Abstract
The topical treatment of nail fungal infections has been a focal point of nail research in the past few decades as it offers a much safer and focused alternative to conventional oral therapy. Although the current focus remains on exploring the ways of enhancing permeation through the formidable nail barrier, the understanding of the nail microstructure and composition is far from complete. This article reviews our current understanding of the nail microstructure, composition and diseases. A few of the parameters affecting the nail permeability and potential causes of the recurrence of fungal nail infection are also discussed.
Keywords: Onychomycosis, Nails, Fungal infections, Anti-fungal, Iontophoresis, Keratin, Laser treatment
Introduction
The nail is modified to a great extent in some mammals, for example, as a prehensile organ – the claw, or as a point of locomotion – the hoof. In humans, the nail plate is the completely keratinized part of the upper surface of the tip of each finger and toe which provides protection to the phalanges, enhances dexterity and facilitates scratching. In addition, nails serve an aesthetic and cosmetic purpose.
The development of nail unit in humans takes place in between the 9th and 17th weeks in utero by an intricate series of mesenchymal-ectodermal interactions [1]. The individual digits are apparent from the 8th week of gestation [2]. Nail anlage, the epidermis overlying the dorsal tip of the digit, presents from 9 weeks and is the first embryonic element of the nail unit. A distinct region called the primary nail field can be seen at 10 weeks overlying the tip of terminal phalanx. At the 13th week, proximal and lateral nail folds develop as a result of differential slow growth of the primary nail field and surrounding tissue. By the 14th week, the nail plate can be seen emerging from under the proximal nail fold. At the 17th week, most of the nail bed is covered by nail plate. From week 20, finger and nail grow approximately in tandem.
The growth of mature adult nails has been studied extensively [3, 4]. Normal fingernail growth varies from less than 1.8 mm to more than 4.5 mm/month and varies distinctly between individuals, but is more consistent among members of the same family [5]. The average nail growth rate is 0.1 mm/day (3 mm/month) and is used to predict nail regrowth time [6]. A normal fingernail grows out completely in about 6 months whereas toenails grow one-third to half the rate of fingernails; thus, toenails take 12–18 months to grow out entirely [7]. The nail growth rate is less than normal in people who are immuno-compromised, immobilized or paralyzed, malnourished, suffering from acute infection or undergoing anti-mitotic drug therapy [8].
Structure of Human Nail Unit
Fig. 1 shows a schematic diagram of human nail unit which is comprised of four main structures: the proximal nail fold (PNF), nail matrix, nail bed and hyponychium. These structures together form nail plate – the flat, rectangular, translucent, keratinized structure sitting on the upper surface of the tip of each finger and toe. The cells in the nail plate are closely-packed, adherent, interdigitating and very flat, lying perpendicular to the plane of the nail plate surface.
Figure 1.
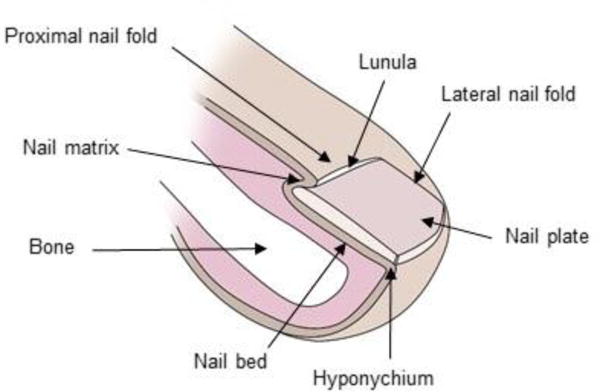
Schematic diagram of human nail plate.
The proximal nail fold (PNF)
The PNF is a wedge shaped fold of skin lying on the dorsum of finger and toes which covers approximately one-fourth of the total nail length. The nail plate appears to emerge out of the PNF. The skin on either side of the nail is an extension of the PNF, and is known as the lateral nail fold. It comprises two layers of epidermis; the dorsal layer which forms the dorsum of finger epidermis and the ventral portion which forms eponychium. The stratum corneum of eponychium forms a thick rim of keratinous material, known as cuticle, around the margins of the PNF which along with the PNF and lateral folds provide protection against penetration of water and toxic chemicals. The disruption of cuticle can lead to bacterial or fungal infection resulting in a nail disease called paronychia, which is characterized by the inflammation of the PNF [9].
The nail matrix and nail plate
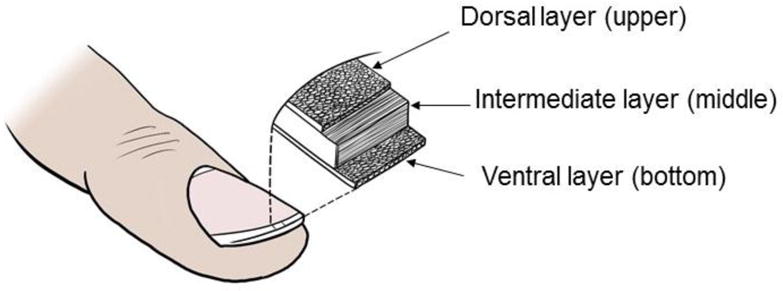
Directly underneath the PNF is a small area of highly proliferative epidermal tissue, known as the nail matrix, which produces the nail plate. Nail plate is made up of three different layers which are well-bonded together. The visible white area of nail matrix is called lunula. The nail plate pigmentation varies with race and is defined by melanocytes present in the nail matrix [8]. Langerhan’s cells are also present in the nail matrix [10]. Nail Matrix produces the nail plate which comprises of an average of 196 cell layers [11] that are distributed among three tightly bound layers – dorsal, intermediate and ventral (Fig. 2). The thickness ratio of the three layers of nail plate was found to be 3:5:2 by Kobayashi et al. [12]. In the same study, dorsal plate was stated to be the rate limiting barrier for permeation of 5-fluorouracil (5-FU), when compared with intermediate and ventral layers [12, 13]. This observation has not been independently confirmed, either with 5-FU or with other compounds. Examination of the biochemistry of nail plate suggests that the interpretation of dorsal layer being the primary barrier for nail permeation should be viewed cautiously. Although the sulfur concentration is equivalent in the dorsal and intermediate plates, sulfhydryl groups are predominant in dorsal plate whereas the amino acid cystine, which contains disulfide bonds, is concentrated in the intermediate plate [9]. This indicates a higher degree of crosslinking in the intermediate plate, which limits swelling and suggests that its barrier properties are likely to be significant.
Figure 2.
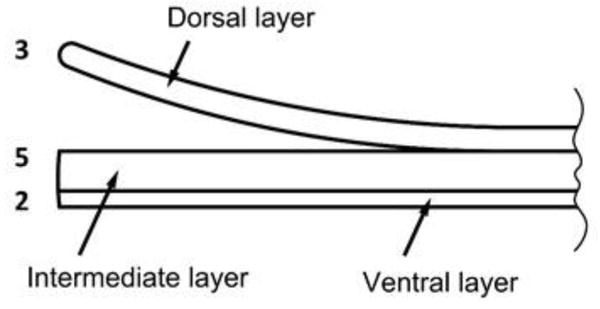
Three layers of the nail plate drawn to scale to represent 3:5:2 thickness ratio.
Of the three main classes of protein (fibrous, globular and conjugated), the nail plate is composed of keratin, a scleroprotein (fibrous protein) which contains large amounts of sulfur. Polypeptide chains are the basic macromolecules which form keratin. These chains exist either in curled helical conformation (α – keratin) or side-by-side pleated conformation (β – keratin). In mammals, α – keratins are the primary constituents of hair, nails, hooves, quills, horns and the epidermal layer of the skin [14]. Two distinct types of α – keratin exist, (a) hard α – keratins, derived embryologically from epithelial cells, are the main constituents of cornified mammalian tissues such as nails, hooves, horns, quills, and (b) soft α – keratin, is the main component of epidermis (of skin) and other epithelial tissues. Human nails are comprised of approximately 80% hard α – keratin and 20% soft α – keratin [15]. The dorsal plate cells have thickened plasma membranes and are anucleated as shown by the lack of acid phosphatase activity. The intermediate plate possesses some eosinophilic cytoplasm and nuclear remnants. They are held firmly together by desmosome glue spots and complex interdigitations. The cells of the ventral plate emerge from the nail bed and are an easy target for disease [16].
The keratin intermediate fibers/filaments (~70 A° diameter) in the intermediate layer are well aligned and oriented in the transverse direction laterally, perpendicular to the axis of nail growth, whereas keratin fibers in the dorsal and ventral plates are packed like overlapping tiles and have no preferred orientation (Fig. 3) [14, 17, 18]. The toughness of the nail plate can be attributed to this alignment of keratin fibers and to the presence of large numbers of disulfide cross-links due to high proportions of cystine [19].
Figure 3.
Orientation of keratin fibers in the three layers of the nail plate.
The microstructure of nail plate was further analyzed in detail by observing effects of keratinase enzyme on nail plate [20]. It was observed that the enzyme acted on the intercellular matrix that holds the nail corneocytes together and the nail corneocytes on the dorsal surface appeared to be separated from each other. In contrast, the nail corneocytes were not differentiated in control nail sample immersed in buffer solution as they were closely bound to each other. The deeper layers of nail plate (intermediate and ventral) were not affected by the enzymatic action probably due to hindered diffusion of large molecular size (MW ~ 33,000) of the enzyme to these layers.
