Abstract
Choroidal hemangioma is a rare congenital ocular tumor that can present as either circumscribed or diffuse. Circumscribed choroidal hemangioma (CCH) typically manifests as a red-orange mass within the posterior pole and appears similar to other ocular conditions, such as choroidal melanoma and choroidal metastasis. Proper diagnosis is crucial and is aided by the use of ancillary testing. CCH itself is benign but can cause secondary complications such as subretinal fluid accumulation and subsequent retinal detachment. If these conditions should arise, several treatment options are available.
Keywords: Circumscribed choroidal hemangioma, Ultrasonography, Photodynamic therapy, Choroidal melanoma
Resumen
El hemangioma coroideo es un raro tumor ocular congénito que puede presentarse de manera circunscrita o difusa. El hemangioma circunscrito de coroides (CCH) se manifiesta típicamente como una masa rojizo-anaranjada en el interior del polo posterior, y parece similar a otras situaciones oculares, tales como el melanoma coroideo y la metástasis coroidea. El diagnóstico adecuado es esencial, y viene asistido por el uso de pruebas complementarias. El CCH en sí mismo es benigno, aunque puede originar complicaciones secundarias tales como la acumulación de fluidos sub-retinianos y el subsiguiente desprendimiento de retina. En caso de producirse dichas situaciones, existen diversas opciones de tratamiento.
Palabras clave: Hemangioma circunscrito de coroides, Ultrasonografía, Terapia fotodinámica, Melanoma coroideo
Choroidal hemangioma is a benign vascular tumor.1, 2 It is considered a congenital condition but can continue to develop into early adulthood.2, 3 Choroidal hemangiomas are classified into two subtypes, circumscribed and diffuse, a distinction made based on the extent of choroidal involvement. Diffuse choroidal hemangiomas involve more than one quadrant of the choroid and are typically associated with systemic conditions such as Sturge–Weber syndrome.1, 2, 3, 4 Circumscribed choroidal hemangioma (CCH) typically presents as a unilateral orange-red choroidal mass without correlation to systemic conditions. CCH may appear similar to other choroidal tumors, some of which are malignant, such as choroidal melanoma and choroidal metastasis.2, 3 For this reason, it is important to be able to differentiate between these lesions. This can be accomplished by employing ancillary testing such as ultrasonography, fluorescein angiography (FA), indocyanine green angiography (ICGA), magnetic resonance imaging (MRI), and optical coherence tomography (OCT).2, 3 Once the diagnosis has been made, referral to a retinal specialist is usually warranted. Treatment options include laser photocoagulation, thermotherapy, radiotherapy, photodynamic therapy (PDT), and anti-vascular endothelial growth factor (VEGF) therapy.1, 5, 6, 7, 8, 9
Case report
Relevant history
A 49-year-old Caucasian male presented to clinic with complaints of blurred vision at distance that was worse in his left eye. His medical history included hypertension, hyperlipidemia, sleep apnea, and post-traumatic stress disorder. His ocular history was unremarkable.
Clinical findings
The patient's best-corrected visual acuities (BVA) were 20/20 OD and 20/100 OS, and he consistently missed letters on the left side of the chart with his left eye. Pupils were equal, round, and reactive to light OU, and there was no afferent pupillary defect. Confrontation fields were full, and extra-ocular muscles had full range of motion. Amsler grid was unremarkable OD and revealed left-sided metamorphopsia OS. Slit lamp examination was unremarkable OU, and intraocular pressures were within normal limits. Dilated fundus exam of the left eye (Fig. 1) revealed a 2DD orange-red elevated lesion superior temporal to the optic nerve head with overlying retinal pigment epithelium change and pigmentation. An associated 3–4 DD central serous retinal detachment was also noted. Several ancillary tests were performed. Ultrasonography showed a 2 mm × 8 mm solid choroidal mass on B-scan with high internal reflectivity on A-scan. OCT revealed a large choroidal mass with overlying subretinal fluid. Fundus photos were also taken.
Figure 1.
