Summary
Ageing is associated with changes in the peripheral T cell immune system, which can be influenced significantly by latent cytomegalovirus (CMV) infection. To what extent changes in circulating T cell populations correlate with T cell composition of the lymph node (LN) is unclear, but is crucial for a comprehensive understanding of the T cell system. T cells from peripheral blood (PB) and LN of end‐stage renal disease patients were analysed for frequency of recent thymic emigrants using CD31 expression and T cell receptor excision circle content, relative telomere length and expression of differentiation markers. Compared with PB, LN contained relatively more CD4+ than CD8+ T cells (P < 0·001). The percentage of naive and central memory CD4+ and CD8+ T cells and thymic output parameters showed a strong linear correlation between PB and LN. Highly differentiated CD28null T cells, being CD27–, CD57+ or programmed death 1 (PD‐1+), were found almost exclusively in the circulation but not in LN. An age‐related decline in naive CD4+ and CD8+ T cell frequency was observed (P = 0·035 and P = 0·002, respectively) within LN, concomitant with an increase in central memory CD8+ T cells (P = 0·033). Latent CMV infection increased dramatically the frequency of circulating terminally differentiated T cells, but did not alter T cell composition and ageing parameters of LN significantly. Overall T cell composition and measures of thymic function in PB and LN are correlated strongly. However, highly differentiated CD28null T cells, which may comprise a large part of circulating T cells in CMV‐seropositive individuals, are found almost exclusively within the circulation.
Keywords: ageing, end‐stage renal disease, lymph node, peripheral blood, T cells
Introduction
One of the dominant aspects of the ageing process in the T cell immune system is a decreased thymic output of newly formed T cells, named recent thymic emigrants (RTE). This is evident from a decrease in circulating naive T cells expressing CD31, and by a decrease in T cell receptor excision circles (TREC) content 1, 2, 3, 4, 5. Furthermore, an increase in age leads to a decline in the frequency of circulating naive T cells, while the memory T cell compartment may contain more terminally differentiated T cells 6, 7. This progressive T cell differentiation is also marked by the loss of the co‐stimulatory molecule CD28 on the surface of memory T cells 8, which enables these cells to become activated without the need for co‐stimulation. Next to the loss of CD28, the loss of CD27 and the expression of CD57 and programmed death 1 (PD‐1) are indicators of terminal T cell differentiation 9, 10, 11. Lastly, the increased number of rounds of T cell proliferation and differentiation reduces the telomere length of T cells 12, 13.
Cytomegalovirus (CMV) infection needs to be taken into account when the T cell compartment is analysed, as this infection may cause a significant imprint on the differentiation status of circulating T cells 8, 14. In general, it leads to an expansion of late‐differentiated CD4+ and CD8+ T cells and an inverted CD4 : CD8 T cell ratio 15. Furthermore, CMV infection may lead to a reduction in telomere length of T cells 16.
The impact of an aged circulating T cell population has been studied, for example, in relation to the risk for infection, decreased vaccination response and cardiovascular events 17, 18, 19. In addition, the presence of highly differentiated CD28null T cells in the peripheral blood has gained considerable interest in the field of solid organ transplantation, as they may be instrumental in co‐stimulatory blockade‐resistant rejection and long‐term allograft (dys)function 17, 20, 21, 22, 23. Analysis of the T cell population in lymph nodes (LNs) might prove to be of additional/higher value, as immune responses are initiated in secondary lymphoid organs. Until now, little is known about how changes in circulatory T cells relate to changes in the LN, and only a few studies have investigated the effect of ageing in secondary lymphoid organs 24, 25.
In this study, we investigated the relation between T cell parameters of the LN and PB compartments in end‐stage renal disease (ESRD) patients. More specifically, ageing parameters such as thymic output, relative telomere length (RTL), terminal differentiation markers and the influence of CMV infection on these were studied. Our results show that overall T cell composition and measures of thymic function are correlated strongly between PB and LN. However, highly differentiated CD28null T cells, which may comprise a large part of circulating T cells in CMV‐seropositive individuals, are virtually absent in LNs.
Materials and methods
Study population
Patients prior to kidney transplantation in the period from August 2015 to March 2016 were eligible for participation. All patients gave written informed consent to participate in this study. The study was approved by the Medical Ethical Committee of the Erasmus MC (MEC number 2015‐301) and was conducted in accordance with the Declaration of Helsinki and the Declaration of Istanbul.
Clinical variables were assessed as shown in Table 1, including age, gender, CMV‐seropositivity, underlying cause of renal failure, dialysis prior to transplantation and dialysis vintage (defined as time on dialysis since the start of any type of dialysis).
Table 1.
Patient characteristics
| RT patients (n = 38) | |
|---|---|
| Age | 58 (45–65) |
| Male | 24 (63%) |
| CMV‐seropositivity | 28 (74%) |
| Underlying disease | |
| Nephrosclerosis/atherosclerosis/hypertension | 11 (29%) |
| Primary glomerulopathies | 5 (13%) |
| Diabetes | 7 (18%) |
| Urinary tract infections/stones | 0 (0%) |
| Reflux nephropathy | 0 (0%) |
| Polycystic kidney disease | 8 (21%) |
| Other | 3 (8%) |
| Unknown | 4 (11%) |
| Dialysis prior to transplantation | 23 (61%) |
| Dialysis vintage (years) | 2.4 (0·9–3·3) |
Data are presented as median (interquartile range) or as number (percentage). RT = renal transplantation; CMV = cytomegalovirus.
PBMC isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples by using Ficoll‐Paque Plus (GE Healthcare, Uppsala, Sweden). Blood was obtained from renal transplant recipients 1 day prior to renal transplantation (RT). The isolated PBMCs were stored at −150°C with a minimum amount of 10 × 106 cells per vial. The median frequency of lymphocytes within heparinized blood amounted to 21·9% (17·7–26·6%) and following density gradient centrifugation this frequency amounted to 72·6% (64·7–76·7%).
LNMC isolation
Iliac LNs were isolated from tissue, which was obtained as waste material from renal transplant recipients at the time of implantation of the renal allograft. Upon arrival of the material at the laboratory, excessive fat tissue around the LNs was removed. Next, the LNs were minced and subsequently mashed through a 70‐µm sieve to obtain a cell suspension. Then, the cell suspension was centrifuged several times to remove platelets and remaining fat tissue. The isolated lymph node mononuclear cells (LNMCs) were stored at −150°C with a minimum amount of 10 × 106 cells per vial. The median yield of a lymph node was 20 × 106 cells. The median frequency of lymphocytes within LNMNCs amounted to 94·8% (92·0–96·3%).