To supplement the microstructural findings of Mohorcic et. al. [20], SEM imaging on nail plate was also performed in collaboration with Forensic Science Lab at Amway Corporation, Ada, MI. (Fig. 4, 5 and 6). SEM imaging was performed on nail clipping under three different conditions – (i) room temperature – Fig. 4 (A–F) and 5(A); (ii) hydrated nail after 24 hrs – Fig. 5(B); and nail treated with 5%w/w Solvable® tissue dissolving fluid (after 4 hrs – Fig. 6(A–B)). The morphological features of the three layers were found to be similar as mentioned earlier.
Figure 4.
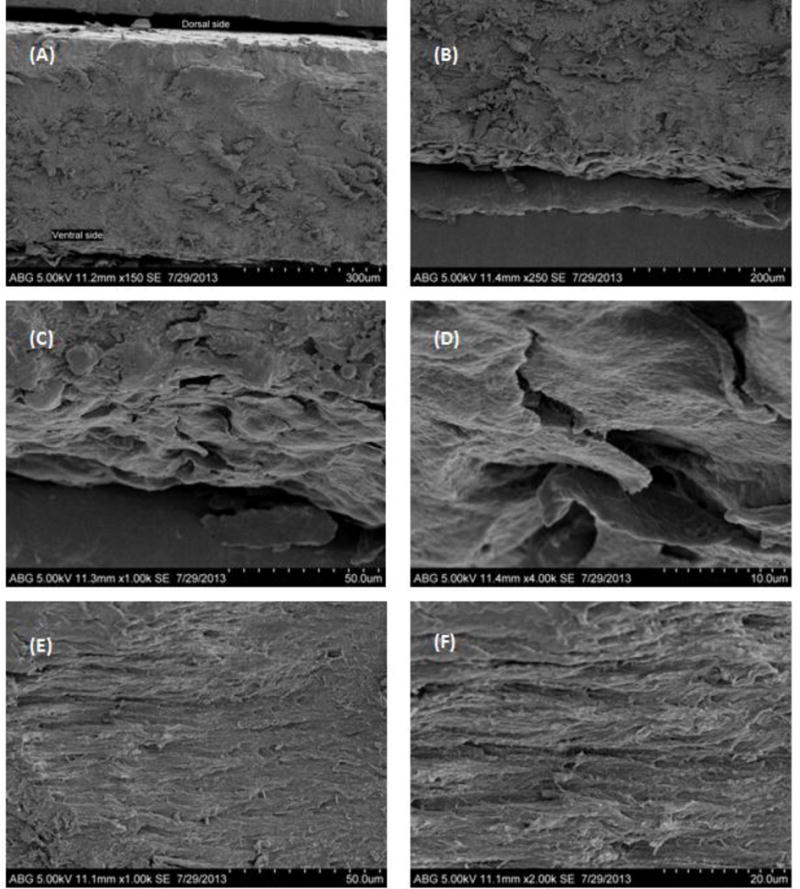
SEM images of the cross-section of nail showing (A) three layers of nail plate (150×); (B) intermediate and ventral layer (250×); (C) ventral layer (1000×); (D) ventral layer cells (4000×); (E) fiber orientation in the intermediate layer (1000×) and (F) fiber orientation in the intermediate layer (2000×).
Figure 5.
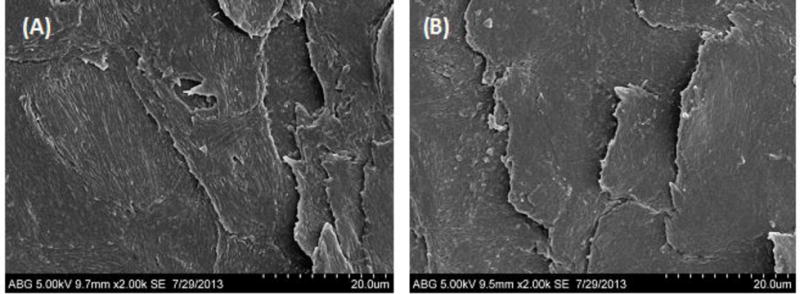
SEM images of (A) dorsal surface of dry nail and (B) dorsal surface of hydrated nail.
Figure 6.
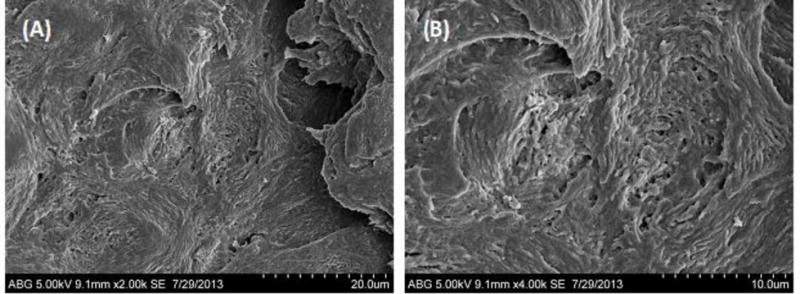
SEM image of a nail immersed in diluted tissue dissolving fluid (Solvable® 5%v/v) for 4 hours. (A) 2000x; (B) 4000x.
The surface appearance of dry nail clipping at room temperature was found to be different than hydrated nail clipping. The dry nail clipping showed stratified corneocyte surface with ridges on the dorsal side, probably representing bundles of keratin fibers (Fig. 5(A)). In comparison, the hydrated nail plate showed less striation/ridges on the dorsal surface (Fig. 5(B)).
Based on the autoradiographic data, the thickness of the nail plate is determined by the length of the matrix [6]. The thickness of the nail plate progressively increases from proximal to free distal end [11]. The distal plate thickness in normal nail can be ranked as thumb > index > middle > ring > little finger [21]. The average nail plate thickness is 0.5 mm in women and 0.6 mm in men. The nail plate thickness progressively increases with age as a result of increasing size of the cells in the plate and decreased growth rate [5].
The nail bed
The nail bed is a pink-colored soft, thin, noncornified epithelium surface, devoid of granular layer and sebaceous glands, which lies below the nail plate and extends from the nail matrix to the hyponychium. Sometimes, it is referred to as the ventral matrix, since it contributes a few layers of cells on the ventral surface of the nail plate. It is considered to be a transitional zone where living cells keratinize and are integrated in the nail plate. Also, the nail bed is known to have corpuscles and nerve endings which are responsible for sensitivity to light, touch and pressure [9]. The pink color of the nail bed is due to the enriched vascular supply just below the nail bed.
The hyponychium
The hyponychium is the thickened epidermis beneath the free distal end of the nail of a digit. It performs the same protective function as provided by the cuticle, PNF and lateral folds.
Formation of Nail Plate
The nail matrix constantly replenishes the nail plate. The nail matrix is comprised of stratified squamous epithelial cells which are held together by long rete ridges and sparse dermis. Mitosis in the basal layer of the nail matrix constantly replaces the matrix. The matrix cells differentiate and move up continuously, and consequently flatten by dumping their organelles and condensing their cytoplasm. The pressure of this new buildup forces the other cells (nail plate) to move dorsally (Fig. 7). In due course, the cells keratinize as the nuclei vanish [22]. Cell division of nail matrix is continuous; thus, nail is formed continually, unlike hair which is formed cyclically. The cells at the dorsal surface of nail plate overlap to form a smooth surface, in contrast to the ventral surface which is irregular allowing interdigitation with the nail bed. The matrix turnover rate defines the growth rate of nail. The nail form is determined primarily by the growth rate of the dorsal, intermediate and ventral matrices, and could be affected by various disease conditions (Fig. 8).
Figure 7.
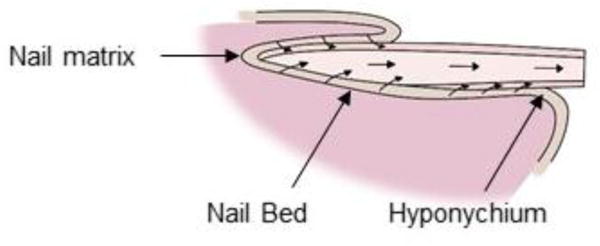
Directions of cell movement in the lumen of nail and its matrices.
Figure 8.
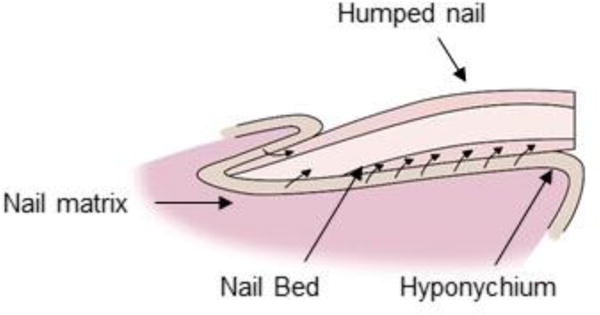
Human nail growth showing humping of nail plate when increasing numbers of ventral plate cells are added. This condition is exacerbated in certain nail disorders.
Biochemistry of the Human Nail Plate
The keratins found in human nail plate are almost identical to those of hair [23, 24], which is primarily composed of α – keratin. The analysis of human nail plate by immunoblotting technique has shown that it is comprised of approximately 10–20% soft epithelial keratins (50K/58K and 48K/56K keratin pairs) with the remainder being hard α-keratin [15]. Despite the existence of different forms of α – keratins, they exhibit many similarities in their chemical and structural features. Figure 9 demonstrates the basic molecular structure of α – keratin. The isolated α helical chains (formed by condensation of amino acids) form a coiled coil dimer with crosslinked disulfide bonds to assemble into a protofibril unit. Eight protofibril units polymerize together to form the basic structural unit, the intermediate filament (IF) or microfibril. In human hair, α – keratins have unique arrangement of aligned IFs (~ 7 nm diameter and spacing of 10 nm) and are enclosed by a non-filamentous protein matrix [25] (Fig. 10). These hair-like intermediate filaments which exist only in the intermediate layer of the nail plate [26] are measured to be approximately 7 nm in diameter by various researchers [14, 27].
