Summary
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by extensive immune response, including over‐activation of T and B cell development of pathogenic autoantibodies, organ damage induced by the formation and deposition of immune complex and the abnormal elevation of type I interferon. Semaphorin5A (Sema5A) is involved essentially in immune cell regulation and is also implicated in the pathogenesis of autoimmune disorders. We aimed to evaluate the role of Sema5A in patients with SLE. Serum levels of Sema5A were tested by enzyme‐linked immunosorbent assay (ELISA) in 152 SLE patients and 48 healthy controls. The message ribonucleic acid (mRNA) expression levels of Sema5A and ADAM metallopeptidase domain 17 (ADAM17) in the peripheral blood mononuclear cells (PBMC) from 43 patients with SLE and 19 healthy controls were detected by the real‐time–quantitative polymerase chain reaction (qPCR). Serum Sema5A levels were increased significantly in SLE patients compared with healthy controls (P < 0·001). Elevated levels of Sema5A were correlated positively with 24‐h proteinuria excretion (r = 0·558, P < 0·0001), SLE disease activity index (SLEDAI) (r = 0·278, P = 0·0006) and C‐reactive protein (CRP) (r = 0·266, P = 0·002), but negatively with planet (PLT) (r = –0·294, P = 0·0003) and complement 3 (C3) (r = –0·287, P = 0·0004) in SLE patients. Patients with elevated Sema5A levels showed higher incidence of rash, serositis and nephritis (P < 0·05 or P < 0·001). Patients with decreased PLT, C3 or positive for proteinuria also showed elevated Sema5A (P < 0·001 or P < 0·05). The mRNA ADAM17 was increased in SLE patients and correlated positively with serum Sema5A levels. Our data demonstrated that elevated serum Sema5A in SLE patients correlated with disease activity and are involved in kidney and blood system damage; ADAM17 might be involved in the release of secreted Sema5A.
Keywords: nephritis, semaphorin5A, SLEDAI, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a prototypical systemic autoimmune disease, which can result in skin rashes, arthritis, leucopenia, nephritis and inflammation of the nervous system 1. Immunologically, SLE is characterized by high B cell reactivity and production of autoantibodies to nuclear and cytoplasmic components 2, and sustained production of autoantibodies leads to the accumulation of immune complex in kidney 3, brain 4 and other organs. SLE begins with self‐tolerance loss and the presence of autoreactive lymphocytes in the peripheral blood, due to both environmental and genetic factors 5, 6. Multiple types of cells and cytokines in the adaptive and innate immune system have been confirmed to contribute to SLE pathogenesis.
Semaphorins are a large family (classified into eight subclasses) of secreted and membrane‐bound proteins, discovered originally in the nervous system, which are involved in repulsive axon guidance during nervous system development 7. They are related to two families of receptors: the neuropilins (NP‐1 and NP‐2), as the primary ligand binding sites and plexins, as the signal transducing components. Several of members of the semaphorin family, the so‐called ‘immune semaphorins’, including semaphorin3A (Sema3A), Sema4D, Sema6D and Sema7A, are involved essentially in immune cell regulation 8 and are also implicated in the pathogenesis of autoimmune disorders 9, 10, 11.
Sema5A, as a membrane‐bound member of this family, comprises transmembrane protein that exhibits a unique extracellular domain containing seven thrombospondin‐specific repeats in addition to the sema domain 12. Receptors for Sema5A are plexin proteins, including plexin‐A1 and plexin‐B3 13, 14. Previously, a soluble form of Sema5A was described 15. It has been described to promote angiogenesis by increasing endothelial cell proliferation and decreasing apoptosis 16; elevated expression levels of Sema5A were associated with high tumorigenic and metastatic potentials in pancreatic and gastric tumours 17, 18, 19. Moreover, further studies revealed Sema5A to be an immune semaphorin for its role in innate immune responses by inducing the expression of tumour necrosis factor (TNF) and interleukin (IL)−8 genes 15, 20.
With respect to the significant role of secreted Sema5A in innate immunity, increasing attention has been paid to its immune regulation in autoimmune diseases. A recent study showed that significantly elevated levels of secreted Sema5A were detected in the serum of patients with rheumatoid arthritis (RA) and other autoimmune diseases compared with control serum. Soluble Sema5A greatly promoted T cell and natural killer (NK) cell proliferation and induced the secretion of T helper type 1 (Th1)/Th17 proinflammatory cytokines 21. In another study, the researchers found that plasma levels of Sema5A were increased significantly in patients with active chronic immune thrombocytopenic purpura (ITP). In addition, plasma levels of Sema5A were correlated negatively with platelet counts in active patients. Therefore, it is plausible that Sema5A might be in positive correlation with disease activity 22. In consideration of the above, we initiated this study to assess the possible role of Sema5A in systemic lupus erythematosus (SLE); that is, to measure its serum level in SLE patients and assess whether this level correlates with disease status.