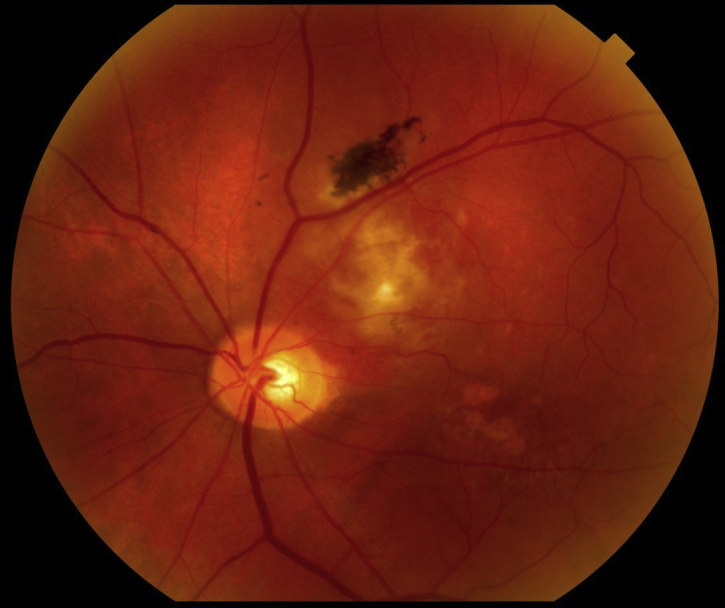
Fundus photo shows a circumscribed choroidal hemangioma superior temporal to the optic nerve in the left eye.
Management/follow-up
The patient was diagnosed with CCH and referred to a retinal specialist. Fluorescein angiography was performed, and it showed hyperflourescence in all stages. The lesion was treated with argon laser photocoagulation over the entire mass. The patient was seen two weeks later, and BVA was 20/50 OS. Dilated fundus exam revealed an improvement in subretinal fluid, but a serous detachment was still present. FA was repeated as well as another session of argon laser photocoagulation.
Over the next 15 years, the patient was seen every three to six months depending on the status of the condition. After three treatments of argon laser photocoagulation, all of the subretinal fluid resolved. The CCH did not shrink in size. Pigment later developed over the lesion. BVA remained around 20/30 at each follow-up visit. The subretinal fluid eventually returned 11 years after the last treatment session, but it did not affect the patient's vision. Therefore, observation was chosen as the treatment at that time. No changes in the amount of subretinal fluid or BVA have been noted at any follow-up visits since.
Literature review
Background
CCH is a rare vascular tumor.1, 3 It is considered to be congenital in nature; however, it often does not cause symptoms until the fourth to sixth decade of life.2, 3 It is observed more frequently in Caucasians and equally between the sexes. CCH is a very uncommon tumor that is likely to go undiagnosed unless the patient becomes symptomatic.
Unlike diffuse choroidal hemangiomas, which are associated with phakomatoses such as Sturge–Weber syndrome, CCH has no correlation with systemic disease.3, 4 CCH is composed of dilated choroidal vessels but does not involve the choriocapillaris. The specific cause of the blood vessel malformation is unknown. CCH is different from other “true hemangiomas” in that it does not show cellular proliferation. In other words, it shows slow to no progression in size over time.2, 3
CCH presents clinically as an orange-red mass in the posterior pole, usually within one to three disk diameters of the macula.2, 3, 4 They typically range in size from 3 to 19 mm in diameter and 1 to 8 mm in thickness. The mass itself is not pigmented but overlying pigment can develop over time. CCH is a slowly progressive tumor. It can appear to increase in size secondary to subretinal fluid accumulation.2, 3
Signs and symptoms
Presenting symptoms can range from no symptoms to significant vision loss. In a 200-patient study by Shields et al., the most common symptom was blurred vision followed by a loss in visual field. Other symptoms can include metamorphopsia, floaters, flashes of light, and rarely ocular pain. Many of these symptoms are caused by subretinal fluid accumulation. This occurs secondary to a breakdown of the blood-retinal barrier, allowing fluid leakage that leads to a serous retinal detachment.3
Clinically, signs include an orange-red dome-shaped lesion with indistinct borders.3 Rarely, the tumor can take on more of a plateau or mushroom shape, which increases the likelihood of misdiagnosis of a more serious ocular condition. Other associated clinical signs can include dilated episcleral vessels, neovascularization of the iris, drusen, RPE hyperplasia, dilated retinal arteries and veins, and retinoschisis.2, 3
Differential diagnosis