T cell differentiation status and absolute numbers of T cell subsets
T cell differentiation status was determined through whole blood staining and on freshly isolated LNMCs, which was based on the study by Sallusto et al. 26, as described in detail previously 27. These cell samples were measured on the fluorescence activated cell sorter (FACS)Canto II (BD Biosciences, San Jose, CA, USA) and analysed with FACS Diva software version 6.1.2 (BD Biosciences). Briefly, differential cell surface expression of CD45RO (pan‐T cell marker for memory T cells) and CCR7 (a chemokine receptor involved in homing of T cells to various secondary lymphoid organs such as lymph nodes) was used to distinguish naive from different memory T cell populations. Memory T cells differentiate into central memory (CM) T cells (CD45RO+CCR7+ T cells, able to access the lymphoid tissue), effector memory (EM) T cells (CD45RO+CCR7– T cells) and EM CD45RA+ (EMRA) T cells (highly differentiated T cells re‐expressing CD45RA but CCR7–) 26. A typical example of the gating strategy is shown in Supporting information, Fig. S1.
In addition to this definition of memory T cells, the expression of CD28, CD27, CD57 and PD‐1 was used to divide the T cell compartment in early or late differentiated T cells. A typical example of the gating strategy is shown in Supporting information, Fig. S2. Essentially, the naive and CM T cells are early differentiated T cells and still express the co‐stimulatory molecules CD28 and CD27. EM and EMRA T cells may progressively lose CD28 and subsequently CD27 expression and gain expression of CD57 and PD‐1 9, 10, 11, 28. Next to this, examining expression of CD28 together with CD27 is of additional value, as the order of loss of these molecules differs between CD4+ and CD8+ T cells (i.e. CD4+ T cells first lose CD27 before losing CD28, while for the CD8+ T cells this is the other way around) 29, 30, 31. Early differentiated T cells were defined as CD28+CD27+, CD28+PD‐1– and CD28+CD57– T cells. T cells that were CD28nullCD27–, CD28nullPD‐1+ and CD28nullCD57+ were defined as late differentiated memory T cells. This measurement was performed on the Navios flow cytometer (Beckman Coulter, Brea, CA, USA) and the obtained data were analysed with Kaluza software version 1.3 (Beckman Coulter). The T cells were analysed with DuraClone IM T cell subsets tubes (Beckman Coulter), which were coated with allophycocyanin (SAPC) Alexa Fluor 750 (APC‐A750) anti‐CD3, APC‐labelled anti‐CD4, Alexa Fluor 700 (A700)‐labelled anti‐CD8, fluorescein isothiocyanate (FITC)‐labelled anti‐CD45RA, phycoerythrin (PE)‐labelled anti‐CCR7, phycoerythrin Texas red (ECD)‐labelled anti‐CD28, phycoerythrin‐cyanine 7 (PE‐Cy7)‐labelled anti‐CD27, phycoerythrin‐cyanine 5.5 (PE‐Cy5.5‐ labelled anti‐PD1, pacific blue (PacB)‐labelled anti‐CD57 and krome orange (KO)‐labelled anti‐CD45.
Relative telomere length
To determine the RTL of CD4+ and CD8+ T cells, flow fluorescence in‐situ hybridization was performed on thawed PBMCs and LNMCs, as described in detail previously 27.
Assesment of recent thymic emigrants using CD31 and TREC content
CD31+ naive T cells were assessed by flow cytometry as a measure of recent thymic emigrants (RTE), as decribed previously 32. TREC content was determined using 1 × 106 snap‐frozen PBMCs and LNMCs. DNA was isolated from these snap‐frozen samples and the TREC content, depicted by ΔCT (which is related inversely to the TREC content), was determined using quantitative polymerase chain reaction (PCR) as described previously 33.
Statistical analysis
All variables are presented as medians with interquartile ranges. The differences between paired samples (i.e. PB and LN T cell ageing parameters of the same ESRD patients) were analysed using the Wilcoxon signed‐rank test. Differences between continuous variables from two independent groups (i.e. CMV‐seropositive versus CMV‐seronegative ESRD patients) were assessed with the Mann–Whitney U‐test. Correlations between PB and LN were calculated using Spearman's rank correlation. Relationships between age and the different T cell ageing parameters were assessed using a linear regression analysis. The significance level (P‐value) was two‐tailed and an α of 0·05 was used for all analyses. Statistical analyses were performed using SPSS version 21.0 for Windows® (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5 (San Diego, CA, USA). Figures were created with GraphPad Prism 5.
Results
Patient characteristics
Patient characteristics (n = 38) are shown in Table 1. The median patient age was 58 years. Most of the patients were CMV immunoglobulin (Ig)G+ (74%). The major cause of ESRD was nephrosclerosis/atherosclerosis/hypertension (29%), followed by polycystic kidney disease (21%), which together accounted for half the cases.
CD4+ T cell composition of the lymph node and peripheral blood
The median frequency of CD4+ T cells was significantly higher in LN samples compared with the PB (P < 0·001) (Fig. 1a), but the relative distribution of CD4+ naive T cells was similar (Fig. 1b). However, compared with PB, the LN contained higher frequencies of CD4+ CM T cells (P < 0·001) (Fig. 1c) and lower frequencies of CD4+ EM T cells and EM CD45RA+ (EMRA) T cells (P < 0·001 and P = 0·026, respectively) (Fig. 1d,e). Except for the frequency of CD4+ EMRA T cells, frequencies of CD4+ T cells, CD4+ naive T cells, CD4+ CM T cells and CD4+ EM T cells showed a remarkably good correlation between PB and LN (Fig. 1).
Figure 1.
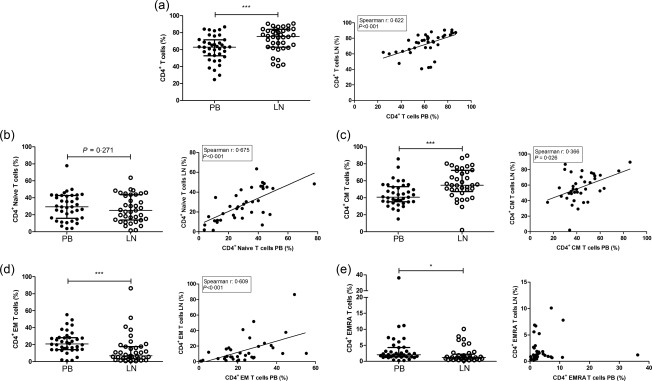
CD4+ T cell subsets in the peripheral blood (PB) and the lymph node (LN) and the correlation between PB and LN. The frequencies and correlations of (a) CD4+ T cells within CD3+ T cells, (b) CD4+ naive T cells within CD4+ T cells, (c) CD4+ central memory (CM) T cells within CD4+ T cells, (d) CD4+ effector memory (EM) T cells within CD4+ T cells and (e) CD4+ effector memory CD45RA+ (EMRA) T cells within CD4+ T cells are represented. Frequencies of cells are depicted as individual percentages as well as median and interquartile range (left plots). A Spearman's rho was shown only when a significant correlation was present; n = 38 for peripheral blood and n = 38/37 for lymph node samples. Significant differences were calculated and shown (*P < 0·05; **P ≤ 0·01; ***P ≤ 0·001).