Materials and methods
Patients and controls
A total of 152 patients with SLE and 48 healthy controls were enrolled at the Department of Rheumatology, The Second Affiliated Hospital of Zhejiang University School of Medicine, and blood samples were obtained from these patients. The diagnosis of SLE was based on the American College of Rheumatology (ACR) 1997 revised classification criteria 23. Healthy controls were recruited from healthy staff members of the hospital. Prior to enrolment, all participants signed the informed consent to donate their blood samples and their clinical information was de‐identified for research. Our study was approved by the ethics committee of the Second Affiliated Hospital of Zhejiang University School of Medicine. The mRNA detection was performed from 43 of 152 patients and 19 of 48 healthy controls.
Clinical data analysis
General and laboratory data were collected from the medical records of these patients, including age, gender, disease duration, clinical symptoms, blood cell counts [leucocyte: white blood cells (WBC); platelets (PLT): thrombocyte], routine chemistry, urinalysis, 24‐h proteinuria excretion, lupus‐associated antoantibodies [anti‐double‐strand DNA antibody (anti‐dsDNA antibody); anti‐nuclear antibody (ANA); anti‐nucleosome antibody (AnuA); anti‐SSA antibody (SSA); anti‐Sm antibody (Sm); anti‐cardiolipin antibody (aCL), immunoglobulins (IgG, IgM, IgA), complement component3 (C3), complement component 4 (C4), C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR)]. White cell and planet counts of less than 4 ×10^9/l and 100 × 10^9/l were regarded as leucocytopenia and thrombocytopenia, respectively. Proteinuria was defined as 24‐h proteinuria excretion more than or equal to 0·5 g. Anti‐dsDNA antibody, ACL, AnuA, C3, C4, IgG, IgM and IgA were tested by enzyme‐linked immunosorbent assay (ELISA), with normal ranges of 0–100 IU/ml, 0–12 RU/ml, 0–20 RU/ml, 0·82–1·8 g/l, 100–400 mg/l, 7–16 g/l, 0·4–2·3 g/l and 0·7–4 g/l. ANA, SSA and Sm were tested by indirect immunofluorescence assay. Positive autoantibodies of anti‐dsDNA antibody, aCL and AnuA were defined as values more than 100 IU/ml, 12 RU/ml and 20 RU/ml, respectively. Decreased C3 and C4 were defined as values less than 0·82 g/l and 100 mg/l. CRP was examined using the immunonephelometry method. Values more than or equal to 10 mg/l were considered positive. ESR were measured by Westergren's method, with values > 15 mm/h for men and > 20 mm/h for women considered abnormal.
Disease activity was calculated by using the SLE disease activity index (SLEDAI) 24. Clinical features defined by the SLEDAI system were seizure, psychiatric symptoms, encephalosis, visual injury, cranial neuropathy, lupus headache, cerebrovascular insult, vasculitis arthritis, myositis, cylindruria [haemoglobin red blood cell (HbRBC) cylinder, granular cast], haemoglobinuria (> 5RBC/haptoglobin (HP)], pyuria (> 5WBC/HP), leucocytopenia and thrombocytopenia.
Enzyme‐linked immunosorbent assay (ELISA)
All serum samples were split into aliquots and stored at −80°C until use. ELISA kits (JYM2074Hu) were used for measuring levels of Sema5A in serum (Jiyinmei, Wuhan, China), according to the manufacturer's instructions. The difference in intra‐ and interassay is less than 9 and 15%, respectively.
RNA isolation and quantitative real‐time–polymerase chain reaction (RT–PCR) analysis
Forty‐three SLE patients and 19 healthy controls were assessed for Sema5A and ADAM metallopeptidase domain 17 (ADAM17) mRNA expression in peripheral blood mononuclear cells (PBMCs). Cells were harvested and total RNA was extracted from PBMCs using an RNA extraction kit (Tiangen, Beijing, China). The cDNA was synthesized according to the instructions indicated in a RevertAid™ first‐strand cDNA synthesis kit (Fermentas, Shenzhen, China). For real‐time PCR, two‐step PCR was performed using Power SYBR® Green PCR Master Mix (Applied Biosystems, Beijing, China), according to the manufacturer's instructions. The sequences of the amplification primers were as follows – Sema5A: forward: 5′‐TGGAAGACACCTGGACCACATTCA‐3′, reverse: 5′‐ATCCAGCTCAGGCAGGAAGAAAGT‐3′; ADAM17, forward: 5′‐CGTTGGGTCTGTCCTGGTTT‐3′, reverse: 5′‐GATTTCGACGTTACTGGGG‐3′; and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH), forward: 5′‐AAGGTGAAGGTCGGAGTCAA‐3′, reverse: 5′‐AATGAAGGGGTCATTGATGG‐3′. The reaction was run on the 7500 Fast Real‐time PCR System (Applied Biosystems). Gene expression was quantified relative to the expression of the housekeeping gene, GAPDH, and normalized to control by standard 2–△△CT calculation.