Several posterior segment lesions can resemble CCH. These include central serous chorioretinopathy, choroidal melanoma, choroidal metastasis, posterior nodular scleritis, and non-specific retinal detachment. The most concerning of these are choroidal melanoma and choroidal metastasis because of their risk of ocular and systemic morbidity and mortality.3
Patients with central serous chorioretinopathy are typically middle-aged adults who present with complaints of decreased vision. Examination reveals an elevated area in the macula, usually with associated subretinal fluid. It is typically the same color as the surrounding retina. This condition is benign and often resolves without treatment, although recurrence is possible.2
Posterior nodular scleritis can present as a tumor-like mass in the posterior pole. This can be distinguished from CCH because other signs and symptoms of ocular inflammation, such as vitreous cells or anterior scleritis, are usually present.10
Choroidal metastases often present as elevated, plateau-shaped lesions. They can be found anywhere in the posterior segment of the eye, including the posterior pole, and are typically a creamy yellow color. Unlike CCH, they can be multifocal or bilateral.2, 3
Choroidal melanoma is typically a large mushroom-shaped, pigmented lesion that can be found anywhere in the posterior segment of the eye. Masses larger than 2 mm are considered suspicious for melanoma. However, most measure greater than 10 mm at diagnosis. The majority of melanomas are darkly pigmented with either a black or gray-green appearance, but amelanotic melanomas also exist.2 This lesion can be confused with a CCH, but its appearance is somewhat different since it is yellow to tan in color and sometimes has overlying drusen.3
It is important to be able to differentiate between different types of ocular tumors in order to provide appropriate treatment. As mentioned previously, some conditions such as choroidal melanoma require correct diagnosis and prompt treatment to reduce the risk of ocular and systemic morbidity and mortality.
Ancillary testing
Since CCH can mimic several other ocular conditions, additional testing is required for proper diagnosis. These tests include ultrasonography, FA, ICGA, MRI, and OCT.2, 3, 12, 13
Ultrasonography can be used to measure the internal reflectivity of an ocular mass. High internal reflectivity on A-scan, or acoustically solid on B-scan, indicates that the mass is composed of a variety of different cell types, which causes more disruption of the sound waves. Normal choroidal tissue is made of several different cell types. Low internal reflectivity on A-scan, or acoustic hollowness on B-scan, is a sign that very similar cells are located within the lesion. CCH is characterized by high internal reflectivity since it is composed of blood vessels and other cells that are similar to the normal choroidal structure.3 This is in contrast to choroidal melanomas, which usually show acoustic hollowness and low to medium internal reflectivity. On the other hand, choroidal metastasis shows similar findings to CCH and is not as easily distinguished from CCH with ultrasonography.2, 3
FA allows viewing of the retinal blood vessels and is often used to locate areas of leakage. CCH shows a characteristic pattern on FA which includes mild early lacy hyperflourescence that increases through all stages. In contrast, choroidal melanoma and choroidal metastasis show slower and less intense hyperflourescence.2, 3 Central serous chorioretinopathy, on the other hand, shows early hypofluorescence followed by the classic smoke stack appearance of fluid leakage.