Next, the early versus late T cell differentiation was analysed by measuring the expression of CD27, PD‐1 and CD57 on CD28+ and CD28null T cells (Table 2). Within the CD4+ T cell population, the frequency of CD28null T cells was significantly lower in LN compared with PB (1·0%, range 0·6–1·6% versus 1·8%, range 0·7–8·8; P = 0·001) and late memory T cell subpopulations, either defined as CD28nullCD27– T cells, CD28nullPD‐1+ T cells or CD28nullCD57+ T cells, were virtually absent in LN (P < 0·001 for all comparisons). Instead, the percentage of CD4+ T cells with full expression of CD28 and CD27 (CD28+CD27+) was significantly higher in the LN (Table 2).
Table 2.
CD27, programmed death 1 (PD‐1) and CD57 expression on CD28null T cells in the peripheral blood and lymph node
| PB (n = 38) | LN (n = 33) | P | |
|---|---|---|---|
| CD4+ T cells | |||
| CD28+CD27+ (%) | 87·4 (81·5–94·3) | 93·8 (91·6–96·1) | < 0·001 |
| CD28nullCD27– (%) | 1·1 (0·2–7·5) | 0·3 (0·1–0·6) | < 0·001 |
| CD28+PD–1– (%) | 78·5 (71·0–84·0) | 74·2 (65·8–81·6) | 0·077 |
| CD28nullPD–1+ (%) | 1·0 (0·3–7·3) | 0·4 (0·3–0·9) | 0·004 |
| CD28+CD57– (%) | 96·7 (90·1–98·7) | 97·4 (96·0–98·2) | 0·063 |
| CD28nullCD57+ (%) | 0·8 (0·1–5·9) | 0·1 (0·0–0·2) | < 0·001 |
| CD8+ T cells | |||
| CD28+CD27+ (%) | 54·8 (36·7–73·7) | 89·8 (84·1–92·6) | < 0·001 |
| CD28nullCD27– (%) | 28·7 (10·8–49·3) | 1·9 (1·0–2·6) | < 0·001 |
| CD28+PD–1– (%) | 43·1 (25·9–57·5) | 63·2 (49·5–75·1) | < 0·001 |
| CD28nullPD–1+ (%) | 12·4 (6·9–18·4) | 2·4 (1·6–5·1) | < 0·001 |
| CD28+CD57– (%) | 56·2 (37·5–73·9) | 87·3 (80·2–89·7) | < 0·001 |
| CD28nullCD57+ (%) | 26·6 (13·4–39·1) | 1·5 (1·0–2·3) | < 0·001 |
Data are presented as medians (interquartile range). PB = peripheral blood; LN = lymph node.
In summary, the composition of the CD4+ T cell compartment in PB and LN are highly inter‐related, except for the absence of late differentiated T cells, which almost exclusively appear in PB.
CD8+ T cell composition of the lymph node and peripheral blood
The frequency of CD8+ T cells was significantly lower in LN than in PB with a relative higher proportion of naive T cells (P < 0·001 for both variables) (Fig. 2a,b). The CD8+ memory T cell population in LN contained relatively more CM T cells (P < 0·001) (Fig. 2c) and fewer highly differentiated CD8+ EMRA T cells (P < 0·001) (Fig. 2e).
Figure 2.
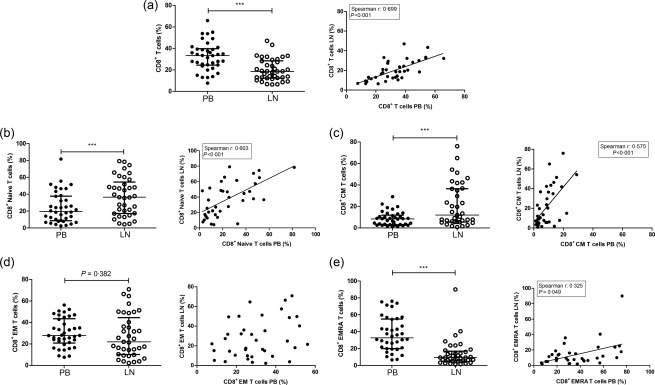
CD8+ T cell subsets in the peripheral blood (PB) and the lymph node (LN) and the correlation between PB and LN. The frequencies and correlations of (a) CD8+ T cells within CD3+ T cells, (b) CD8+ naive T cells within CD8+ T cells, (c) CD8+ central memory (CM) T cells within CD8+ T cells, (d) CD8+ effector memory (EM) T cells within CD8+ T cells and (e) CD8+ effector memory CD45RA+ (EMRA) T cells within CD8+ T cells are represented. Frequencies of cells are depicted as individual percentages as well as median and interquartile range (left plots). A Spearman's rho was shown only when a significant correlation was present; n = 38 for peripheral blood and n = 38/37 for lymph node samples. Significant differences were calculated and shown (*P < 0·05; **P ≤ 0·01; ***P ≤ 0·001).
All CD8+ T cell subsets, except for EM T cells, were correlated positively between the two different compartments (Fig. 2). Specifically, the frequency of total CD8+ T cells, CD8+ naive T cells and CD8+ CM T cells showed a very close correlation.
Differentiated CD8 + CD28null T cells were significantly less frequent in LN than in PB (10·6%, range 7·5–15·0% versus 41·5%, range 23·8–64·0%; P < 0·001).
The expression of CD27, PD‐1 and CD57 in relation to CD28 expression showed similar results within the CD8+ T cell population as those obtained for the CD4+ T cells (Table 2). The frequencies of late differentiated T cells (CD8+CD28null T cells lacking CD27–, expressing PD‐1 or CD57) were high in PB, but few of these cells were found in LN (P < 0·001). In contrast, LN contained significantly more T cells expressing CD28 and CD27.
In summary, similar to the CD4+ T cells, the composition of the CD8+ T cell compartment of PB and LN was highly inter‐related, but late differentiated CD8+CD28null T cells were confined to the circulating CD8+ T cell pool.
Recent thymic emigrants and RTL in the lymph node and peripheral blood
Frequencies of CD31+ naive T cells were significantly lower in LN than in PB for both CD4+ and CD8+ T cells (P < 0·001 and P = 0·008, respectively) (Fig. 3,b). Frequencies of CD31+CD4+ and CD31+CD8+ naive T cells in PB and LN were correlated highly (P < 0·001 for both variables) (Fig. 3a,b). Furthermore, the TREC content in the lymphocyte population, which is another measure of the number of RTE, was significantly higher (i.e. lower ΔCT) in the LN (P = 0·002) (Fig. 3c) compared with PB. The TREC content also showed a significant positive correlation between PB and LN (Fig. 3c). Combining both parameters, a significant negative correlation between the frequency of CD31+CD4+ and CD8+ naive T cells and ΔCT in both the PB and LN was observed (Supporting information, Fig. S3). Interestingly, the RTL of both CD4+ as well as CD8+ T cells was similar between PB and LN (Fig. 3d,e).
Figure 3.
