High‐dose dexamethasone shifts the balance of stimulatory and inhibitory Fcgamma receptors on monocytes in patients with primary immune thrombocytopenia. Blood 2011; 117:2061–9. [DOI] [PubMed] [Google Scholar]
- 32. Crow AR, Song S, Freedman J et al IVIg‐mediated amelioration of murine ITP via FcgammaRIIB is independent of SHIP1, SHP‐1, and Btk activity. Blood 2003; 102:558–60. [DOI] [PubMed] [Google Scholar]
- 33. Leontyev D, Katsman Y, Branch DR. Mouse background and IVIG dosage are critical in establishing the role of inhibitory Fcgamma receptor for the amelioration of experimental ITP. Blood 2012; 119:5261–4. [DOI] [PubMed] [Google Scholar]
- 34. Wu Z, Zhou J, Prsoon P, Wei X, Liu X, Peng B. Low expression of FCGRIIB in macrophages of immune thrombocytopenia‐affected individuals. Int J Hematol 2012; 96:588–93. [DOI] [PubMed] [Google Scholar]
- 35. Shimomura M, Hasegawa S, Seki Y, Fukano R, Hotta N, Ichiyama T. Intravenous immunoglobulin does not increase FcgammaRIIB expression levels on monocytes in children with immune thrombocytopenia. Clin Exp Immunol 2012; 169:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu XG, Liu S, Feng Q et al Thrombopoietin receptor agonists shift the balance of Fcgamma receptors toward inhibitory receptor IIb on monocytes in ITP. Blood 2016; 128:852–61. [DOI] [PubMed] [Google Scholar]
- 37. Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa‐158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa‐48L/R/H phenotype. Blood 1997; 90:1109–14. [PubMed] [Google Scholar]
- 38. Catani L, Fagioli ME, Tazzari PL et al Dendritic cells of immune thrombocytopenic purpura (ITP) show increased capacity to present apoptotic platelets to T lymphocytes. Exp Hematol 2006; 34:879–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Splenic macrophage phenotype between controls and immune thrombocytopenia (ITP) patients. (a) Macrophage phenotype was determined by flow cytometry. Splenocytes were first gated on the expression of CD14. The expression of the different FcγR (CD16, CD32A, CD32B, CD64), of activation markers [CD86, human leucocyte antigen D‐related (HLA‐DR)], of scavenger receptor (CD163) and of mannose receptor (CD206) were measured. Results of one representative ITP patient are depicted in grey shaded histograms. Dashed lines represent control isotype staining. The mean percentage of positive macrophages for each staining in overall patients is given. (b) Expression of CD32b was determined using clone 2B6. Five patients and one control (dashed square) had high median fluorescence intensity (MFI) compared to others that was associated with the presence of the FCGR2C–ORF polymorphism. (c) The expression of the different FcγR, CD86, HLA‐DR, CD163 and CD206 measured as the median fluorescence intensity (MFI) was compared between controls (n = 6) and ITP patients (n = 24), with exclusion of FCGR2C–ORF patients for CD32b and CD32a/CD32b expression. Results are summarized in dot‐plots. The horizontal bar represents the mean with the standard error of the mean. P‐value derived by Student's t‐test; n.s. = non‐significant; *P < 0·05; **P < 0·01.