Figure 9.
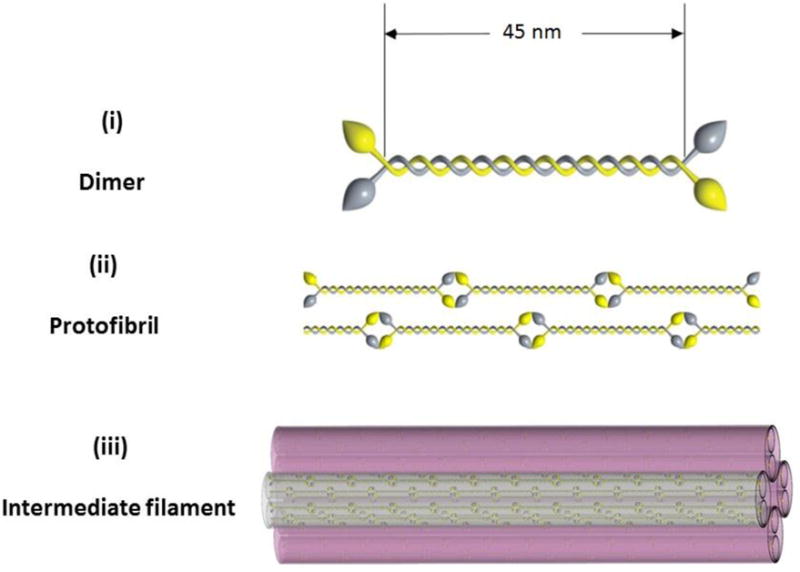
Molecular structure of α – keratin. (i) A dimeric coiled coil formed by two keratin polypeptides; (ii) tail-to-head association of coiled coils to form protofibril and (iii) eight protofibrils associate together to form an intermediate filament (IF).
Figure 10.
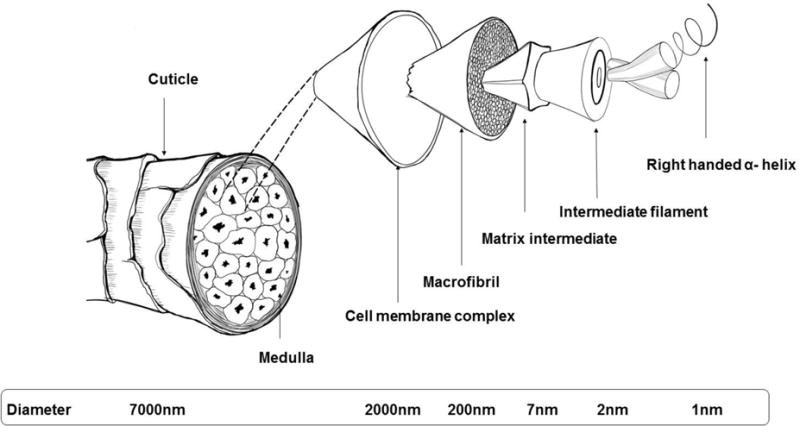
Schematic diagram of a human hair fiber.
The polypeptide chains in keratin are formed by condensation of several amino acids. Although all are derived from the ectodermal cells, fundamental physical and chemical differences in fully differentiated forms of nail, hair and stratum corneum exist. A comparison of amino acid composition of human nail, hair and stratum corneum is shown in Table 1 [19, 28]. Hair and nail are differentiated along the same lines despite their different morphological characteristics [9]. Nail contains 10–20% more basic soft keratins, in addition to the sulfur-rich hard keratins present in both tissues [15]. A significant proportion of glutamic acid, half-cystine, arginine, aspartic acid, serine and leucine are present in the human nail plate. It is noteworthy to observe that the content of glycine and half-cystine is similar in human hair and nail, but not stratum corneum. An outstanding degree of physical and chemical stability is conferred to the keratin filaments by the extensively cross-linked disulfide structures corresponding to the high proportions of cystine [19].
Table 1.
Amino acid composition (as residues per 100 residues) of human nail, hair and stratum corneum [19, 28].
| Amino acid | pI a | Nail (%) | Hair (%) | Stratum corneum (%) |
|---|---|---|---|---|
| Lysine | 9.74 | 3.1 | 2.5 | 4.2 |
| Histidine | 7.59 | 1.0 | 0.9 | 1.5 |
| Arginine | 10.76 | 6.4 | 6.5 | 3.8 |
| Aspartic acid | 2.77 | 7.0 | 5.4 | 7.9 |
| Threonine | 5.60 | 6.1 | 7.6 | 3.0 |
| Serine | 5.68 | 11.3 | 12.2 | 13.6 |
| Glutamic acid | 3.22 | 13.6 | 12.2 | 12.6 |
| Proline | 6.30 | 5.9 | 8.4 | 3.0 |
| Glycine | 5.97 | 7.9 | 5.8 | 24.5 |
| Alanine | 6.00 | 5.5 | 4.3 | 4.4 |
| Valine | 5.96 | 4.2 | 5.5 | 3.0 |
| Methionine | 5.74 | 0.7 | 0.5 | 1.1 |
| Isoleucine | 6.02 | 2.7 | 2.3 | 2.7 |
| Leucine | 5.98 | 8.3 | 6.1 | 6.9 |
| Tyrosine | 5.66 | 3.2 | 2.2 | 3.4 |
| Phenylalanine | 5.48 | 2.5 | 1.7 | 3.2 |
| Half-cystine | 5.07 | 10.6 | 15.9 | 1.2 |
pI is the isoelectric point of the individual amino acids.
The proteins of nail and hair are alike and have extensive folding supported by extremely stable disulfide bonds and in addition, have Van der Waals interactions, hydrogen bonds and coulombic interactions. This explains the high degree of chemical and physical resistance of nail and hair, in contrast to the skin. Although these disulfide bonds are also found to exist in skin to a lesser extent, the geometric spatial configuration of these bonds is different for nail/hair and skin. As determined by Raman spectroscopy, hair and nail have these bonds in gauche-gauche-gauche conformation and those for stratum corneum are in gauche-gauche-trans conformation, with the latter being less stable [29].
The extreme folding of protein molecules and different geometries of disulfide bonds in nail/hair and skin has resulted in a different degree of hydration. Water has been stated to exist in the nail plate in either free or bound form, with the latter accounting for most of the nail’s water content [29]. In comparison with the normal nail structure, the loose skin structure allows more free water than bound water. More quantitative analysis indicates that very little of the water in the nail plate can be considered to be “free” [30]. The uptake or rate of loss of water is not affected by the extraction of lipids which suggests that the role played by the lipid pathway in diffusion process of water across the nail plate is insignificant [31]. Although the detailed characterization of nail keratins has not been done to date, one can approximate a weighted average isoelectric point (pI) of the nail plate by using the percent composition and the individual pI’s of the amino acids from Table 1. This value was calculated to be 4.7.
The amounts of minerals and electrolytes found in nail plate are highly variable. They include elements such as sodium, potassium, nitrogen, magnesium, calcium, iron, copper, zinc, phosphorus, selenium, sulfur and several others [9, 10, 22]. Table 2 gives an overview of different methods employed to determine minerals and other exogenous materials in the nail plate. Cholesterol is the primary lipid component of the nail plate. In contrast to the 10% lipid content and 20–40% water content in the stratum corneum, nail plate contains 0.1–1% lipids and 7–12% water (varies with relative humidity) [9].
Table 2.
Different methods for constituent analysis of nail plate.
| Method | Element | Reference |
|---|---|---|
| I. Structural and mineral constituents | ||
|
| ||
| Raman spectroscopy | Water, protein and lipid | [29] |
| Immunohistochemistry | Keratin | [32] |
| Polymerase chain reaction | Deoxyribonucleic acid | [33, 34] |
| Electron microscopy | Cystine | [35] |
| X-ray diffraction | Mg, Cl, Na, Ca, S, Cu | [36, 37] |
| Colorimetry | Fe | [38] |
|
| ||
| II. Exogenous materials | ||
|
| ||
| Atomic absorption spectroscopy | Cd, Pb, Zn, Ca, Cr, Fe, Cu, Cr, Fe, Mn, Ni, Co, Na, K | [36, 39, 40] |
| Mass fragmentography | Metamphetamine | [41] |
| Gas chromatography | Amphetamine, Cocaine | [42, 43] |
| Flow injection hydride generation atomic absorption spectrometry | Arsenic | [44] |
Biophysical Properties of Human Nail Plate
Robson et al. measured the Knoop hardness number (KHN – in kg/mm2) of nail plate and found it to be 24.25 and 34.09 for healthy and malnourished individuals, respectively [45]. Newman and Young also found the KHNs of the human nail in the range of 20 – 40 kg/mm2 [46]. When the nail was cut parallel and perpendicular to the axis, the Young’s modulus of elasticity was measured at 4.3 × 1010 and 2.1 × 1010 dynes/cm2, respectively [31]. It was also demonstrated by Forslind that the effective elastic modulus of nail is directly related to cell arrangement, cell adhesion, microstructure of keratin fibers and hydration level [36]. Farren et al. showed that cutting nail longitudinally requires double the energy (~ 6 kJ/m2) than cutting it transversely (~ 3kJ/m2) and that the upper layers of nail plate provide most of the nail resistance against tearing [17, 28].