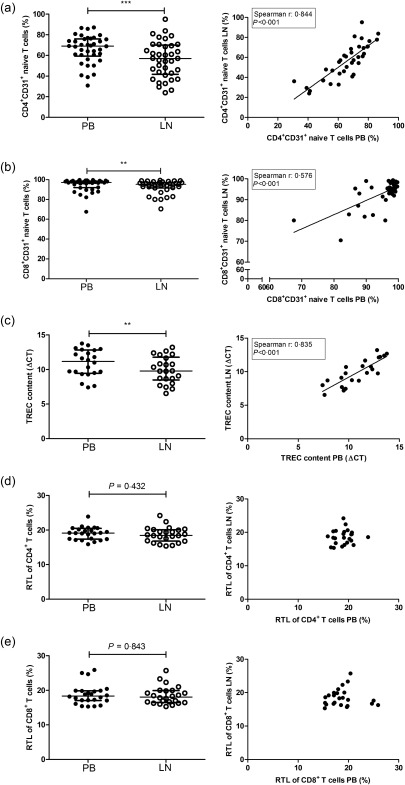
CD31+ naive T cell frequency, T cell receptor excision circles (TREC) content and relative telomere length (RTL) in the peripheral blood (PB) and lymph node (LN) with correlations. The frequencies and correlations of (a) CD4+CD31+ naive T cells, (b) CD8+CD31+ naive T cells, (c) TREC content, (d) CD4+ RTL and (e) CD8+ RTL for both the peripheral blood and the lymph node are represented. Frequencies of CD4+ and CD8+CD31+ naive T cells and RTL are depicted as percentages as well as median and interquartile range. The ΔCT is related inversely to the TREC content; n = 38 for peripheral blood and n = 37 for lymph node samples with regard to the analysis of CD4+CD31+ naive T cells and CD8+CD31+ naive T cells; n = 22 for analysis of the TREC content and n = 24 for the analysis of RTL in both T cell subsets. Significant differences were calculated and shown (*P < 0·05; **P ≤ 0·1; ***P ≤ 0·001).
Age‐related changes of T cell characteristics in the lymph node
Figure 4 shows the T cell characteristics that were associated significantly with age. A negative association between age and frequency of CD4+ naive T cells was observed (B = –0·42, P = 0·035) (Fig. 4a), while the frequencies of CD4+ memory T cells increased with age (B = 0·42, P = 0·035) (Fig. 4b). The expression of CD27, PD‐1 and CD57 in combination with CD28 expression within the CD4+ T cell population did not show any associations with age (data not shown).
Figure 4.
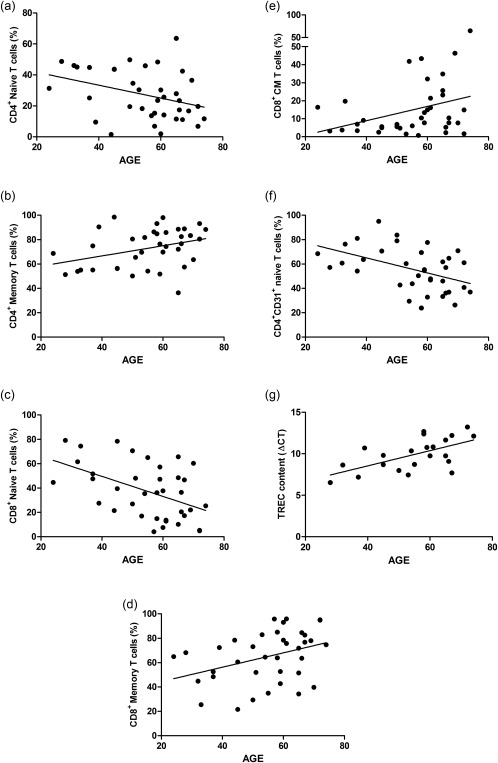
Effect of age on T cell ageing parameters in the lymph node (LN). Linear regression analysis between age and (a) CD4+ naive T cells, (b) CD4+ memory T cells, (c) CD8+ naive T cells, (d) CD8+ memory T cells, (e) CD8+ central memory (CM) T cells and (f) CD4+CD31+ naive T cells and (g) T cell receptor excision circles (TREC) content are represented. Frequencies of cells are depicted with percentages and the TREC content is depicted with ΔCT. Only significant associations are shown.
Similarly, frequencies of naïve CD8+ T cells were associated negatively with age (B = –0.82, P = 0·002) (Fig. 4c), whereas those of CD8+ memory T cells revealed a positive association with age (B = 0·59, P = 0·023) (Fig. 4d). Next to this, also the frequency of CD8+ CM T cells was associated positively with age (B = 0·40, P = 0·033) (Fig. 4e). Within the CD8+ T cell population, frequencies of early differentiated T cells, i.e. CD28+CD27+ and CD28+PD1– T cells were decreased with increasing age (B = –0·20, P = 0·028 and B = –0·50, P = 0·043, respectively).
Assessment of thymic output also revealed age‐associated changes within the LN. Frequencies of CD4+ RTE decreased with age (B = –0·62, P = 0·004) (Fig. 4f), as did the TREC content (i.e. higher ΔCT) (B = 0·09, O = 0·003) (Fig. 4g). Conversely, the RTL of both the CD4+ and the CD8+ T cells in the LN showed no association with age (data not shown).
CMV infection does not change lymph node T cell characteristics
In accordance with previous studies 8, 14, 15, CMV infection altered the peripheral T cell compartment leading to relatively more CD8+ T cells, as well as increased frequencies of CD4+CD28null and CD8+CD28null T cells showing decreased expression of CD27 and increased expression of PD‐1 and CD57 (Tables 3 and 4).
Table 3.