ICGA gives similar data to FA, but ICGA allows visualization of the choroidal vasculature as opposed to the overlying retinal vessels. CCH characteristically shows extreme hyperflourescence in the early stages which decreases in the late stages, also known as the “wash-out” phenomenon. In contrast, choroidal melanoma and metastasis demonstrate slower and less intense filling.2, 3
MRI can be used to locate lesions within the orbit. Unfortunately, both CCH and choroidal melanomas show similar characteristics on MRI. Both are hyperintense on T1-weighted images. On T2-weighted images, both are isointense compared to the vitreous and tend to disappear on the image.3 Although MRI does allow visualization of the location of the mass, it is not a very useful test for helping to differentiate CCH from choroidal melanoma.2
If all other testing is inconclusive, a biopsy of the lesion can be performed. This is a more invasive procedure and therefore carries greater risks. It is usually reserved for cases where the diagnosis cannot be made with less involved methods.3
OCT is a newer technology which shows high definition imaging of the posterior segment of the eye. It is especially helpful in detecting lesions which are less than 1 mm thick. Clinically, it is used most often to monitor for complications of CCH such as macular edema and serous retinal detachment. It can also be used to evaluate the efficacy of treatment.2, 13
Treatment options
CCH is often an asymptomatic lesion. In this case, close observation is the only treatment warranted. Although the lesion itself is benign, it can lead to sight-threatening complications, including macular edema, serous retinal detachment, or neovascular glaucoma. The most common symptom in patients with CCH is blurred vision secondary to macular edema. If complications do arise, there are several treatment options available. The goal of any treatment is resolution of the macular edema.3 Any decrease in tumor size is a benefit but is not the primary purpose of the treatment.11
In the 1960s and 1970s, the traditional treatment for CCH was xenon arc photocoagulation. This was performed over the entire surface of the tumor. It produced good resolution of subretinal fluid but often left residual scarring that could limit the patient's visual recovery.9, 12 After the 1970s, the argon laser became the preferred laser for photocoagulation of CCH. Argon laser can also cause scarring of the choroid and retina, however, which can lead to decreased visual acuity. Re-treatments are often necessary as subretinal fluid tends to recur. Photocoagulation can sometimes lead to significant, if not complete, resolution of subretinal fluid but typically has a little to no effect on the tumor size.1, 3, 9, 12 Most patients’ visual acuity remains stable or improves after treatment. This success rate, along with its wide availability and cost-effectiveness, makes argon laser photocoagulation a reasonable treatment choice for many patients.1, 3
Another treatment available for CCH is transpupillary thermotherapy (TTT), where a diode laser with a broad beam and long exposure time is used to treat the lesion. This laser penetrates deeper, causing hyperthermia and occlusion of blood vessels with less damage to the surrounding retina. This leads to a decrease in subretinal fluid as well as a decrease in overall tumor size. Since this therapy penetrates deeper, it can lead to damage of the retinal pigment epithelium. Complications include branch retinal vein occlusion, recurrent macular edema, scarring, and pre-retinal fibrosis. If macular edema recurs, the treatment can be repeated every two to three months. However, the risk of complication increases with every re-treatment.2, 3, 9 For this reason, TTT is usually reserved for lesions less than 10 mm in diameter, less than 4 mm in thickness, and more than 3 mm from the foveola. TTT is also not effective for lesions with large amounts of overlying fluid or large retinal detachments.3, 9
Since the late 1980s, radiation therapy has been available for the treatment of CCH. The three most common types of radiation therapy include episcleral plaque radiotherapy, external beam irradiation, and proton beam irradiation. Radiation therapy is usually used in cases of CCH where photocoagulation cannot be applied, such as for large extensive retinal detachments or subfoveal lesions. Episcleral plaque radiotherapy, or brachytherapy, is the process of placing a radioactive plaque under the tumor.2, 3, 9 The plaque is surgically attached to the episclera then left in place until the desired amount of radiation is delivered. Later, a second surgical procedure is performed to remove the plaque. The radiation therapy results in a decrease of subretinal fluid cause by shrinkage of the tumor itself. The success rate is very high for this treatment, even in eyes in which previous treatments have failed.3, 9 Another form of radiation therapy that has been employed for treatment of CCH is external beam radiotherapy. This technique is often used for diffuse choroidal hemangiomas but can be used for CCH as well.13 It is used as a stand-alone treatment or in conjunction with other treatments such as argon laser photocoagulation. One possible complication of this treatment is cataracts, so a lens-sparing technique is often used.1, 3, 9 The newest form of radiation therapy is proton beam radiotherapy, treatment that uses charged particles which are slowed down at the tumor site. This allows a homogeneous dose of radiation at the tumor while sparing the surrounding tissues. Like other radiotherapies, proton beam radiation causes a decrease in tumor size and subsequent resolution of subretinal fluid. This procedure, however, is very expensive and has very limited availability.7, 9 Generally, the radiation therapy of choice is episcleral plaque radiotherapy. This method allows the radiation to be placed at the base of the tumor right under the blood supply. It also takes less time, typically requiring only 2–4 days of therapy before removal of the plaque. This type of procedure also decreases the amount of anterior segment damage from radiation. The main drawback to this method is that it requires two separate surgical procedures to place and later remove the plaque.2, 3, 9 All types of radiation therapies carry some risk of complications from the radiation. These include cataracts, radiation retinopathy, dry eye, and neovascular glaucoma.2 For this reason, radiotherapy is rarely chosen as a first-line therapy for CCH.