Electrical properties of Human Nail Plate
In vivo and in vitro resistance of nail plate immersed in phosphate-buffered saline (PBS) solutions as a function of time (hydration) was studied by Hao et al. [47]. The resistance of nail plate in both in vivo and in vitro experiments decreased significantly upon 2-h nail hydration and then slowly decreased to a constant value over time. A significant decrease in nail resistance with hydration supports the notion that nail hydration plays a significant role in the diffusion process through the nail.
Permeability of Human Nail Plate
Differences in the physical and chemical characteristics of nail and stratum corneum translate to differences in solute permeation through the membrane. Although the nail plate has been historically considered as a fiber matrix system rather than a lipophilic membrane [48, 49], a recent report by Baswan et al. [50, 51] suggested that there might be additional structure to the nail plate such as residual lipids which leads to lower permeability of hydrophilic to moderately lipophilic solutes. The diffusion of hydrophilic solutes may be partially impeded by the remnants of the cell membranes which may form a discontinuous lipid barrier. Permeation of drugs through the nail plate depends on various parameters such as the physico-chemical properties of the drug, nail properties such as hydration and thickness, the nature of the formulation (e.g. pH and use of chemical penetration enhancers) and the binding of drug to keratin matrix, which have been widely studied by various researchers [12, 47–49, 52–62].
Effect of Lipophilicity and Molecular Weight of the Permeant
Walters et al. performed transungual permeation studies for a homologous series of alcohol ranging from methanol to dodecanol and compared it to stratum corneum data [48, 49]. As the alkyl chain length increased from 1 to 8, the permeability coefficient through the nail plate decreased. This result was attributed to a decrease in the partitioning of solutes in the nail plate. They also suggested the existence of a parallel lipid pathway for extremely lipophilic compounds, since the permeability coefficient of alkanols after n-octanol increased with chain length. Studies conducted by Kobayashi et al. suggested that the dependence of permeability on molecular weight is more significant than lipophilicity [55]. This conclusion was also supported by a more comprehensive dataset of 42 compounds analyzed by Baswan et al. [50, 51]. Studies conducted by Mertin and Lippold on ten anti-fungal drugs also showed that hoof permeability decreased with an increase in molecular weight of the drug [57]. The existence of a parallel lipid pathway as proposed by Walters et al. was not supported by studies conducted by Kobayashi and others.
Effect of pH
The effects of pH on nail permeability using ionizable drugs have been studied by various researchers. The ionization of drug has been associated with a decrease in transungual permeability due to an apparent increase in the hydrated radius of the molecule [55, 57]. Studies conducted by Soong [59] in nail plate demonstrated that the permeability coefficient of benzoic acid (a weak acid) decreased by 95.5% as pH was increased from 2.0 to 8.5, indicating that maximum permeation rate was achieved for undissociated benzoic acid at a lower pH. Similar studies were conducted by Mertin and Lippold [57] in bovine hoof membrane using benzoic acid and pyridine (a weak base) at pH 2.0 and 7.4. The authors attributed the enhanced permeability of benzoic acid at pH 2.0 and pyridine at 7.4 to Donnan equilibrium effect of nail plate. As the isoelectric point of the keratin is approximately 5, it is negatively charged at pH 7.4 and positively charged at pH 2.0. Thus, it hinders transport of positively charged pyridine molecule at pH 2.0 and enhances the permeation of undissociated benzoic acid and vice-versa at pH 7.4. Kobayashi et al. demonstrated that the dissociation of the drug leads to reduction of permeability in nail plate, irrespective of the charge on the drug [55]. A magnitude of difference on log scale in the regression lines (permeability vs. molecular weight) of ionic and non-ionic drug was observed. On the contrary, Walters et al. studied the nail permeation of anti-fungal drug miconazole and concluded that both ionized and neutral form penetrated the nail at comparable rates [48, 61].
Similar to passive diffusion, transungual iontophoresis is also affected by the change in pH. The nail plate exhibited iontophoretic permselectivity for the delivery of glucose and griseofulvin [63], similar to that observed in human skin [56, 64]. The permselectivity of nail plate is attributed to the nature of keratin protein whose isoelectric point is around pH 5 and thus, the nail is net negatively charged at pH above the isoelectric point. The anodal iontophoresis was favored at pH > 5 for both glucose and griseofulvin, as the nail plate is negatively charged and enhanced the transport of glucose by convective water flow from anode to cathode direction. It is also noteworthy to observe that passive flux of griseofulvin did not vary significantly with pH. The extent of keratin binding with griseofulvin (33 ± 3.1%) was found to be independent of pH.
Effect of Nail Hydration
The nail plate hydration plays a significant role in permeability of the nail plate. Water flux in fully hydrated nail plate was found to be 12.6 ± 5.6 mg/cm2/h or 126 ± 56 g/m2/h (permeability coefficient of (16.5 ± 5.9) × 10−3 cm/h) by Walters et al. [60], which is almost 6.5 times the value of 2.5 mg/cm2/h determined by Burch and Windson in dry nails [65]. This is attributed to the fact that hydrated nail plate holds 0.3–0.5 g of water/g of dry nail weight [30, 31, 53, 66, 67]. Environmental factors such as temperature also influence the permeation in nail plate. Malhotra et al. found that the water permeability through nail plate is enhanced significantly with an increase in temperature (test conditions: 37°C, 47°C and 57°C) [68, 69]. The activation energy of water permeation through nail plate (7.2 kcal/mol) was found to be closer to the value of delipidized human skin (6.3 kcal/mol) than for intact stratum corneum (14.3 kcal/mol). The transonychial water loss (TOWL) was found to be lower for diseased nails (6.9 g/m2/h) than healthy nails (19.4 g/m2/h) [70, 71]. Jemec et al. did not find any correlation between TOWL and nail thickness, whereas Murdan et al. [72] and Dutet et al. [73] found an inverse linear relationship of TOWL with nail thickness. Also, the values of TOWL in the literature vary from 18 – 80 g/m2/h [70, 72, 73]. This large variation is attributed to inter- and intra- (different digits) subject variability, environmental conditions and method of measurement. In spite of the difference in TOWL for healthy and diseased nails, Kobayashi et al. demonstrated that permeability in nail with fungal infection can be estimated by data on healthy nail plate [55]. Although transepidermal water loss (TEWL) is considered as a marker for epidermal damage, TOWL has not been validated as a marker for nail plate damage.
Other Enhancement Methods
Chemical enhancers such as urea, thioglycolic acid, hydrophobins, 2-mercaptoethanol, N-acetyl-L-cysteine, keratolytic agents and surfactants [53, 66, 74, 75] have shown some promising results in enhancing nail permeability. Other physical methods such as iontophoresis [52, 63, 76–80], nail abrasion [81–83], acid etching [84] and nail micro-drilling [85] also enhance the permeability of the nail plate. Combination of one or more of the above mentioned methods has been reported to yield permeability enhancement synergistically [53, 86].
Nail Diseases – Onychomycosis
The term onychomycosis is derived from the Greek word “onyx” which means nail and “mykes” meaning fungus. The term is used to describe fungal infection of one or more nail units which can be caused by dermatophytes, yeasts or nondermatophyte molds. The susceptibility of toenail to this disease is 25 times more than the fingernails due to the brunt of the body pressure and occlusive nature of footwear [87]. It is usually asymptomatic and thus patients usually do not have any physical complaints and assume it as a cosmetic problem. As the disease progresses, it can cause pain, discomfort, loss of dexterity and sensation.
Etiology
There are three groups of fungi associated with onychomycosis: dermatophytes, non-dermatophytic molds and yeast. The rate of involvement of various fungal species in onychomycosis are given in Fig. 11 [88]. Dermatophytes are the primary causative agents in onychomycosis, accounting for nearly 90% of toenail infections and 50% of fingernail infections. Common fungal agents associated with onychomycosis are:
Dermatophytes: Trichophyton rubrum, Trichophyton mentagrophytes and Epidermophyton floccosum
Nondermatophyte fungi: Acremonium species, Alternaria species, Aspergillus species, Botryodiplodia theobromae, Fusarium species, Onycochola Canadensis, Scytalidium dimidiatum, Scytalidium hyalinum, Geotrichum candidum, Cladosporium carrionii andScopulariopsis brevicaulis
Yeast: Candida albicans
Figure 11.
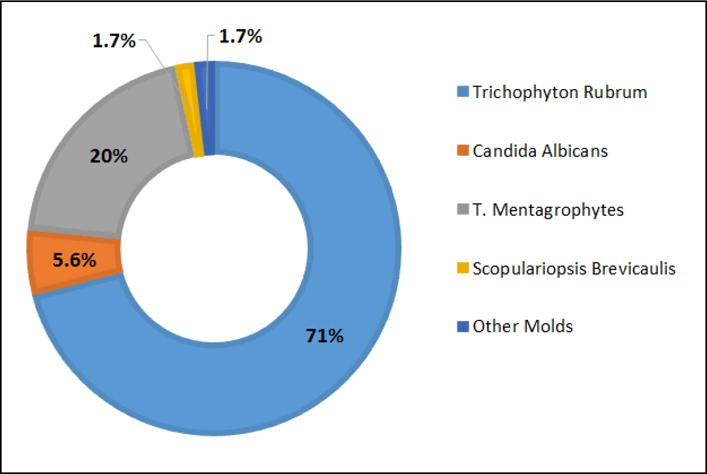
The fungi involved in onychomycosis. Figure based on data from [88].