Statistical analyses
Data analyses were performed using spss software for Windows version 16.0. For normally distributed data expressed as mean values ± standard deviation (s.d.), differences between groups were analysed using Student's t‐test. Comparisons of categorical variables were conducted using Pearson's χ2 tests. For non‐parametric data, results were expressed as median (range) values, and the differences between groups were analysed by the Mann–Whitney U‐test. Pearson's correlation coefficient was applied to detect the correlation between two groups. P‐values less than 0·05 were considered significant.
Results
Clinical and demographic features
Demographic and clinical characteristics of SLE patients and healthy controls are shown in Table 1. One hundred and fifty‐two SLE patients and 48 age‐ and gender‐matched controls were recruited into this study (age, 37·42 ± 14·73 versus 37·05 ± 14·51, P = 0·1; gender, χ2 = 0·15, P = 0·69). The SLE patients had a median disease duration of 48 months, ranging from 1 to 480, and the mean SLEDAI scores of these patients was 9·21, ranging from 0 to 32.
Table 1.
Clinical and laboratory characteristics in patients with systemic lupus erythematosus (SLE) and healthy controls
| Characteristics | Controls (n = 48) | SLE cases (n = 152) |
|---|---|---|
| Female (n = 139, %) | 89·5 | 91·4 |
| Age (mean ± s.d. years) | 37·05 ± 14·51 | 37·42 ± 14·73 |
| Disease duration (months, median) | – | (1–480, 48) |
| Clinical manifestations (%) | ||
| Fever (n = 152) | 21·7 | |
| LN (n = 152) | – | 40·8 |
| NPSLE (n = 152) | – | 5·3 |
| Rash (n = 152) | – | 35·5 |
| Photosensitivity (n = 152) | – | 11·8 |
| Oral ulcers (n = 152) | – | 5·9 |
| Raynaud (n = 152) | – | 17·8 |
| Arthritis (n = 152) | – | 23·7 |
| Serositis (n = 152) | 16·3 | |
| Leucopenia (n = 151) | – | 32·5 |
| Thrombopenia (n = 150) | – | 20·7 |
| Decreased C3 (n = 151) | 77·5 | |
| Decreased C4 (n = 86) | – | 57·3 |
| Autoantibody positivity (%) | ||
| ANA (n = 150) | – | 97·3 |
| Anti‐dsDNA antibody (n = 95) | – | 63·3 |
| AnuA (n = 142) | 43·7 | |
| ACL (n = 29) | 21·3 | |
| Anti‐Ro (SSA) (n = 111) | – | 74·5 |
SLE = systemic lupus erythematosus; LN = lupus nephritis; NPSLE = neuropsychiatric systemic lupus erythematosus; ANA = anti‐nuclear antibody; anti‐dsDNA antibody = anti‐double‐strand DNA antibody; ACL = anti‐cardiolipin antibody; AnuA = anti‐nucleosome antibody; SSA = anti‐SSA antibody; C3 = complement component 3; C4 = complement component 4; s.d. = standard deviation.
Serum Sema5A levels in SLE patients and controls
Serum Sema5A levels were significantly higher in patients with SLE (9·48 ± 0·77 ng/ml) than in healthy controls (2·93 ± 0·34ng/ml, P < 0·0001, Fig. 1a). The concentrations of serum Sema5A were identified to be correlated positively with age and disease duration (for age: r = 0·244, P = 0·0028; for disease duration: r = 0·213, P = 0·0085) (Fig. 1b, c).
Figure 1.
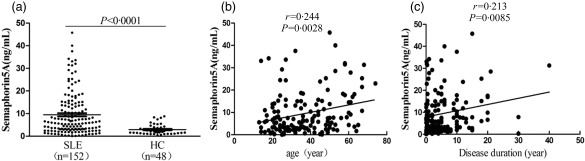
Serum semaphorin5A (Sema5A) levels in systemic lupus erythematosus (SLE) patients and healthy controls. (a) Serum Sema5A levels were significantly higher in patients with SLE than healthy controls. The concentrations of serum Sema5A were identified to be correlated positively with age and disease duration (b) and (c).
Increased serum Sema5A levels were associated with increased activity and severe clinical manifestations in SLE
In patients with SLE, there was a weak but significant positive correlation between serum Sema5A levels and SLEDAI score (r = 0·278, P = 0·0006) (Fig. 2a, Table 2). The concentrations of serum Sema5A were also found to be correlated positively with 24‐h proteinuria excretion (r = 0·558, P < 0·0001) and CRP (r = 0·266, P = 0·002) (Table 2, Fig. 2a), but negatively with PLT (r = –0·294, P = 0·0003) and C3 (r = –0·287, P = 0·0004) (Table 2, Fig. 2a) in SLE patients, which implied that patients with elevated Sema5A levels were prone to develop more severe inflammation or symptoms such as thrombocytopenia and proteinuria. Other laboratory data, including leucocyte number, C4 level, IgA, IgG, IgM, ESR and anti‐dsDNA antibody, did not show a significant correlation with serum Sema5A levels (Table 2).