CD4+ T cell differentiation status in lymph node and peripheral blood for CMV+ and CMV– patients
| n | CMV+ | n | CMV– | P | |
|---|---|---|---|---|---|
| Lymph node | |||||
| CD4+ T cells (%) | 28 | 74·3 (62·5–80·1) | 10 | 83·0 (68·6–87·0) | 0·094 |
| CD4+ naive T cells (%) | 27 | 30·2 (13·6–43·7) | 10 | 18·9 (12·3–30·7) | 0·313 |
| CD4+ memory T cells (%) | 27 | 69·8 (56·3–86·4) | 10 | 81·1 (69·3–87·7) | 0·313 |
| CD4+ CM T cells (%) | 27 | 54·8 (43·1–68·3) | 10 | 63·8 (49·5–76·5) | 0·383 |
| CD4+ EM T cells (%) | 27 | 10·5 (4·6–17·8) | 10 | 4·7 (1·9–22·4) | 0·494 |
| CD4+ EMRA T cells (%) | 27 | 1·1 (0·8–1·9) | 10 | 1·8 (0·6–2·6) | 0·620 |
| CD4+CD28null T cells (%) | 27 | 1·1 (0·7–1·8) | 10 | 0·7 (0·3–1·1) | 0·078 |
| CD4+CD28+CD27+ (%) | 23 | 93·9 (92·2–97·3) | 10 | 92·4 (89·9–96·0) | 0·248 |
| CD4+CD28nullCD27– (%) | 23 | 0·3 (0·1–0·6) | 10 | 0·4 (0·1–0·5) | 0·739 |
| CD4+CD28+PD–1– (%) | 23 | 73·9 (66·8–78·2) | 10 | 79·7 (64·4–84·6) | 0·281 |
| CD4+CD28nullPD–1+ (%) | 23 | 0·4 (0·3–1·0) | 10 | 0·6 (0·3–0·9) | 0·681 |
| CD4+CD28+CD57– (%) | 23 | 97·1 (96·6–98·3) | 10 | 97·6 (93·4–98·2) | 0·652 |
| CD4+CD28nullCD57+ (%) | 23 | 0·1 (0·0–0·2) | 10 | 0·0 (0·0–0·2) | 0·246 |
| Peripheral blood | |||||
| CD4+ T cells (%) | 28 | 60·3 (48·8–64·8) | 10 | 76·7 (68·5–83·7) | < 0·001 |
| CD4+ naive T cells (%) | 28 | 28·9 (13·1–42·2) | 10 | 32·4 (19·9–42·8) | 0·703 |
| CD4+ memory T cells (%) | 28 | 71·1 (57·8–86·9) | 10 | 67·6 (57·2–80·2) | 0·703 |
| CD4+ CM T cells (%) | 28 | 39·5 (35·3–50·9) | 10 | 44·0 (33·8–54·8) | 0·655 |
| CD4+ EM T cells (%) | 28 | 22·5 (15·7–30·1) | 10 | 17·3 (13·8–25·1) | 0·380 |
| CD4+ EMRA T cells (%) | 28 | 2·0 (1·3–4·9) | 10 | 2·1 (1·4–3·3) | 0·934 |
| CD4+CD28null T cells (%) | 28 | 5·6 (1·3–11·5) | 10 | 0·6 (0·3–0·8) | < 0·001 |
| CD4+CD28+CD27+ (%) | 28 | 83·9 (77·8–92·7) | 10 | 94·2 (89·7–9·5) | 0·012 |
| CD4+CD28nullCD27– (%) | 28 | 3·9 (0·7–9·2) | 10 | 0·1 (0·0–0·2) | < 0·001 |
| CD4+CD28+PD–1– (%) | 28 | 77·7 (71·0–82·8) | 10 | 81·4 (71·1–89·0) | 0·214 |
| CD4+CD28nullPD–1+ (%) | 28 | 2·3 (0·6–9·0) | 10 | 0·1 (0·0–0·3) | < 0·001 |
| CD4+CD28+CD57– (%) | 28 | 93·9 (88·7–97·4) | 10 | 98·9 (97·8–99·4) | < 0·001 |
| CD4+CD28nullCD57+ (%) | 28 | 2·7 (0·6–6·5) | 10 | 0·0 (0·0–0·2) | < 0·001 |
Data are presented as medians (interquartile range). CMV = cytomegalovirus; CM = central memory; EM = effector memory; EMRA = effector memory CD45RA+.
Table 4.
CD8+ T cell differentiation status in lymph node and peripheral blood for CMV+ and CMV– patients
| n | CMV+ | n | CMV– | P | |
|---|---|---|---|---|---|
| Lymph node | |||||
| CD8+ T cells (%) | 28 | 19·0 (13·2–29·5) | 10 | 13·0 (8·7–22·9) | 0·159 |
| CD8+ naive T cells (%) | 27 | 39·5 (20·5–51·6) | 10 | 28·5 (11·0–58·4) | 0·281 |
| CD8+ memory T cells (%) | 27 | 60·5 (48·4–79·6) | 10 | 71·5 (41·6–89·1) | 0·281 |
| CD8+ CM T cells (%) | 27 | 12·0 (5·6–36·5) | 10 | 11·3 (4·6–44·2) | 0·959 |
| CD8+ EM T cells (%) | 27 | 21·9 (9·9–36·1) | 10 | 26·5 (14·3–53·7) | 0·442 |
| CD8+ EMRA T cells (%) | 27 | 8·8 (5·0–17·7) | 10 | 12·7 (7·4–19·2) | 0·330 |
| CD8+CD28null T cells (%) | 27 | 10·7 (7·4–16·7) | 10 | 9·1 (6·8–13·4) | 0·365 |
| CD8+CD28+CD27+ (%) | 23 | 90·9 (83·3–92·7) | 10 | 86·5 (84·1–91·4) | 0·357 |
| CD8+CD28nullCD27– (%) | 23 | 1·9 (1·2–3·4) | 10 | 1·4 (0·8–2·5) | 0·240 |
| CD8+CD28+PD–1– (%) | 23 | 63·2 (50·2–74·8) | 10 | 63·7 (46·4–76·0) | 0·953 |
| CD8+CD28nullPD–1+ (%) | 23 | 2·2 (1·5–5·4) | 10 | 3·5 (2·1–5·3) | 0·240 |
| CD8+CD28+CD57– (%) | 23 | 87·3 (81·6–90·1) | 10 | 87·4 (73·6–89·3) | 0·681 |
| CD8+CD28nullCD57+ (%) | 23 | 1·6 (1·1–2·7) | 10 | 1·3 (1·0–1·8) | 0·248 |
| Peripheral blood | |||||
| CD8+ T cells (%) | 28 | 35·5 (27·9–41·4) | 10 | 18·3 (13·1–27·3) | 0·002 |
| CD8+ naive T cells (%) | 27 | 18·7 (7·7–25·8) | 10 | 32·5 (14·2–49·0) | 0·123 |
| CD8+ memory T cells (%) | 27 | 81·3 (74·2–92·2) | 10 | 67·5 (51·0–85·8) | 0·123 |
| CD8+ CM T cells (%) | 27 | 5·9 (2·6–9·4) | 10 | 14·7 (7·5–19·7) | 0·006 |
| CD8+ EM T cells (%) | 27 | 27·0 (16·3–37·5) | 10 | 27·9 (24·8–49·2) | 0·159 |
| CD8+ EMRA T cells (%) | 27 | 42·2 (29·6–57·1) | 10 | 19·7 (12·8–26·4) | < 0·001 |
| CD8+CD28null T cells (%) | 27 | 47·0 (35·8–69·1) | 10 | 22·2 (15·1–32·2) | < 0·001 |
| CD8+CD28+CD27+ (%) | 28 | 47·9 (29·5–62·1) | 10 | 76·1 (70·3–85·0) | < 0·001 |
| CD8+CD28nullCD27– (%) | 28 | 35·6 (26·6–57·9) | 10 | 7·4 (3·7–11·0) | < 0·001 |
| CD8+CD28+PD–1– (%) | 28 | 38·7 (24·8–49·2) | 10 | 57·8 (40·6–69·9) | 0·026 |
| CD8+CD28nullPD–1+ (%) | 28 | 13·1 (6·9–18·7) | 10 | 10·3 (6·9–19·5) | 0·804 |
| CD8+CD28+CD57– (%) | 28 | 49·7 (30·6–63·2) | 10 | 77·3 (66·9–84·0) | < 0·001 |
| CD8+CD28nullCD57+ (%) | 28 | 30·5 (21·3–49·1) | 10 | 11·6 (7·9–14·9) | < 0·001 |
Data are presented as medians (interquartile range). CMV = cytomegalovirus; CM = central memory; EM = effector memory; EMRA = effector memory CD45RA+.