Clinical Presentation of Fungal Infection in Onychomycosis
There are four main clinical presentations of onychomycosis, many of which can be further subdivided into four major types as follows:
Superficial Onychomycosis (SO) – This superficial onychomycosis is primarily caused by dermatophyte T. mentagrophytes and sometimes by nondermatophytic molds like Acremonium spp., Aspergillus terreus and Fusarium oxysporum. The superficial layers of nail plate are directly invaded by fungi and can be recognized by the presence of opaque yellowish-white islands on the external nail plate. As the viable tissue is not involved in SO, inflammation is minimal in patients.
Proximal Subungual Onychomycosis (PSO) – In PSO, the fungal organisms invade the nail unit through proximal nail fold, via cuticle, and penetrate the newly formed nail plate migrating distally resulting in subungual hyperkeratosis, proximal onycholysis and destruction of the nail plate. This fungal infection is visible through the cuticle as yellowish-white discoloration, while the distal nail plate remains normal. It is primarily caused by dermatophyte, T. rubrum.
Distal Lateral Subungual Onychomycosis (DLSO) – DLSO is characterized by the invasion of nail bed and the underside of nail plate by fungi and can be best described as “nail bed dermatophytosis”. The infecting fungi migrate proximally through the underlying nail matrix. It causes mild inflammation and often results in onycholysis and subungual thickening. The common dermatophytes responsible for DLSO are T. rubrum, T. mentagrophytes, T. tonsurans and E. floccosum.
Total Dystrophic Onychomycosis (TDO) – There are two forms of TDO: primary and secondary. The entire nail unit is involved in TDO and is considered as a combination of all types of the disease.
Epidemiology of Onychomycosis
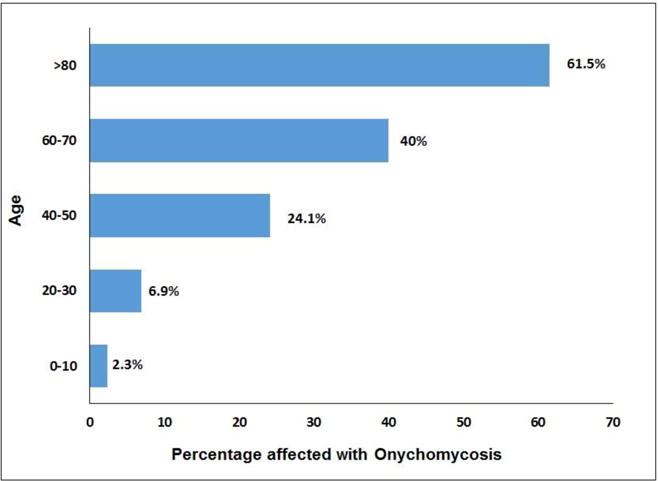
The literature available on the epidemiology of onychomycosis is varied. It accounts for approximately 50% of all nail fungal infections and affects 2–18.5% or more of the world’s population [87, 89–93]. Age, social class, climate, occupation, predisposing factors, frequency of travel and living conditions determine the prevalence rate of onychomycosis. Studies have shown that prevalence of onychomycosis increases with age (Fig. 12) [88, 92, 94]. The reasons for this age-dependent prevalence may be poor peripheral circulation, smoking habits, diabetes, nail trauma, long exposure to pathogenic fungi, suppressed immune functions and inability to maintain good foot care [89, 95]. It is found to be 2–3 times more common in diabetics than nondiabetics [96, 97]. The prevalence of onychomycosis among children varies from 0.44% (United States, Wales and Finland) to 2.6% (Guatemala) and this 30-fold decrease in prevalence rate relative to adults may be attributed to faster nail growth, small surface area of nail, less time spent in environment containing pathogens and lower prevalence of tinea pedis [88, 98].
Figure 12.
Clinical Diagnosis and Treatments of Onychomycosis
In the present world where advanced medical healthcare is available for most of the conditions, the high prevalence of nail fungal infections suggests that either the treatments currently available are not efficacious or the patients do not realize the condition in early stages. The clinical diagnosis of onychomycosis is based on a physical examination, microscopy culture of nail specimens and PAS (periodic acid-Schiff) examination of subungual debris (highest sensitivity of all methods). The etiologic agent is identified by culture of sampled nail specimen [87].
The treatment of onychomycosis range from palliative to curative therapies which involve the use of oral anti-fungals, topical therapy and clinical debridement or nail avulsion. The newer oral anti-fungal agents like terbinafine hydrochloride, itraconazole, etc., have shown promising efficacies (~ 60%) in the treatment of fungal nail infections but has high relapse rates ranging from 25–50% [90, 93, 95, 96, 98, 99]. In addition, adverse effects such as headache, risk of hepatotoxicity, potential drug–drug interactions limit the use of oral medications, leading to only 35–65% of physicians prescribing oral therapy for the treatment of onychomycosis [93]. Topical therapies range from the use of topical medicated nail lacquer formulations such as ciclopirox solution (8%) to physical treatments like nail debridement or nail avulsion. In a placebo-controlled, double-blind clinical study, once-a-day application of 8% ciclopirox solution for 48 consecutive weeks resulted in mycological cure rates of 29–36% and complete cure rates of 5.5–8.5% [93]. The complete cure rates of 8% ciclopirox solution reported to the FDA during the registration process were as low as 2–3% for different stages of onychomycosis [100]. Ciclopirox topical nail lacquer is most effective in treating superficial and mild, distal lateral onychomycosis. When used alone, topical anti-fungal agents are of limited efficacy but may result in synergistic action when used in combination with oral anti-fungals.
Photodynamic therapy and laser treatments are approved by Food and Drug Administration (FDA) for temporarily increasing the clear nail in patients with onycomycosis. Although, the use of these devices is claimed to have minimum side effects such as feeling of warmth during treatment and rare side effects such as redness of treated skin around the nail, discoloration of nail surface, blistering and scarring, very little is known about its potential of thermal damage and long term safety. Examples of the lasers commercially available for the treatment are PinPointe™ FootLaser, JOULE ClearSense™, Q-Clear™, CoolTouch™ VARIATM and CuteraGenesisPlus™[101]. Very scarce data exist on the efficacy of these laser treatments [102, 103]. The Pinpointe™ Foot Laser claims to cure 70% of the patients with one time treatment but these results have not been validated independently. Most recently, FDA issued warning letter to Nuvolase Inc. indicating that PinPointe™ FootLaser should not be referred as a “treatment for onychomycosis” either on the company website or in the product manuals [104]. In the most recently published study by Hollmig et al. [105], no significant fungal clearance in the mycological culture and the clinical nail plate was observed when treated with 1064-nm Nd:YAG laser (neodymium:yttrium-aluminum-garnet) in comparison to the control. Another study also demonstrated that the Nd:YAG laser treatment failed to inhibit the growth of in vitro colonies of Trichophyton rubrum [106].
Although the condition of onychomycosis is treatable, the current therapeutic options are limited due to long courses of drug treatment required to achieve an effect, potential hepatotoxicity complications of oral formulations and low efficacy for commercial topical formulations. New techniques such as topical anti-fungal formulations using chemical penetration enhancers [53, 66, 74, 75], anti-fungal drug delivery through micro-drilling [85] and iontophoresis [74] are also being evaluated as an alternative treatment for onychomycosis.
Transungual Iontophoretic Drug Delivery
The drug/device combination which uses iontophoresis to deliver high concentrations of medication directly to the site of action can greatly improve the treatment of onychomycosis. The literature demonstrating transungual iontophoresis dates back almost three decades. In 1986, James et al. tried transungual delivery of prednisolone across thumbnail to achieve peak plasma levels of about one third of that achieved by oral ingestion [107]. Various studies were performed by Nair et al. on transungual delivery of terbinafine [74, 79, 108] and in one of the studies, a 21- and 37- fold enhancement in terbinafine delivery was reported after 1 h (0.5 mA/cm2) of iontophoresis in cadaverous human finger and toe nail, respectively [108]. A 150-fold increase in iontophoretic delivery of terbinafine through folded porcine epidermis as a model of proximal nail fold was demonstrated by Manda et al. [78]. An in vivo transungual iontophoretic experiment enhanced the flux of chloride and sodium ions by 8- and 27- fold, respectively, when compared to passive diffusion [73]. Hao et al. demonstrated an enhancement of 10-fold in delivering ciclopirox olamine through cadaverous human nail plate iontophoretically, when compared to passive transport [54].
Amichai et al. performed maiden clinical trials on iontophoretic delivery of terbinafine in human nails for the treatment of onychomycosis [76] and showed a significant decrease in frequency of patients showing presence of nail fungus at week 12 who wore 1% terbinafine iontophoretic patch (n=19) than compared to the passive treatment group wearing just 1% terbinafine patch (n=16). Amichai et al. attributed the enhanced efficacy of the iontophoretic patch to greater loading of drug in the nail plate, which helped in maintaining the levels of drug above the minimum inhibitory concentration (0.1–1μg/cm2) required to be effective against fungal infection. Two of the patients (out of 19) reported local irritation and discomfort whereas some of the patients reported tingling sensation. The iontophoretic patch treatment was considered to be overall tolerable and safe as none of the patients who reported tingling sensation or discomfort stopped the patch treatment.
Why is the nail fungal infection so difficult to treat?