PDT has been shown to be a safe and effective treatment for CCH. PDT uses a photochemical to selectively occlude vascular channels in the CCH. The photochemical binds to low density lipoproteins which are present in large amounts in the tumor's endothelium. Following the injection of the photochemical, a laser is applied, and this causes a reaction only in areas that are bound to the photochemical. The objective is to allow very specific treatment with minimal damage to surrounding tissue.5, 9 Since the most common symptom in patients with CCH is blurred vision due to serous retinal detachment involving the macula or macular edema, the goal of treatment is resolution of the macular edema and subretinal fluid. Some patients who undergo PDT also show a regression in tumor size as well. One possible side effect is choroidal atrophy from repeated treatments. Overall, PDT is a safe and effective primary or secondary treatment for CCH.5, 14, 15
Intravitreal injection of anti-VEGF medication has been a frequently used treatment for wet macular degeneration and diabetic macular edema for several years. Recently, it has also been used to help treat macular edema secondary to CCH. This treatment is minimally invasive and has a little to no effect on the surrounding retinal tissue. Anti-VEGF medication has been shown to be effective in resolving macular edema in some patients with CCH. One drawback to this treatment is that it needs to be repeated often. Most patients had return of some macular edema three to six months after treatment. Its relative lack of efficacy may be due to the nature of the mature vessels composing the hemangioma, as opposed to neovascular vessels or vessels that have been compromised by diabetic disease. Due to its short-term effect, this treatment is often combined with other previously mentioned therapies for CCH.7, 9, 16
Enucleation is a last resort treatment for CCH. Most eyes that undergo enucleation are result of the misdiagnosis of a choroidal melanoma.7, 13 This underscores the importance of ancillary testing and proper diagnosis. Other reasons for enucleation include a total retinal detachment or development of neovascular glaucoma resulting in a blind painful eye.7, 13
Conclusion
Although CCH is a rare retinal finding, it is important to be able to distinguish it from other similar retinal tumors.7, 13 Clinical presentation as well as the use of ancillary testing can be used to properly diagnose this condition. These tests include ultrasonography (A/B scan), FA, ICGA, MRI, and OCT.1, 3, 6, 7 CCH is difficult to diagnosis in some cases because it mimics several other posterior segment findings. Prompt diagnosis to a retinal specialist is necessary if any uncertainty exists.7 Treatment is only indicated for CCH when subretinal fluid affects the macular area and causes decreased vision for the patient. When macular subretinal fluid or macular edema is present, a retinal specialist can then make the decision of which treatment option to use based on several factors, including tumor size and location, as well as characteristics of the subretinal fluid.7, 13 Despite its status as a benign tumor, CCH can lead to profound vision loss in about half of all cases secondary to long-term macular edema.12 The earlier that treatment is implemented, the better the visual outcome for the patient.13 Several treatment options are available presently, including argon laser photocoagulation, episcleral plaque radiation therapy, external beam radiotherapy, proton beam radiotherapy, TTT, PDT, and intravitreal anti-VEGF therapy.2, 3, 4, 6, 8, 9, 11, 12, 13, 14, 15 Enucleation is reserved for blind painful eyes after other therapies have failed. Treatment options continue to evolve. Long-term studies are difficult to conduct on such an uncommon condition, but would be useful in guiding treatment for patients with CCH.