However, CMV infection did not affect the composition of CD4+ (Table 3) and CD8+ T cells (Table 4) within LN.
In addition, no association was seen between CMV infection and thymic output parameters in LN, with the frequencies of CD4+CD31+ and CD8 +CD31+ naive T cells and the TREC content being similar between CMV+ and CMV– patients (data not shown). Similarly, no effect of CMV on RTL of CD4+ and CD8+ T cells within LN was observed (data not shown).
In summary, despite a major effect of CMV infection on peripheral blood T cell composition, this was not paralleled by similar effects within LN.
Discussion
In this study we investigated whether the ageing parameters of the circulating T cell population, correlates with the T cell characteristics of LN and the effect of CMV on PB and LN composition. Our main conclusions are that the T cell composition and measures of thymic function in PB and LN are inter‐related, but that the highly differentiated CD28null T cells, which may comprise a large part of circulating T cells in CMV‐seropositive individuals, are found almost exclusively within the circulation. This study is the first in which a pairwise and a more in depth comparison was performed between PB and LN for different T cell ageing parameters in ESRD patients. Next to the different T cell subsets, we included analyses of other differentiation markers such as CD27, PD‐1 and CD57 in combination with CD28. Furthermore, we also analysed the TREC content and RTL of the T cells in both the lymph node and the peripheral blood.
In previous studies it was shown that the PB compartment of ESRD patients displayed distinct changes that resemble the T cell ageing process in elderly healthy individuals. It is associated with loss of naive T cells, an inverted CD4 : CD8 ratio and an increase in highly differentiated T cells, lacking the co‐stimulatory molecule CD28 27, 33, 34, 35, 36. The T cell composition of LNs of the same patients showed a higher frequency of CD4+ T cells and a lower frequency of CD8+ T cells compared with PB. The high frequency of CD4+ T cells was also found in the lymphoid tissues of deceased paediatric and young adult organ donors in a study by Thome et al. 37. Furthermore, there was a lower frequency of highly differentiated T cells within LN samples in contrast to PB. This finding was supported further by the other T cell memory differentiation markers (CD28, CD27, PD‐1 and CD57). It is known that CD4+ CD28null are more differentiated than CD8+CD28null, as the latter can still contain CD27 in contrast to the CD4+ T cell fraction 29, 30, 31. However, both the CD8+CD28null T cells as well as the CD8+CD28nullCD27– T cells were more frequent in PB. Even though a positive correlation existed between LN and PB samples for the majority of the different T cell subset frequencies, the highly differentiated T cells (i.e. CD28null T cells) were far more abundant in PB. The low number of highly differentiated T cells in LN might be explained by the lack of co‐expression of CCR7, which T cells use for homing to secondary lymphoid organs 21.
The parameters for thymic output showed similar results for both the CD31 and the TREC assay. The results indicate a remarkably high correlation between the data obtained in the circulation and within LN. In addition, CD31+ T cells and TREC content in LN T cell population showed a relation with the age of the individual as we and others have described previously for circulating T cells 3, 5, 27, 33, 38. Taken together, it can be concluded that the loss of thymic function with increasing age is also reflected in the secondary lymphoid tissue. Primary immune responses (e.g. vaccination) are initiated within LN. The intricate relation between the amount of naive T cells in PB and LN therefore probably explains why the number of circulating naive T cells is associated with the response to vaccination 39.
In this study we did not find any differences regarding the RTL between PB and LN. This could be explained by the depth of the analysis of RTL. We took the CD4+ and CD8+ T cell population as a whole, instead of focusing on the different T cell subsets. Because of this, differences in RTL due to differences in frequencies of subsets might be overlooked. Furthermore, we cannot exclude fully that the isolation procedure resulted in a shift in T cell subsets, possibly influencing the analysis of the RTL.
Age‐related changes were observed in both CD4+ and CD8+ T cell compartments within the lymph node. The frequency of naive T cells declined, while the frequency of memory T cells increased. Other studies also showed an age‐related decline in naive T cells within LNs of healthy individuals and from deceased organ donors 24, 37, 40. Furthermore, a study by Thome et al. found that EM T cells were less abundant in paediatric lymphoid tissues, while these were more frequent in young adult lymphoid tissues 37. Next to this, the TREC content also decreased with increasing age in the LN, supporting the T cell ageing process in LN. These results indicate that age‐related changes in T cell composition are similar for PB and LN, with the exception of the late differentiated T cells, which appear to remain outside the LN.
CMV infection changes the composition of circulating T cells dramatically and almost doubles the total number of CD8+ T cells, in particular with a late differentiation phenotype 8, 14, 15. However, the T cell differentiation status within the LNs did not differ between CMV+ and CMV– patients. In a study by Remmerswaal et al., the frequency of CMV‐specific CD8+ T cells was significantly lower in the LN compared with the PB, which is in line with our findings 41. Next to this, a study by Havenith et al. also showed the absence of highly differentiated CD4+CD28nullCD27— T cells in the lymph node, which can appear due to a CMV infection 42. These findings suggest that a latent CMV infection does not have a significant impact on T cells within LN, while the opposite is true for PB T cells.
In conclusion, the T cell composition in PB is related strongly to T cell composition of the LN, while the presence of highly differentiated CD28null T cells is more exclusive to the PB compartment. In addition, CMV changed the T cell differentiation status in PB drastically, while no significant alterations were observed within LN with regard to T cell differentiation and ageing parameters. This study provides a new perspective on the T cell immune system, especially of those with ESRD. PB seems to harbour the majority of terminally differentiated T cells, while LNs do not harbour this specific subset of T cells. These findings suggest that during an immune response the late differentiated T cells access the tissue and exert their immune function directly. This is consistent with the observation that many of these cells carry the chemokine receptor CX3CR1 for the endothelium‐derived chemokine fractalkine 41. Fractalkine is considered a major player in inflammatory responses, recruiting leucocytes into the inflamed tissue 43, 44, 45. These late differentiated T cells then can respond to IL‐15 produced by the inflamed parenchyma, proliferate and exert their cytotoxic function 46, 47, 48. Whether the T cell characteristics in the LN might provide a better predictive model for clinical outcomes, such as the development of (co‐stimulatory blockade‐resistant) rejection and infection after, for example, kidney transplantation is subject to further investigation.