Although the relative permeability per unit thickness of the nail plate is much higher than the stratum corneum [50], the thickness of the nail plate renders itself as a formidable barrier to anti-fungal drug delivery. In addition, the relapse rate of the patients treated for nail fungal infection can be as high as 50% [90, 93, 95, 96, 98, 99]. There might be multiple factors such as patients’ genetic predisposition, lifestyle and occupation which may lead to this high rate of fungal recurrence. Patients with compromised immune system or with concomitant diseases are more predisposed to onychomycosis and are unlikely to attain a lasting cure [109]. The recurrence could also be associated either with failure to completely treat the infected nail or subsequent re-infection due to exposure to a different fungal strain. Nail fungal infection could also spread within household by being in close proximity with the infected family member. One of the most recently published studies demonstrated that members of the same household were infected with same strain of T. Rubrum [110]. Even if the fungal infection is clinically eradicated, the patient still remains at risk of relapse due to exposure to fungal environment from public showers in gyms and hotel rooms. Thus, one must avoid being barefoot at places where the potential of fungal infection is high. As a preventive measure, one could spray the shoes with a topical anti-fungal spray or treat it with a commercially available UV shoe sanitizer [111] after each use to avoid the re-infection.
Conclusions
Although our knowledge of topical transungual delivery and nail permeability properties has enhanced considerably recently, much information is still unknown like the fine microstructure of the nail plate, structural mathematical model for predicting nail delivery and drug-keratin binding properties. The therapeutic approach to treat the nail fungal infections could possibly be achieved by a combination of topical therapy with physical or chemical enhancement methods. In addition, special precautionary steps must be taken to avoid the recurrence of the nail infection.
Acknowledgments
This work was supported in part by a grant from the NIH (GM063559). The opinions expressed in this paper are those of authors and not endorsed by the NIH. The authors thank Mark Smith at Amway Corporation for providing assistance with nail SEM imaging.
Footnotes
Disclosure Statement
The author declares no conflict of interest.
References
- 1.Holbrook K. Human epidermal embryogenesis. Int J Dermatol. 1979;18:329–85. doi: 10.1111/ijd.1979.18.5.329. [DOI] [PubMed] [Google Scholar]
- 2.Lewis B. Microscopic studies of fetal and mature nail and surrounding soft tissue. AMA Arch Derm Syphilol. 1954;70:733–80. [PubMed] [Google Scholar]
- 3.Bean W. A note on fingernail growth. J Invest Dermatol. 1953;20:27–58. doi: 10.1038/jid.1953.5. [DOI] [PubMed] [Google Scholar]
- 4.Bean W. Nail growth: 30 years of observation. Arch Internal Med. 1974;134:497–999. [PubMed] [Google Scholar]
- 5.Hamilton J, Terada H, Mestler G. Studies of growth throughout the lifespan in Japanese: growth and size of nails and their relationship to age, sex, heredity, and other factors. J Gerontol. 1955;10:401–16. doi: 10.1093/geronj/10.4.401. [DOI] [PubMed] [Google Scholar]
- 6.Zaias N. The nail in health and disease. Springer Science & Business Media; 2012. [Google Scholar]
- 7.Samman PD, Feton D. The nails in disease. London: Williams Heinmann; 1986. [Google Scholar]
- 8.Fleckman P. Structure and function of the nail unit. In: Scher RK, Daniel CR, editors. Nails: therapy, diagnosis, surgery. WB Saunders Company; 1997. pp. 13–23. [Google Scholar]
- 9.Dawber RP, De Berker DR, Baran R. Science of nail apparatus. In: Baran R, Dawber RPR, De Berker D, Haneke E, Tosti A, editors. Baran & Dawber’s Diseases of the Nails and Their Management. Wiley-Blackwell; 2001. pp. 1–47. [Google Scholar]
- 10.Baden HPKJ. The nail. In: Goldsmith LA, editor. Physiology, biochemistry, and molecular biology of the skin. Oxford University Press; 1991. pp. 697–711. [Google Scholar]
- 11.De Berker D, Mawhinney B, Sviland L. Quantification of regional matrix nail production. Br J Dermatol. 1996;134:1083–9. [PubMed] [Google Scholar]
- 12.Kobayashi Y, Miyamoto M, Sugibayashi K, Morimoto Y. Drug permeation through the three layers of the human nail plate. J Pharm Pharmacol. 1999;51:271–9. doi: 10.1211/0022357991772448. [DOI] [PubMed] [Google Scholar]
- 13.Runne U, Orfanos C. The human nail: structure, growth and pathological changes. Curr Prob Dermatol. 1981;9:102–51. [PubMed] [Google Scholar]
- 14.McKittrick J, Chen PY, Bodde SG, Yang W, Novitskaya EE, Meyers MA. The structure, functions, and mechanical properties of keratin. JOM. 2012;64:449–68. [Google Scholar]
- 15.Lynch M, O’Guin W, Hardy C, Mak L, Sun T. Acidic and basic hair/nail (“hard”) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to “soft” keratins. J Cell Bio. 1986;103:2593–606. doi: 10.1083/jcb.103.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzpatrick TB. Dermatology in general medicine. New York: Mcgraw-Hill, Inc; 1993. [Google Scholar]
- 17.Farren L, Shayler S, Ennos A. The fracture properties and mechanical design of human fingernails. J Exp Bio. 2004;207:735–76. doi: 10.1242/jeb.00814. [DOI] [PubMed] [Google Scholar]
- 18.Dawber RP. The ultrastructure and growth of human nails. Arch Dermatol Res. 1980;269:197–204. doi: 10.1007/BF00406540. [DOI] [PubMed] [Google Scholar]
- 19.Gupchup GV, L ZJ. Structural characteristics and permeability properties of the human nail : A review. Anglais. 1999;50:363–85. [Google Scholar]
- 20.Mohorcic M, Torkar A, Friedrich J, Kristl J, Murdan S. An investigation into keratinolytic enzymes to enhance ungual drug delivery. Int J Pharm. 2007;332:196–201. doi: 10.1016/j.ijpharm.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 21.Finlay A, Moseley H, Duggan T. Ultrasound transmission time: an in vivo guide to nail thickness. Br J Dermatol. 1987;117:765–835. doi: 10.1111/j.1365-2133.1987.tb07358.x. [DOI] [PubMed] [Google Scholar]
- 22.Spearman R. The physiology of the nail. In: Arthur J, editor. The Physiology and Pathophysiology of the Skin. Academic Press; 1978. pp. 1812–3665. [Google Scholar]
- 23.Baden HP, Kubilus J. Fibrous proteins of bovine hoof. J Invest Dermatol. 1983;81:220–4. doi: 10.1111/1523-1747.ep12518002. [DOI] [PubMed] [Google Scholar]
- 24.Marshall RC. Characterization of the proteins of human hair and nail by electrophoresis. J Invest Dermatol. 1983;80:519–24. doi: 10.1111/1523-1747.ep12535117. [DOI] [PubMed] [Google Scholar]
- 25.Marshall RC, Orwin DFG, Gillespie JM. Structure and biochemistry of mammalian hard keratin. Electron Microscopy Rev. 1991;4:47–83. doi: 10.1016/0892-0354(91)90016-6. [DOI] [PubMed] [Google Scholar]
- 26.Murdan S. Drug delivery to the nail following topical application. Int J Pharm. 2002;236:1–26. doi: 10.1016/s0378-5173(01)00989-9. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto K. Ultrastructure of the human toenail II. Keratinization and formation of the marginal band. J Ultrastructure Res. 1971;36:391–410. doi: 10.1016/s0022-5320(71)80112-0. [DOI] [PubMed] [Google Scholar]
- 28.Baden HP, Goldsmith LA, Fleming B. A comparative study of the physicochemical properties of human keratinized tissues. Biochimica et Biophysica Acta. 1973;322:269–78. doi: 10.1016/0005-2795(73)90303-6. [DOI] [PubMed] [Google Scholar]
- 29.Gniadecka M, Faurskov Nielsen O, Christensen DH, Wulf HC. Structure of water, proteins, and lipids in intact human skin, hair, and nail. J Invest Dermatol. 1998;110:393–8. doi: 10.1046/j.1523-1747.1998.00146.x. [DOI] [PubMed] [Google Scholar]
- 30.Gunt HB, Miller MA, Kasting GB. Water diffusivity in human nail plate. J Pharm Sci. 2007;96:3352–62. doi: 10.1002/jps.20988. [DOI] [PubMed] [Google Scholar]
- 31.Baden H. The physical properties of nail. J Invest Dermatol. 1970;55:115–37. doi: 10.1111/1523-1747.ep12291628. [DOI] [PubMed] [Google Scholar]
- 32.Heid HW, Moll I, Franke WW. Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. II. Concomitant and mutually exclusive synthesis of trichocytic and epithelial cytokeratins in diverse human and bovine tissues (hair follicle, nail bed and matrix, lingual papilla, thymic reticulum) Differentiation. 1988;37:215–30. doi: 10.1111/j.1432-0436.1988.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 33.Kaneshige T, Takagi K, Nakamura S, Hirasawa T, Sada M, Uchida K. Genetic analysis using fingernail DNA. Nucleic Acids Res. 1992;20:5489–90. doi: 10.1093/nar/20.20.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tahir MA, Watson N. Typing of DNA HLA-DQ alpha alleles extracted from human nail material using polymerase chain reaction. J Forensic Sci. 1995;40:634–6. [PubMed] [Google Scholar]