Figure 2.
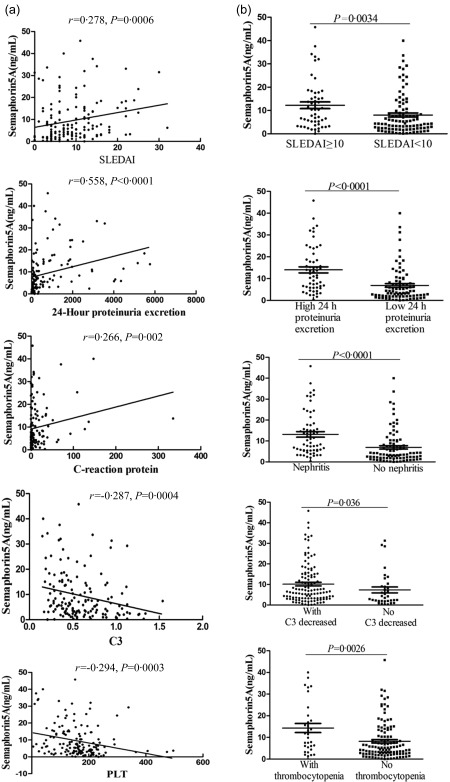
Increased serum semaphorin5A (Sema5A) levels were associated with increased activity and severe clinical manifestations in systemic lupus erythematosus (SLE). (a) Correlations of the Sema5A concentrations with SLE disease activity index (SLEDAI) score and laboratory parameters from SLE patients; (b) serum Sema5A concentrations in patients with SLEDAI score more than or equal to 10, 24‐h proteinuria excretion more than or equal to 0·1 g/day and with C3 decreased, thrombocytopenia or lupus nephritis.
Table 2.
Correlation of the semaphorin5A (Sema5A) in serum with the studied parameters in patients with systemic lupus erythematosus (SLE)
| Clinical manifestation | Sema5A (ng/ml) | |
|---|---|---|
| Spearman's r | P‐value | |
| SLEDAI | 0·278 | 0·0006 |
| 24‐h proteinuria excretion | 0·558 | < 0·0001 |
| Leucocytes | 0·013 | 0·877 |
| Thrombocytes | −0·294 | 0·0003 |
| Anti‐dsDNA antibody | 0·104 | 0·207 |
| IgG | −0·117 | 0·156 |
| IgM | −0·143 | 0·083 |
| IgA | −0·041 | 0·618 |
| ESR | 0·148 | 0·077 |
| CRP | 0·266 | 0·002 |
| C3 | −0·287 | 0·0004 |
| C4 | −0·119 | 0·148 |
SLEDAI = systemic lupus erythematosus disease activity index; anti‐dsDNA antibody = anti‐double‐strand DNA antibody; ESR = erythrocyte sedimentation rate; CRP = C‐reactive protein; C3 = complement component 3; C4 = complement component 4; Ig = immunoglobulin.
We compared further the serum Sema5A levels between patients with and without particular clinical features. In SLE patients with SLEDAI ≥ 10, serum Sema5A levels were significantly higher than those with SLEDAI < 10 (12·23 ± 1·44 ng/ml versus 8·05 ± 0·86 ng/ml, P = 0·0034). Serum Sema5A was elevated significantly in patients with nephritis, more 24‐h proteinuria excretion, thrombocytopenia or decreased C3 than without (with lupus nephritis: 13·14 ± 1·32 ng/ml versus without lupus nephritis: 6·96 ± 0·83 ng/ml, P < 0·0001; proteinuria excretion ≥ 0·1 g: 14·02 ± 1·39 ng/ml versus proteinuria excretion < 0·1 g: 6·85 ± 0·85 ng/ml, P < 0·0001; with thrombocytopenia: 14·33 ± 2·12 ng/ml versus without thrombocytopenia: 8·21 ± 0·77 ng/ml, P = 0·0026; decreased C3: 10·15 ± 0·89 ng/ml versus normal 7·35 ± 1·44 ng/ml, P = 0·036) (Fig. 2b). In summary, elevated Sema5A levels in serum were associated significantly with increased SLE disease activity and severity.