Author contributions
B. D., N. H. R. L., M. K., W. V., D. R. and L. H. performed experiments; B. D., N. H. R. L. and D. R. analysed results and made the figures; A. E. de W., M. G. H. B. and N. H. R. L. designed the research; B. D., A. E. de W., L. H., A. W. L., F. J. D. M. K., W. V., D. R., C. C. B., N. H. R. L. and M. G. H. B. wrote the paper.
Disclosure
The authors declare no competing financial and commercial interests.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Gating strategy of the T cell subsets. First, lymphocytes were selected based on the forward‐ and side‐scatter, and CD3+ T cells were selected from these lymphocytes. The CD3+ T cells were then divided further into CD4+ and CD8+ T cell fractions. Each population was dissected into different subsets using CCR7 and CD45RO. After this division, CD4+ (only this is shown) and further CD8+ naive T cells were analysed for CD31 expression. Besides this, total CD4+ (only this is shown) and total CD8+ T cells were analysed for frequencies of cells lacking CD28 expression.
Fig. S2. Gating strategy of the T cell differentiation markers. First, CD45+ leucocytes were gated. From these cells, lymphocytes were selected based on the forward‐ and side‐scatter; CD3+ T cells were selected from these lymphocytes. The CD3+ T cells were then divided further into CD4+ and CD8+ T cell fractions. Each fraction was analysed further for the expression of CD27, programmed death 1 (PD‐1) or CD57 in combination with CD28 (only the CD8+ T cell fraction is shown). From these analyses only the least (CD28+CD27+, CD28+PD‐1– and CD28+CD57–) and most differentiated (CD28nullCD27–, CD28nullPD‐1+ and CD28nullCD57+) T cells were selected (indicated within the black frames).
Fig. S3. Correlation between CD31+ naive T cells and the T cell receptor excision circles (TREC) content in peripheral blood (PB) and the lymph node. The Spearman's rho correlation analysis is shown between CD4+CD31+ naive T cells within the PB and the TREC content within the PB (a), between CD8+CD31+ naive T cells within the PB and the TREC content within the PB (b), between CD4+CD31+ naive T cells within the LN and the TREC content within the LN (c) and between CD8+CD31+ naive T cells within the LN and the TREC content within the LN (d). Frequencies of cells are depicted on the y‐axis, and the ΔCT which is related inversely to the TREC content is depicted on the x‐axis.
Acknowledgements
We would like to thank the technicians from our laboratory and the surgeons from the surgery department for their contribution to this study.
References
- 1. Kohler S, Thiel A. Life after the thymus: CD31+ and CD31– human naive CD4+ T‐cell subsets. Blood 2009; 113:769–74. [DOI] [PubMed] [Google Scholar]
- 2. Palmer DB. The effect of age on thymic function. Front Immunol 2013; 4:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zubakov D, Liu F, van Zelm MC et al Estimating human age from T cell DNA rearrangements. Curr Biol 2010; 20:R970–1. [DOI] [PubMed] [Google Scholar]
- 4. Gurkan S, Luan Y, Dhillon N et al Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant 2010; 10:2132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pujol‐Borrell R, Herrero‐Mata MJ, Palou E, Armengol MP. Immunological senescence and thymic function in transplantation. Transplantation 2009; 88:S8–13. [DOI] [PubMed] [Google Scholar]
- 6. Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG. Changes in CD8 and CD4 lymphocyte subsets, T‐cell proliferation responses and non‐survival in the very old: the Swedish longitudinal OCTO‐immune study. Mech Ageing Dev 1998; 102:187–98. [DOI] [PubMed] [Google Scholar]
- 7. Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age‐related change in peripheral blood T‐lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev 2000; 121:187–201. [DOI] [PubMed] [Google Scholar]
- 8. Weng NP, Akbar AN, Goronzy J. CD28(–) T‐cells: their role in the age‐associated decline of immune function. Trends Immunol 2009; 30:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bandres E, Merino J, Vazquez B et al The increase of IFN‐gamma production through aging correlates with the expanded CD8(+high)CD28(–)CD57(+) subpopulation. Clin Immunol 2000; 96:230–5. [DOI] [PubMed] [Google Scholar]
- 10. Sauce D, Almeida JR, Larsen M et al PD‐1 expression on human CD8 T cells depends on both state of differentiation and activation status. Aids 2007; 21:2005–13. [DOI] [PubMed] [Google Scholar]
- 11. Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T‐cell subsets in young and old people. Immun Ageing 2008; 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weng NP, Levine BL, June CH, Hodes RJ. Human naive and memory T‐lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci USA 1995; 92:11091–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verdun RE, Karlseder J. Replication and protection of telomeres. Nature 2007; 447:924–31. [DOI] [PubMed] [Google Scholar]
- 14. Fletcher JM, Vukmanovic‐Stejic M, Dunne PJ et al Cytomegalovirus‐specific CD4+ T‐cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol 2005; 175:8218–25. [DOI] [PubMed] [Google Scholar]
- 15. Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res 2011; 157:175–9. [DOI] [PubMed] [Google Scholar]
- 16. van de Berg PJ, Griffiths SJ, Yong SL et al Cytomegalovirus infection reduces telomere length of the circulating T‐cell pool. J Immunol 2010; 184:3417–23. [DOI] [PubMed] [Google Scholar]
- 17. Mou D, Espinosa J, Lo DJ, Kirk AD. CD28 negative T cells: is their loss our gain? Am J Transplant 2014; 14:2460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liuzzo G, Biasucci LM, Trotta G et al Unusual CD4+CD28null T‐lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol 2007; 50:1450–8. [DOI] [PubMed] [Google Scholar]
- 19. McElhaney JE, Zhou X, Talbot HK et al The unmet need in the elderly: how immunosenescence, CMV infection, co‐morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012; 30:2060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lo DJ, Weaver TA, Stempora L et al Selective targeting of human alloresponsive CD8+ effector memory T‐cells based on CD2 expression. Am J Transplant 2011; 11:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Betjes MG, Meijers RW, de Wit LE, Litjens NH. A killer on the road: circulating CD4(+)CD28null T‐cells as cardiovascular risk factor in ESRD patients. J Nephrol 2012; 25:183–91. [DOI] [PubMed] [Google Scholar]
- 22. Kato M, Ono Y, Kinukawa T, Hattori R, Kamihira O, Ohshima S. Long time follow up of CD28– CD4+ T cells in living kidney transplant patients. Clin Transplant 2004; 18:242–6. [DOI] [PubMed] [Google Scholar]