- 35.Salamon T, Lazovic-Tepavac O, Nikulin A, Grujic M, Plavsic B. Sudan-IV-positive material of the nail plate related to plasma triglycerides. Dermatologica. 1988;176:52–4. doi: 10.1159/000248670. [DOI] [PubMed] [Google Scholar]
- 36.Forslind B. Biophysical studies of the normal nail. Acta Derm Venereol. 1970;50:161–8. [PubMed] [Google Scholar]
- 37.Sirota L, Straussberg R, Fishman P, Dulitzky F, Djaldetti M. X-ray microanalysis of the fingernails in term and preterm infants. Pediatr Dermatol. 1988;5:184–6. doi: 10.1111/j.1525-1470.1988.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs A, Jenkins DJ. The iron content of finger nails. Br J Dermatol. 1960;72:145–8. doi: 10.1111/j.1365-2133.1960.tb13863.x. [DOI] [PubMed] [Google Scholar]
- 39.Wilhelm M, Hafner D, Lombeck I, Ohnesorge FK. Monitoring of cadmium, copper, lead and zinc status in young children using toenails: comparison with scalp hair. Sci Total Environ. 1991;103:199–207. doi: 10.1016/0048-9697(91)90145-5. [DOI] [PubMed] [Google Scholar]
- 40.Nowak B. Occurrence of heavy metals, sodium, calcium, and potassium in human hair, teeth, and nails. Biol Trace Elem Res. 1996;52:11–22. doi: 10.1007/BF02784086. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki S, Inoue T, Hori H, Inayama S. Analysis of methamphetamine in hair, nail, sweat, and saliva by mass fragmentography. J Anal Toxicol. 1989;13:176–8. doi: 10.1093/jat/13.3.176. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki O, Hattori H, Asano M. Nails as useful materials for detection of methamphetamine or amphetamine abuse. Forensic Sci Int. 1984;24:9–16. doi: 10.1016/0379-0738(84)90146-4. [DOI] [PubMed] [Google Scholar]
- 43.Cirimele V, Kintz P, Mangin P. Detection of amphetamines in fingernails: an alternative to hair analysis. Arch Toxicol. 1995;70:68–9. doi: 10.1007/BF03035462. [DOI] [PubMed] [Google Scholar]
- 44.Das D, Chatterjee A, Mandal BK, Samanta G, Chakraborti D, Chanda B. Arsenic in ground water in six districts of West bengal, India: the biggest arsenic calamity in the world. Part 2. Arsenic concentration in drinking water, hair, nails, urine, skin-scale and liver tissue (biopsy) of the affected people. Analyst. 1995;120:917–24. doi: 10.1039/an9952000917. [DOI] [PubMed] [Google Scholar]
- 45.Robson J, el-Tahawi H. Hardness of human nail as an index of nutritional status: a preliminary communication. Br J Nutrition. 1971;26:233–9. doi: 10.1079/bjn19710030. [DOI] [PubMed] [Google Scholar]
- 46.Newman S, Young R. Indentation hardness of the fingernail. J Invest Dermatol. 1967;49:103–8. doi: 10.1038/jid.1967.110. [DOI] [PubMed] [Google Scholar]
- 47.Hao J, Smith KA, Li SK. Time-dependent electrical properties of human nail upon hydration in vivo. J Pharm Sci. 2010;99:107–18. doi: 10.1002/jps.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walters KA, Flynn GL, Marvel JR. Physicochemical characterization of the human nail: solvent effects on the permeation of homologous alcohols. J Pharm Pharmacol. 1985;37:771–5. doi: 10.1111/j.2042-7158.1985.tb04966.x. [DOI] [PubMed] [Google Scholar]
- 49.Walters KA, Flynn GL, Marvel JR. Physicochemical characterization of the human nail: permeation pattern for water and the homologous alcohols and differences with respect to the stratum corneum. J Pharm Pharmacol. 1983;35:28–33. doi: 10.1111/j.2042-7158.1983.tb04258.x. [DOI] [PubMed] [Google Scholar]
- 50.Baswan SM, Li SK, Kasting GB. Diffusion of uncharged solutes through human nail plate. Pharm Dev and Tech. 2016;21:255–60. doi: 10.3109/10837450.2014.991876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baswan S. Pharmaceutical Sciences/Biopharmaceutics. University of Cincinnati; Ohio: 2014. Transport of charged and uncharged solutes in hydrated human nail plate; pp. 53–81. [Google Scholar]
- 52.Delgado-Charro MB. Iontophoretic drug delivery across the nail. Expert Opin Drug Deliv. 2012;9:91–103. doi: 10.1517/17425247.2012.642364. [DOI] [PubMed] [Google Scholar]
- 53.Hao J, Smith KA, Li SK. Chemical method to enhance transungual transport and iontophoresis efficiency. Int J Pharm. 2008;357:61–9. doi: 10.1016/j.ijpharm.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao J, Smith KA, Li SK. Iontophoretically enhanced ciclopirox delivery into and across human nail plate. J Pharm Sci. 2009;98:3608–16. doi: 10.1002/jps.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi Y, Komatsu T, Sumi M, et al. In vitro permeation of several drugs through the human nail plate: relationship between physicochemical properties and nail permeability of drugs. Eur J Pharm Sci. 2004;21:471–7. doi: 10.1016/j.ejps.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Luzardo-Alvarez A, Rodriguez-Fernandez M, Blanco-Mendez J, Guy RH, Delgado-Charro MB. Iontophoretic permselectivity of mammalian skin: characterization of hairless mouse and porcine membrane models. Pharm Res. 1998;15:984–7. doi: 10.1023/a:1011909623019. [DOI] [PubMed] [Google Scholar]
- 57.Mertin D, Lippold BC. In-vitro permeability of the human nail and of a keratin membrane from bovine hooves: influence of the partition coefficient octanol/water and the water solubility of drugs on their permeability and maximum flux. J Pharm Pharmacol. 1997;49:30–4. doi: 10.1111/j.2042-7158.1997.tb06747.x. [DOI] [PubMed] [Google Scholar]
- 58.Mertin D, Lippold BC. In-vitro permeability of the human nail and of a keratin membrane from bovine hooves: prediction of the penetration rate of antimycotics through the nail plate and their efficacy. J Pharm Pharmacol. 1997;49:866–72. doi: 10.1111/j.2042-7158.1997.tb06127.x. [DOI] [PubMed] [Google Scholar]
- 59.Soong MH. Transport properties of drug and model compounds across human nail. University of Minnesota; 1991. [Google Scholar]
- 60.Walters KA, Flynn GL, Marvel JR. Physicochemical characterization of the human nail: I. Pressure sealed apparatus for measuring nail plate permeabilities. J Invest Dermatol. 1981;76:76–9. doi: 10.1111/1523-1747.ep12525318. [DOI] [PubMed] [Google Scholar]
- 61.Walters KA, Flynn GL, Marvel JR. Penetration of the human nail plate: the effects of vehicle pH on the permeation of miconazole. J Pharm Pharmacol. 1985;37:498–9. doi: 10.1111/j.2042-7158.1985.tb03050.x. [DOI] [PubMed] [Google Scholar]
- 62.Baswan S, Kasting GB. Characterization of ion transport across human nail plate. Int J Cosmetic Sci. 2012:360. [Google Scholar]
- 63.Murthy SN, Waddell DC, Shivakumar HN, Balaji A, Bowers CP. Iontophoretic permselective property of human nail. J Dermatol Sci. 2007;46:150–2. doi: 10.1016/j.jdermsci.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Marro D, Guy RH, Delgado-Charro MB. Characterization of the iontophoretic permselectivity properties of human and pig skin. J Control Rel. 2001;70:213–7. doi: 10.1016/s0168-3659(00)00350-3. [DOI] [PubMed] [Google Scholar]
- 65.Burch Ge WT. DIffusion of water through dead plantar, palmar and torsal human skin and through toe nails. Arch Derm. 1946;53:39–41. doi: 10.1001/archderm.1946.01510300042009. [DOI] [PubMed] [Google Scholar]
- 66.Vejnovic I, Huonder C, Betz G. Permeation studies of novel terbinafine formulations containing hydrophobins through human nails in vitro. Int J Pharm. 2010;397:67–76. doi: 10.1016/j.ijpharm.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 67.Baswan SM, Li SK, LaCount TD, Kasting GB. Size and Charge Dependence of Ion Transport in Human Nail Plate. Journal of pharmaceutical sciences. 2016;105:1201–8. doi: 10.1016/j.xphs.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malhotra GG, Zatz JL. Characterization of the physical factors affecting nail permeation using water as a probe. J Cosmet Sci. 2000;51:367–77. [Google Scholar]
- 69.Malhotra GG, Zatz JL. Investigation of nail permeation enhancement by chemical modification using water as a probe. J Pharm Sci. 2002;91:312–23. doi: 10.1002/jps.10058. [DOI] [PubMed] [Google Scholar]
- 70.Jemec G, Agner T, Serup J. Transonychial water loss: relation to sex, age and nail-plate thickness. Br J Dermatol. 1989;121:443–9. doi: 10.1111/j.1365-2133.1989.tb15511.x. [DOI] [PubMed] [Google Scholar]