Comparison of clinical features between Sema5A‐positive and ‐negative SLE patients
The cut‐off value for positivity was established as 2 s.d. above the mean value for healthy controls. Among the 152 patients with SLE, there were no significant differences between the Sema5A‐positive and ‐negative subgroups with respect to sex, autoantibodies and most other features (Table 3). However, the Sema5A‐positive group showed a higher incidence of rash (17 of 64 versus 37 of 88; P = 0·048), serositis (19 of 64 versus six of 88; P < 0·001) and nephritis (37 of 64 versus 25 of 88; P < 0·001) compared with the Sema5A‐negative group. Moreover, the values of age, disease duration, SLEDAI and CRP were significantly higher in the Sema5A‐positive group compared with the Sema5A‐negative group (age: 41·79 ± 16·36 versus 33·92 ± 12·47; P = 0·002; disease duration: 60 (1–480) versus 30 (1–360); P = 0·003; SLEDAI: 10·50 ± 7·02 versus 8·26 ± 5·59; P = 0·038; CRP: 6·6 (0·1–334·7) versus 2 (0·1–93·1); P = 0·013;), but the values of IgG, C3 and PLT counts were lower in the Sema5A‐positive group compared with the Sema5A‐negative group (IgG: 13·61 (2·75–29·6) versus 16·3 (5·85–52·4); P = 0·023; C3: 0·52 ± 0·26 versus 0·65 ± 0·31; P = 0·007; PLT count: 130 (10–339) versus 171·5 (5–196); P < 0·001). (Table 3).
Table 3.
Comparison of clinical features between the semaphorin5A (Sema5A)‐positive group and Sema5A‐negative systemic lupus erythematosus (SLE) patients
| Sema5A (+) (n = 64) | Sema5A (–) (n = 88) | P‐value | |
|---|---|---|---|
| Age (mean ± s.d. years) | 41·79 ± 16·36 | 33·92 ± 12·47 | 0·002 |
| Sex | 60/64 | 79/88 | 0·387 |
| Disease duration (months, median) | 60 (1–480) | 30 (1–360) | 0·003 |
| Fever | 17/64 | 16/88 | 0·215 |
| Rash | 17/64 | 37/88 | 0·048 |
| Raynaud's phenomenon | 14/64 | 13/88 | 0·258 |
| Alopecia | 7/64 | 14/88 | 0·380 |
| Photosensitization | 4/64 | 14/88 | 0·069 |
| Oral ulcer | 2/64 | 7/88 | 0·213 |
| Arthritis | 11/64 | 25/88 | 0·108 |
| Pleuritis | 19/64 | 6/88 | < 0·001 |
| Lupus nephritis | 37/64 | 25/88 | < 0·001 |
| Neuropsychological lupus | 4/64 | 4/88 | 0·642 |
| ANA (+) | 61/64 | 85/88 | 0·689 |
| dsDNA antibody (+) | 40/64 | 55/88 | 1·000 |
| SSA (+) | 49/64 | 62/88 | 0·402 |
| AnuA (+) | 26/64 | 36/88 | 0·972 |
| ACL (+) | 12/64 | 17/88 | 0·930 |
| SLEDAI | 10·50 ± 7·02 | 8·26 ± 5·59 | 0·038 |
| ESR, median, mm/h | 33 (2–140) | 26 (2–140) | 0·269 |
| CRP level, median mg/l | 6·6 (0·1–334·7) | 2 (0·1–93·1) | 0·013 |
| IgA, mean ± s.d., g/l | 2·71 ± 1·75 | 2·71 ± 1·45 | 0·983 |
| IgG, median g/l | 13·61 (2·75–29·6) | 16·3 (5·85–52·4) | 0·023 |
| IgM, median g/l | 0·74 (0·18–2·91) | 0·97 (0·95–4·91) | 0·051 |
| C3, mean ± s.d., g/l | 0·52 ± 0·26 | 0·65 ± 0·31 | 0·007 |
| C4, median, g/l | 69 (20–475) | 75 (15–455) | 0·709 |
| WBC, median, ×109 | 4·7 (1·5–14·7) | 4·95 (0·4–19·8) | 0·917 |
| PLT, median, ×109 | 130 (10–339) | 171·5 (5–196) | < 0·001 |
ANA = anti‐nuclear antibody; anti‐dsDNA antibody = anti‐double strand DNA antibody; ACL = anti‐cardiolipin antibody; AnuA = anti‐nucleosome antibody; SSA = anti‐SSA antibody; SLEDAI = systemic lupus erythematosus disease activity index; ESR = erythrocyte sedimentation rate; CRP = C‐reactive protein; Ig = immunoglobulin; C3 = complement component 3; C4 = complement component 4; WBC = white blood cells; PLT = platelets; s.d. = standard deviation.
The mRNA expression of Sema5A and ADAM17 in PBMCs of SLE patients
Sema5A and ADAM17 mRNA expression were detected in both SLE patients and healthy controls. Inconsistent with serum Sema5A levels, quantitative RT–PCR analysis showed that there was no significant difference in Sema5A mRNA expression between SLE patients and healthy controls (2·55 ± 0·76 versus 1·07 ± 0·19; P > 0·05, Fig. 3a). The ADAM17 mRNA level in PBMC was significantly higher in SLE patients than in healthy controls (13·20 ± 1·40 versus 1·37 ± 0·31, P < 0·0001; Fig. 3b). There was a positive correlation between ADAM17 mRNA and serum Sema5A levels (r = 0·86, P = 0·024; Fig. 4).