References
- 1.Madreperla S., Hungerford J., Plowman N.P., Laganowski H.C., Gregory P.T.S. Choroidal hemangiomas: visual anatomical results of treatment by photocoagulation or radiation therapy. Ophthalmology. 1997;104:1773–1779. doi: 10.1016/s0161-6420(97)30027-x. [DOI] [PubMed] [Google Scholar]
- 2.Mashayekhi A., Shields C.L. Circumscribed choroidal hemangioma. Curr Opin Ophthalmol. 2003;14:142–149. doi: 10.1097/00055735-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Shields C.L., Hanaver S.G., Shields J.A., Carter J., Demirci H. Choroidal hemangioma: clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108:2237–2247. doi: 10.1016/s0161-6420(01)00812-0. [DOI] [PubMed] [Google Scholar]
- 4.Witschel H., Font R.L. Hemangioma of the choroid: a clinicopathologic study of 71 cases and a review of literature. Surv Ophthalmol. 1976;20:425–431. doi: 10.1016/0039-6257(76)90067-9. [DOI] [PubMed] [Google Scholar]
- 5.Bioxandera A., Arumi J., Martinex-Castillo V. Prospective clinical trial evaluating the efficacy of photodynamic therapy for symptomatic circumscribed choroidal hemangioma. Ophthalmology. 2009;116:100–105. doi: 10.1016/j.ophtha.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Blasi M., Tiberti A.C., Scupola A., Balestrazzi A., Colangelo E. Photodynamic therapy with verteporfin for symptomatic circumscribed choroidal hemangioma: five-year outcomes. Ophthalmology. 2010;117:1630–1637. doi: 10.1016/j.ophtha.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 7.Zeisberg A., Seibel I., Cordini D. Long-term (4 years) results of choroidal hemangioma treated with proton beam irradiation. Graefes Arch Clin Exp Ophthalmol. 2014;252:1165–1170. doi: 10.1007/s00417-014-2635-1. [DOI] [PubMed] [Google Scholar]
- 8.Minoru F., Sekiryu T., Kasai A., Oguchi Y. Morphologic changes of the fovea and visual acuity associated with retinal detachment secondary to circumscribed choroidal hemangioma. Saudi J Ophthalmol. 2013;27:209–213. doi: 10.1016/j.sjopt.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunduz K. Transpupillary thermotherapy in the management of circumscribed choroidal hemangioma. Surv Ophthalmol. 2004;49:316–326. doi: 10.1016/j.survophthal.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R., Lavric A., Resotri M., Pavesio C., Sagoo M.S. Nodular posterior scleritis: clinic-sonographic characteristics and proposed diagnostic criteria. Retina. 2015;0:1–10. doi: 10.1097/IAE.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 11.Kivela T., Tenhunen M., Joensuu T., Tommila P., Joensuu H., Kouri M. Stereostatic radiotherapy of symptomatic circumscribed choroidal hemangiomas. Ophthalmology. 2003;110:1977–1982. doi: 10.1016/S0161-6420(03)00483-4. [DOI] [PubMed] [Google Scholar]
- 12.Shields J.A., Shields C.L., Materin M.A., Marr B.P., Demirci H., Mashayekhi A. Changing concepts in management of circumscribed choroidal hemangioma. Ophthalmic Surg Lasers. 2004:383–392. [PubMed] [Google Scholar]
- 13.Torres V.L.L., Brugnoni N., Kaiser P.K., Singh A.D. Optical coherence tomography enhanced depth imaging of choroidal tumors. Am J Ophthalmol. 2011;151:586–593. doi: 10.1016/j.ajo.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Cerman E., Cekic O. Clinical use of photodynamic therapy in ocular tumors. Surv Ophthalmol. 2015;60:557–574. doi: 10.1016/j.survophthal.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Su Z., Tang X., Zhang L., Su X. Comparison of outcomes between overlapping-spot and single-spot photodynamic therapy for circumscribed choroidal hemangioma. Int J Ophthalmol. 2014;7:66–70. doi: 10.3980/j.issn.2222-3959.2014.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan L.W., Hsieh Y.T. Photodynamic therapy for choroidal hemangioma unresponsive to ranibizumab. Optom Vis Sci. 2014;91:e226–e229. doi: 10.1097/OPX.0000000000000349. [DOI] [PubMed] [Google Scholar]