- 71.Kronauer C, Gfesser M, Ring J, Abeck D. Transonychial water loss in healthy and diseased nails. Acta Derm Venereol. 2001;81:175–7. doi: 10.1080/000155501750376249. [DOI] [PubMed] [Google Scholar]
- 72.Murdan S, Hinsu D, Guimier M. A few aspects of transonychial water loss (TOWL): inter-individual, and intra-individual inter-finger, inter-hand and inter-day variabilities, and the influence of nail plate hydration, filing and varnish. Eur J Pharm Biopharm. 2008;70:684–9. doi: 10.1016/j.ejpb.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 73.Dutet J, Delgado-Charro MB. In vivo transungual iontophoresis: effect of DC current application on ionic transport and on transonychial water loss. J Control Rel. 2009;140:117–25. doi: 10.1016/j.jconrel.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 74.Nair AB, Sammeta SM, Kim HD, Chakraborty B, Friden PM, Murthy SN. Alteration of the diffusional barrier property of the nail leads to greater terbinafine drug loading and permeation. Int J Pharm. 2009;375:22–7. doi: 10.1016/j.ijpharm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Brown MB, Khengar RH, Turner RB, et al. Overcoming the nail barrier: A systematic investigation of ungual chemical penetration enhancement. Int J Pharm. 2009;370:61–7. doi: 10.1016/j.ijpharm.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 76.Amichai B, Nitzan B, Mosckovitz R, Shemer A. Iontophoretic delivery of terbinafine in onychomycosis: a preliminary study. Br J Dermatol. 2010;162:46–50. doi: 10.1111/j.1365-2133.2009.09414.x. [DOI] [PubMed] [Google Scholar]
- 77.Dutet J, Delgado-Charro MB. Electroosmotic transport of mannitol across human nail during constant current iontophoresis. J Pharm Pharmacol. 2010;62:721–9. doi: 10.1211/jpp.62.06.0008. [DOI] [PubMed] [Google Scholar]
- 78.Manda P, Sammeta SM, Repka MA, Murthy SN. Iontophoresis across the proximal nail fold to target drugs to the nail matrix. J Pharm Sci. 2012;101:2392–7. doi: 10.1002/jps.23139. [DOI] [PubMed] [Google Scholar]
- 79.Nair AB, Vaka SR, Sammeta SM, et al. Trans-ungual iontophoretic delivery of terbinafine. J Pharm Sci. 2009;98:1788–96. doi: 10.1002/jps.21555. [DOI] [PubMed] [Google Scholar]
- 80.Narasimha Murthy S, Wiskirchen DE, Bowers CP. Iontophoretic drug delivery across human nail. J Pharm Sci. 2007;96:305–11. doi: 10.1002/jps.20757. [DOI] [PubMed] [Google Scholar]
- 81.Maeda N, Mizuno N, Ichikawa K. Nail abrasion: a new treatment for ingrown toe-nails. J Dermatol. 1990;17:746–9. doi: 10.1111/j.1346-8138.1990.tb03023.x. [DOI] [PubMed] [Google Scholar]
- 82.Behl PN. Abrasion in the treatment of nail disorders. Indian J Dermatol. 1973;18:77–9. [PubMed] [Google Scholar]
- 83.Jeremiasse HP. Treatment of nail infections with griseofulvin combined with abrasion. Trans St Johns Hosp Dermatol Soc. 1960;45:92–3. [PubMed] [Google Scholar]
- 84.Repka MA, Mididoddi PK, Stodghill SP. Influence of human nail etching for the assessment of topical onychomycosis therapies. Int J Pharm. 2004;282:95–106. doi: 10.1016/j.ijpharm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 85.Salter SA, Ciocon DH, Gowrishankar TR, Kimball AB. Controlled nail trephination for subungual hematoma. Am J Emerg Med. 2006;24:875–7. doi: 10.1016/j.ajem.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 86.Murdan S. Enhancing the nail permeability of topically applied drugs. Exp Opin Drug Delv. 2008;5:1267–82. doi: 10.1517/17425240802497218. [DOI] [PubMed] [Google Scholar]
- 87.Kaur R, Kashyap B, Bhalla P. Onychomycosis–epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26:108–16. doi: 10.4103/0255-0857.40522. [DOI] [PubMed] [Google Scholar]
- 88.Faergemann J, Baran R. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol. 2003;149(Suppl 65):1–4. doi: 10.1046/j.1365-2133.149.s65.4.x. [DOI] [PubMed] [Google Scholar]
- 89.Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172–3. [PubMed] [Google Scholar]
- 90.Gupta AK, Jain HC, Lynde CW, Watteel GN, Summerbell RC. Prevalence and epidemiology of unsuspected onychomycosis in patients visiting dermatologists’ offices in Ontario, Canada–a multicenter survey of 2001 patients. Int J Dermatol. 1997;36:783–7. doi: 10.1046/j.1365-4362.1997.00349.x. [DOI] [PubMed] [Google Scholar]
- 91.Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: The frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641–8. doi: 10.1067/mjd.2000.107754. [DOI] [PubMed] [Google Scholar]
- 92.Pierard G. Onychomycosis and other superficial fungal infections of the foot in the elderly: a pan-European survey. Dermatol. 2001;202:220–4. doi: 10.1159/000051640. [DOI] [PubMed] [Google Scholar]
- 93.Botek G. Fungal nail infection: assessing the new treatment options. Cleve Clin J Med. 2003;70:110–4. 7–8. doi: 10.3949/ccjm.70.2.110. [DOI] [PubMed] [Google Scholar]
- 94.Charif MA, Elewski BE. Prevalence of onychomycosis in the United States: results of a population based survey. J Invest Dermatol. 1996;106:892. [Google Scholar]
- 95.Gupta AK, Gupta MA, Summerbell RC, et al. The epidemiology of onychomycosis: possible role of smoking and peripheral arterial disease. J Eur Acad Dermatol Venereol. 2000;14:466–9. doi: 10.1046/j.1468-3083.2000.00124.x. [DOI] [PubMed] [Google Scholar]
- 96.Gupta AK, Konnikov N, MacDonald P, et al. Prevalence and epidemiology of toenail onychomycosis in diabetic subjects: a multicentre survey. Br J Dermatol. 1998;139:665–71. doi: 10.1046/j.1365-2133.1998.02464.x. [DOI] [PubMed] [Google Scholar]
- 97.Dogra S, Kumar B, Bhansali A, Chakrabarty A. Epidemiology of onychomycosis in patients with diabetes mellitus in India. Int J Dermatol. 2002;41:647–51. doi: 10.1046/j.1365-4362.2002.01528.x. [DOI] [PubMed] [Google Scholar]
- 98.Gupta AK, Sibbald RG, Lynde CW, et al. Onychomycosis in children: Prevalence and treatment strategies. J Am Acad Dermatol. 1997;36:395–402. doi: 10.1016/s0190-9622(97)80215-0. [DOI] [PubMed] [Google Scholar]
- 99.Adams B. Personal communication. 2014 Oct 17; [Google Scholar]
- 100.US FDA. Drug approval databases, Penlac Nail Lacquer Topical Solution. 1999 http://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21-022_Penlac.cfm, Accessed: Aug 21, 2016.
- 101.Bhatta AK, Huang X, Keyal U, Zhao JJ. Laser treatment for onychomycosis: a review. Mycoses. 2014;57:734–40. doi: 10.1111/myc.12225. [DOI] [PubMed] [Google Scholar]
- 102.Hochman L. Laser treatment of onychomycosis using a novel 0.65-millisecond pulsed Nd:YAG 1064-nm laser. J cosm laser therapy. 2011;13:2–7. doi: 10.3109/14764172.2011.552616. [DOI] [PubMed] [Google Scholar]
- 103.Watanabe D, Kawamura C, Masuda Y, Akita Y, Tamada Y, Matsumoto Y. Successful treatment of toenail onychomycosis with photodynamic therapy. Arch Dermatol. 2008;144:19. doi: 10.1001/archdermatol.2007.17. [DOI] [PubMed] [Google Scholar]
- 104.FDA Warning Letter. 2014 May; http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2014/ucm396631.htm Accessed: July 25, 2016.
- 105.Hollmig ST, Rahman Z, Henderson MT, Rotatori RM, Gladstone H, Tang JY. Lack of efficacy with 1064-nm neodymium:yttrium-aluminum-garnet laser for the treatment of onychomycosis: A randomized, controlled trial. J American Acad Derm. 2014;70:911–7. doi: 10.1016/j.jaad.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 106.Hees H, Raulin C, Bäumler W. Laser treatment of onychomycosis: an in vitro pilot study. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2012;10:913–7. doi: 10.1111/j.1610-0387.2012.07997.x. [DOI] [PubMed] [Google Scholar]
- 107.James MP, Graham RM, English J. Percutaneous iontophoresis of prednisolone-a pharmacokinetic study. Clinical Exp Dermatol. 1986;11:54–61. doi: 10.1111/j.1365-2230.1986.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 108.Nair AB, Vaka SR, Murthy SN. Transungual delivery of terbinafine by iontophoresis in onychomycotic nails. Drug Deliv Ind Pharm. 2011;37:1253–8. doi: 10.3109/03639045.2011.568946. [DOI] [PubMed] [Google Scholar]
- 109.Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149:5–9. doi: 10.1046/j.1365-2133.149.s65.5.x. [DOI] [PubMed] [Google Scholar]
- 110.Ghannoum MA, Mukherjee PK, Warshaw EM, Evans S, Korman NJ, Tavakkol A. Molecular analysis of dermatophytes suggests spread of infection among household members. Cutis. 2013;91:237–45. [PubMed] [Google Scholar]
- 111.Ghannoum MA, Isham N, Long L. Optimization of an Infected Shoe Model for the Evaluation of an Ultraviolet Shoe Sanitizer Device. J American Pod Med Ass. 2012;102:309–13. doi: 10.7547/1020309. [DOI] [PubMed] [Google Scholar]