Figure 3.
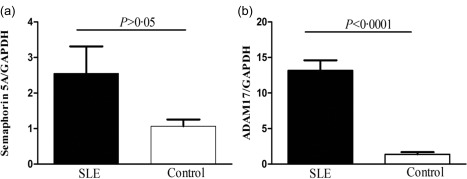
The mRNA expression of semaphorin5A (Sema5A) and ADAM metallopeptidase domain 17 (ADAM17) in peripheral blood mononuclear cells (PBMC) systemic lupus erythematosus (SLE) SLE patients. (a) There was no significant difference in Sema5A mRNA expression between SLE patients and healthy controls. (b) The ADAM17 mRNA level in peripheral blood mononuclear cells (PBMCs) was significantly higher in SLE patients than in healthy controls.
Figure 4.
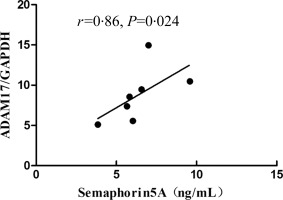
There was a positive correlation between semaphorin5A (Sema5A) levels in serum and ADAM metallopeptidase domain 17 (ADAM17) mRNA expression in peripheral blood mononuclear cells (PBMCs).
Discussion
The semaphorins were discovered originally in the nervous system, where they have been involved in repulsive axon guidance during nervous system development 25, 26. They are a large family (classified into eight subclasses) of secreted and membrane‐bound proteins, characterized by a distinct cysteine‐rich domain of approxinately 500 amino acids, called the ‘sema domain’. Classes 2 and 3 semaphorins are secreted proteins, classes 1, 4, 5 and 6 semaphorins are membrane proteins and class 7 semaphorins are glycosylphosphatidylinositol (GPI)‐anchored proteins. Two families of semaphoring receptors have been identified: the neuropilins (Np‐1 and Np‐2) and plexins 27.
In recent years, there has been a large growing body of data regarding the involvement of semaphorins in the regulation of the immune system (‘immune semaphorins’). They were found in the microenvironmental cells of the developing murine thymus, suggesting that they are involved in the guidance of T precursor cells into the thymus by chemorepulsion 28.
Several semaphorin members were found in association with autoimmune diseases. The expression of the Sema4D molecule on peripheral blood lymphocytes of lupus nephritis patients correlated with clinical status 29. Altered serum Sema3A levels were found to be in inverse correlation with SLE disease activity, mainly with renal damage and the presence of anti‐cardiolipin antibodies; it has a regulatory mode of action, with proven abilities of decreasing Toll‐like receptor (TLR)−9 expression in memory B cells of SLE patients 30, 31.
As one of the semaphorin family members, Sema5A and its receptors were characterized originally as constituents of the complex regulatory system responsible for the wiring of neural networks during the development of the central nervous system, and were found subsequently to participate in activities outside the nervous system, such as migration of neural crest cells and heart development, to name but a few examples. A recent study suggested a role for Sema5A in innate immune signalling pathways during mastitis in dairy cows. Moreover, Sema5A potently modulate immune cell responses in relation to disease activity in RA and ITP patients.
Deregulation of innate immunity and clearance of apoptotic cells have been implicated in the pathogenesis of SLE 32, 33. In SLE, cell debris produced by impaired apoptosis may serve as danger signals to break immune tolerance and result in autoimmune inflammation and autoantibody production 34. In this study, we demonstrated for the first time, to our knowledge, that serum levels of Sema5A were increased significantly in SLE patients compared with healthy controls (P < 0·001). Serum levels of Sema5A were correlated positively with 24‐h proteinuria excretion (r = 0·558, P < 0·0001), SLEDAI (r = 0·278, P < 0·001) and CRP (r = 0·266, P = 0·002), but negatively with PLT (r = –0·294, P < 0·001) and C3 (r = –0·287, P < 0·001) in SLE patients. Taken together, these results demonstrate that Sema5A might play an important role in the development of SLE.
In terms of clinical and laboratory variables, Sema5A‐positive and negative SLE patients were similar, with the notable exception that the Sema5A‐positive group showed a higher incidence of rash, serositis and nephritis compared with the Sema5A‐negative group. Moreover, the values of age, disease duration, SLEDAI and CRP were significantly higher in the Sema5A‐positive group than the Sema5A‐negative group, but the values of IgG, C3 and PLT counts were lower in the Sema5A‐positive than the Sema5A‐negative group.
Evidence of elevated Sema5A expression has been reported in PBMCs at the RNA level, suggesting that PBMCs might be a major source of secreted Sema5A 21. In our study, contrary to increased serum Sema5A levels in SLE patients, Sema5A mRNA expression in PBMCs was not up‐regulated significantly, which indicated that increased soluble Sema5A did not result from Sema5A expression, but was associated with more protein off to serum. Membrane Sema5A was reported to be shed into soluble forms through ADAM‐17‐dependent cleavage and circulated in plasma. Our study showed clearly that ADAM17 expression in PBMCs was correlated positively with Sema5A levels in serum, which might explain the inconsistency between the serum levels of Sema5A and the mRNA expression of Sema5A in our study.