- 23. Betjes MG, Weimar W, Litjens NH. Circulating CD4(+)CD28null Tcells may increase the risk of an atherosclerotic vascular event shortly after kidney transplantation. J Transplant 2013; 2013:841430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck‐Loebenstein B. Age‐related loss of naive T cells and dysregulation of T‐cell/B‐cell interactions in human lymph nodes. Immunology 2005; 114:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martinet KZ, Bloquet S, Bourgeois C. Ageing combines CD4 T cell lymphopenia in secondary lymphoid organs and T‐cell accumulation in gut associated lymphoid tissue. Immun Ageing 2014; 11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T‐lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708–12. [DOI] [PubMed] [Google Scholar]
- 27. Betjes MG, Langerak AW, van der Spek A, de Wit EA, Litjens NH. Premature aging of circulating T‐cells in patients with end‐stage renal disease. Kidney Int 2011; 80:208–17. [DOI] [PubMed] [Google Scholar]
- 28. Appay V, Dunbar PR, Callan M et al Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 2002; 8:379–85. [DOI] [PubMed] [Google Scholar]
- 29. Amyes E, Hatton C, Montamat‐Sicotte D et al Characterization of the CD4+ T cell response to Epstein–Barr virus during primary and persistent infection. J Exp Med 2003; 198:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamann D, Roos MT, van Lier RA. Faces and phases of human CD8 T cell development. Immunol Today 1999; 20:177–80. [DOI] [PubMed] [Google Scholar]
- 31. van Leeuwen EM, Remmerswaal EB, Vossen MT et al Emergence of a CD4+CD28– granzyme B+, cytomegalovirus‐specific T‐cell subset after recovery of primary cytomegalovirus infection. J Immunol 2004; 173:1834–41. [DOI] [PubMed] [Google Scholar]
- 32. Meijers RW, Litjens NH, de Wit EA, Langerak AW, Baan CC, Betjes MG. Uremia‐associated immunological aging is stably imprinted in the T‐cell system and not reversed by kidney transplantation. Transpl Int 2014; 27:1272–84. [DOI] [PubMed] [Google Scholar]
- 33. Meijers RW, Litjens NH, de Wit EA et al Uremia causes premature ageing of the T‐cell compartment in end‐stage renal disease patients. Immun Ageing 2012; 9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Litjens NH, van Druningen CJ, Betjes MG. Progressive loss of renal function is associated with activation and depletion of naive T‐lymphocytes. Clin Immunol 2006; 118:83–91. [DOI] [PubMed] [Google Scholar]
- 35. Betjes MG, Huisman M, Weimar W, Litjens NH. Expansion of cytolytic CD4+CD28– T cells in end‐stage renal disease. Kidney Int 2008; 74:760–7. [DOI] [PubMed] [Google Scholar]
- 36. Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Naive and central memory T‐cell lymphopenia in end‐stage renal disease. Kidney Int 2006; 70:371–6. [DOI] [PubMed] [Google Scholar]
- 37. Thome JJ, Bickham KL, Ohmura Y et al Early‐life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med 2016; 22:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pido‐Lopez J, Imami N, Aspinall R. Both age and gender affect thymic output: more recent thymic migrants in females than males as they age. Clin Exp Immunol 2001; 125:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weinberger B, Herndler‐Brandstetter D, Schwanninger A, Weiskopf D, Grubeck‐Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis 2008; 46:1078–84. [DOI] [PubMed] [Google Scholar]
- 40. Thome JJ, Yudanin N, Ohmura Y et al Spatial map of human T‐cell compartmentalization and maintenance over decades of life. Cell 2014; 159:814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Remmerswaal EB, Havenith SH, Idu MM et al Human virus‐specific effector‐type T‐cells accumulate in blood but not in lymph nodes. Blood 2012; 119:1702–12. [DOI] [PubMed] [Google Scholar]
- 42. Havenith SH, Remmerswaal EB, Idu MM et al CXCR5+CD4+ follicular helper T‐cells accumulate in resting human lymph nodes and have superior B cell helper activity. Int Immunol 2014; 26:183–92. [DOI] [PubMed] [Google Scholar]
- 43. Bazan JF, Bacon KB, Hardiman G et al A new class of membrane‐bound chemokine with a CX3C motif. Nature 1997; 385:640–4. [DOI] [PubMed] [Google Scholar]
- 44. Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Mol Interv 2010; 10:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Umehara H, Bloom ET, Okazaki T, Nagano Y, Yoshie O, Imai T. Fractalkine in vascular biology: from basic research to clinical disease. Arterioscler Thromb Vasc Biol 2004; 24:34–40. [DOI] [PubMed] [Google Scholar]
- 46. Alonso‐Arias R, Moro‐Garcia MA, Vidal‐Castineira JR et al IL‐15 preferentially enhances functional properties and antigen‐specific responses of CD4+CD28(null) compared to CD4+CD28+ T‐cells. Aging Cell 2011; 10:844–52. [DOI] [PubMed] [Google Scholar]
- 47. Echeverria A, Moro‐Garcia MA, Asensi V, Carton JA, Lopez‐Larrea C, Alonso‐Arias R. CD4(+)CD28null T‐lymphocytes resemble CD8(+)CD28null T‐lymphocytes in their responses to IL‐15 and IL‐21 in HIV‐infected patients. J Leukoc Biol 2015; 98:373–84. [DOI] [PubMed] [Google Scholar]
- 48. White L, Krishnan S, Strbo N et al Differential effects of IL‐21 and IL‐15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T‐cells of patients with human immunodeficiency virus‐1 (HIV). Blood 2007; 109:3873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Gating strategy of the T cell subsets. First, lymphocytes were selected based on the forward‐ and side‐scatter, and CD3+ T cells were selected from these lymphocytes. The CD3+ T cells were then divided further into CD4+ and CD8+ T cell fractions. Each population was dissected into different subsets using CCR7 and CD45RO. After this division, CD4+ (only this is shown) and further CD8+ naive T cells were analysed for CD31 expression. Besides this, total CD4+ (only this is shown) and total CD8+ T cells were analysed for frequencies of cells lacking CD28 expression.
Fig. S2. Gating strategy of the T cell differentiation markers. First, CD45+ leucocytes were gated. From these cells, lymphocytes were selected based on the forward‐ and side‐scatter; CD3+ T cells were selected from these lymphocytes. The CD3+ T cells were then divided further into CD4+ and CD8+ T cell fractions. Each fraction was analysed further for the expression of CD27, programmed death 1 (PD‐1) or CD57 in combination with CD28 (only the CD8+ T cell fraction is shown). From these analyses only the least (CD28+CD27+, CD28+PD‐1– and CD28+CD57–) and most differentiated (CD28nullCD27–, CD28nullPD‐1+ and CD28nullCD57+) T cells were selected (indicated within the black frames).
Fig. S3. Correlation between CD31+ naive T cells and the T cell receptor excision circles (TREC) content in peripheral blood (PB) and the lymph node. The Spearman's rho correlation analysis is shown between CD4+CD31+ naive T cells within the PB and the TREC content within the PB (a), between CD8+CD31+ naive T cells within the PB and the TREC content within the PB (b), between CD4+CD31+ naive T cells within the LN and the TREC content within the LN (c) and between CD8+CD31+ naive T cells within the LN and the TREC content within the LN (d). Frequencies of cells are depicted on the y‐axis, and the ΔCT which is related inversely to the TREC content is depicted on the x‐axis.