To demonstrate further that increased serum levels of sema5A are due to increased shedding, we assessed the expression of Sema5A on the membrane of PBMCs and compared this between SLE and healthy controls. Western blot analysis showed that membrane Sema5A levels were decreased significantly in SLE patients compared with healthy controls (ratio of Sema5A/Na/K ATPase SLE: 0·29 ± 0·14; HC: 1·17 ± 0·62, P < 0·001, Supporting information, Fig. S1), which indicated that increased serum levels of Sema5A in SLE patients rely upon increased shedding.
In conclusion, although our results stem from a single‐centre study with a relatively small sample size that could bias the association between Sema5A and SLE, we present initial evidence that serum Sema5A increased significantly in SLE and correlated positively with disease activity and severity. The up‐regulation of serum Sema5A may serve as a biomarker of disease activity and severity of SLE. Further investigation into the exact role of Sema5A signalling will provide novel insights into the pathogenesis of SLE.
Disclosure
The authors declare no conflicts of interest regarding this work.
Author contributions
Y. D. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: Y. D., J. L., H. W.; acquisition of data: Y. D., X. W., M. C., W. W., L. Y., H. W., D. W., J. X., W. S., H. W.; analysis and interpretation of data: Y. D., H. Wu; manuscript preparation: Y. D., J. L., H. W.; statistical analysis: Y. D., H. W.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's website.
Fig. S1. Decreased membrane semaphorin5A (Sema5A) on peripheral blood mononuclear cells (PBMCs) in patients with systemic lupus erythematosus (SLE) was detected. Membrane protein was extracted from the PBMCs of 11 SLE patients and 11 healthy controls. Sema5A protein expression levels were detected using Western blot. Na/K ATPase was used as a protein loading control. A set of random data from six SLE patients and six healthy controls is presented (P < 0·001).
Acknowledgements
This study was supported in part by Zhejiang Provincial Public Technology Applied Research Project (no. 2015C33177), Zhejiang Provincial Medical Science and Technology Plan Project (no. 2017KY381), National Natural Science Foundation of China (no. 81501388), Jiangsu Provincial Special Program of Medical Science (no. BE2015631), Natural Science Foundation of Jiangsu (no. BK20151174), Scientific Research of Changzhou (no. CJ20159038; no.CE20155046) and Changzhou High Level Medical Talents Training Project (no. 2016C2BJ016).
References
- 1. Liu CC, Kao AH, Manzi S, Ahearn JM. Biomarkers in systemic lupus erythematosus: challenges and prospects for the future. Ther Adv Musculoskelet Dis 2013; 5:210–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Plotz PH. The autoantibody repertoire: searching for order. Nat Rev Immunol 2003; 3:73–8. [DOI] [PubMed] [Google Scholar]
- 3. Takazoe K, Shimada T, Nakano H et al Massive uncomplicated vascular immune complex deposits in the kidney of a patient with systemic lupus erythematosus. Clin Nephrol 1997; 48:195–8. [PubMed] [Google Scholar]
- 4. Kozora E, Filley CM, Zhang L et al Immune function and brain abnormalities in patients with systemic lupus erythematosus without overt neuropsychiatric manifestations. Lupus 2012; 21:402–11. [DOI] [PubMed] [Google Scholar]
- 5. Kumar KR, Li L, Yan M et al Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science 2006; 312:1665–9. [DOI] [PubMed] [Google Scholar]
- 6. Crow MK. Collaboration, genetic associations, and lupus erythematosus. N Engl J Med 2008; 358:956–61. [DOI] [PubMed] [Google Scholar]
- 7. Kolodkin AL. Semaphorins: mediators of repulsive growth cone guidance. Trends Cell Biol 1996; 6:15–22. [DOI] [PubMed] [Google Scholar]
- 8. Takamatsu H, Okuno T, Kumanogoh A. Regulation of immune cell responses by semaphorins and their receptors. Cell Mol Immunol 2010; 7:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X, Kumanogoh A, Watanabe C, Shi W, Yoshida K, Kikutani H. Functional soluble CD100/Sema4D released from activated lymphocytes: possible role in normal and pathologic immune responses. Blood 2001; 97:3498–504. [DOI] [PubMed] [Google Scholar]
- 10. Kim CW, Cho EH, Lee YJ, Kim YH, Hah YS, Kim DR. Disease‐specific proteins from rheumatoid arthritis patients. J Korean Med Sci 2006; 21:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okuno T, Nakatsuji Y, Kumanogoh A. The role of immune semaphorins in multiple sclerosis. FEBS Lett 2011; 585:3829–35. [DOI] [PubMed] [Google Scholar]
- 12. Adams RH, Betz H, Puschel AW. A novel class of murine semaphorins with homology to thrombospondin is differentially expressed during early embryogenesis. Mech Dev 1996; 57:33–45. [DOI] [PubMed] [Google Scholar]
- 13. Matsuoka RL, Chivatakarn O, Badea TC et al Class 5 transmembrane semaphorins control selective mammalian retinal lamination and function. Neuron 2011; 71:460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Artigiani S, Conrotto P, Fazzari P et al Plexin‐B3 is a functional receptor for semaphorin 5A. EMBO Rep 2004; 5:710–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadanandam A, Sidhu SS, Wullschleger S et al Secreted semaphorin 5A suppressed pancreatic tumour burden but increased metastasis and endothelial cell proliferation. Br J Cancer 2012; 107:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sadanandam A, Rosenbaugh EG, Singh S, Varney M, Singh RK. Semaphorin 5A promotes angiogenesis by increasing endothelial cell proliferation, migration, and decreasing apoptosis. Microvasc Res 2010; 79:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sadanandam A, Varney ML, Singh S et al High gene expression of semaphorin 5A in pancreatic cancer is associated with tumor growth, invasion and metastasis. Int J Cancer 2010; 127:1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sadanandam A, Varney ML, Kinarsky L, Ali H, Mosley RL, Singh RK. Identification of functional cell adhesion molecules with a potential role in metastasis by a combination of in vivo phage display and in silico analysis. Omics 2007; 11:41–57. [DOI] [PubMed] [Google Scholar]
- 19. Pan GQ, Ren HZ, Zhang SF, Wang XM, Wen JF. Expression of semaphorin 5A and its receptor plexin B3 contributes to invasion and metastasis of gastric carcinoma. World J Gastroenterol 2009; 15:2800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sugimoto M, Fujikawa A, Womack JE, Sugimoto Y. Evidence that bovine forebrain embryonic zinc finger‐like gene influences immune response associated with mastitis resistance. Proc Natl Acad Sci USA 2006; 103:6454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gras C, Eiz‐Vesper B, Jaimes Y et al Secreted semaphorin 5A activates immune effector cells and is a biomarker for rheumatoid arthritis. Arthritis Rheumatol 2014; 66:1461–71. [DOI] [PubMed] [Google Scholar]
- 22. Lyu M, Li Y, Hao Y et al Elevated Semaphorin 5A correlated with Th1 polarization in patients with chronic immune thrombocytopenia. Thromb Res 2015; 136:859–64. [DOI] [PubMed] [Google Scholar]
- 23. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 24. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992; 35:630–40. [DOI] [PubMed] [Google Scholar]
- 25. Yazdani U, Terman JR. The semaphorins. Genome Biol 2006; 7:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Semaphorin Nomenclature Committee. Unified nomenclature for the semaphorins/collapsins. Cell 1999; 97:551–2. [DOI] [PubMed] [Google Scholar]
- 27. Negishi M, Oinuma I, Katoh H. Plexins: axon guidance and signal transduction. Cell Mol Life Sci CMLS 2005; 62:1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takahashi K, Ishida M, Hirokawa K, Takahashi H. Expression of the semaphorins Sema 3D and Sema 3F in the developing parathyroid and thymus. Dev Dyn 2008; 237:1699–708. [DOI] [PubMed] [Google Scholar]
- 29. Vadasz Z, Attias D, Kessel A, Toubi E. Neuropilins and semaphorins – from angiogenesis to autoimmunity. Autoimmun Rev 2010; 9:825–9. [DOI] [PubMed] [Google Scholar]
- 30. Vadasz Z, Haj T, Halasz K et al Semaphorin 3A is a marker for disease activity and a potential immunoregulator in systemic lupus erythematosus. Arthritis Res Ther 2012; 14:R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vadasz Z, Toubi E. Semaphorin 3A – a marker for disease activity and a potential putative disease‐modifying treatment in systemic lupus erythematosus. Lupus 2012; 21:1266–70. [DOI] [PubMed] [Google Scholar]
- 32. Choi J, Kim ST, Craft J. The pathogenesis of systemic lupus erythematosus – an update. Curr Opin Immunol 2012; 24:651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaipl US, Munoz LE, Grossmayer G et al Clearance deficiency and systemic lupus erythematosus (SLE). J Autoimmun 2007; 28:114–21. [DOI] [PubMed] [Google Scholar]
- 34. Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol 2010; 6:280–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's website.
Fig. S1. Decreased membrane semaphorin5A (Sema5A) on peripheral blood mononuclear cells (PBMCs) in patients with systemic lupus erythematosus (SLE) was detected. Membrane protein was extracted from the PBMCs of 11 SLE patients and 11 healthy controls. Sema5A protein expression levels were detected using Western blot. Na/K ATPase was used as a protein loading control. A set of random data from six SLE patients and six healthy controls is presented (P < 0·001).


