Abstract
Purpose
Amphotericin B (AmB) and 5-fluorocytosine (5-FC) exhibit additive to synergistic activity against systemic mycoses. Incompatibility of prescribed formulations precludes concomitant IV administration, a route with distinct advantages. Previously, we used PEG-DSPE micelles to produce a reformulation of Fungizone (AmB-SD), AmB solubilized by sodium deoxycholate, called mAmB-90. Herein, we describe a second reformulation that facilitates co-delivery of mAmB-90 and 5-FC, and evaluate the effect of PEG-DSPE micelles on the combination’s activity against Candida albicans.
Methods
We assessed the effect of 5-FC addition on the stability, in vitro toxicity, and antifungal efficacy of mAmB-90. The aggregation state and particle size of mAmB-90 combined with 5-FC (FmAmB-90) was evaluated over 48 hours. Hemolytic activity was measured in vitro. Antifungal activity was determined in vitro against C. albicans. The efficacy of monotherapy and combination treatment was evaluated in a neutropenic mouse model of disseminated candidiasis.
Results
The aggregation state, particle size, and hemolytic activity of mAmB-90 were unaffected by 5-FC. While antifungal activity was similar in vitro, mAmB-90 alone and combined with 5-FC was more potent than AmB-SD in vivo.
Conclusions
Short-term stability and in vivo efficacy of our formulation suggest potential to simultaneously deliver AmB and 5-FC for potent antifungal efficacy.
Keywords: 5-fluorocytosine, Aggregation State Hypothesis, Amphotericin B, Candida albicans, PEG-DSPE
Introduction
Amphotericin B (AmB) and 5-fluorocytosine (5-FC) are used in combination to treat life-threatening systemic fungal infections, specifically cryptococcal meningitis and invasive candidiasis (1). The combination of AmB and 5-FC exerts additive to synergistic antifungal activity in vitro (2–6) and is more effective than treatment with AmB alone in clinical trials comparing treatments for cryptococcal meningitis (7). Furthermore, combination therapy both minimizes the nephrotoxicity of AmB by administration of lower doses of the drug for a shorter length of time and reduces development of resistance to 5-FC (8–10).
Broad spectrum antifungal activity is a valued characteristic of AmB, which initiates membrane disruption by binding to ergosterol, a sterol in fungal cell membranes (11). The prodrug 5-FC is converted to its biologically active metabolites by cytosine deaminase, an enzyme present in susceptible fungi but not in human cells (9). In combination, more than one mechanism is postulated to account for the synergy between AmB and 5-FC. Synergism has been attributed to enhanced uptake of 5-FC resulting from AmB-induced membrane disruption (12) and sequential drug action (13). In the United States, 5-FC is dosed orally as a capsule under the trade name Ancobon. Side effects of orally administered 5-FC, including myelosuppression and liver damage, may stem from conversion of the prodrug to 5-fluorouracil, a precursor of DNA and protein synthesis inhibitors, by gut microflora (14, 15). Intravenous (IV) administration of 5-FC may result in less toxicity than oral delivery because it has the potential for less gut exposure and, therefore, reduced nonspecific conversion to 5-FU (16). Additionally, IV administration of 5-FC prevents variation in bioavailability that occurs across different patient populations. An IV formulation of 5-FC is available for clinical use in Europe. Opposing hydrophilic and hydrophobic regions in the structure of AmB cause it to self-aggregate, a property that reduces its selectivity for ergosterol and leads to toxic interactions with mammalian cells (17). The original AmB formulation, Fungizone (AmB-SD), contains highly aggregated AmB solubilized by sodium deoxycholate. Once the gold standard for treating fungal infections, its clinical use is limited by dose-dependent renal toxicity (18–20). Development of monomeric AmB formulations (21–25) and salt supplementation (26, 27) have minimized the drug’s potentially life-threatening toxicity. Previously, we combined these strategies and described a simple method of preparing AmB-SD with micelles of the FDA-approved PEG-lipid poly(ethylene glycol)-distearoylphosphatidylethanolamine (PEG-DSPE) in saline. AmB-SD in a 1:90 mole ratio with PEG-DSPE micelles in saline (mAmB-90) allowed for co-delivery of monomeric AmB and sodium supplementation, a combination that caused significantly less renal toxicity than AmB-SD alone in rats dosed with 2 mg/kg AmB for three days (28).
Currently, combination delivery of AmB-SD and 5-FC by the IV route is precluded by risk of drug precipitation and, consequently, the potential for an embolism. We sought to determine the feasibility of delivering both antifungals via the IV route by adding 5-FC to mAmB-90, producing FmAmB-90 (Figure 1). In line with a perspective of clinical application, our formulation is prepared with 10 mg/ml 5-FC in 0.9% NaCl-USP, the concentration of the drug’s infusion solution clinically available as Ancotil. Additionally, we were interested in comparing the antifungal efficacy of our reformulation containing deaggregated AmB with AmB-SD, both alone and combined with 5-FC, against the clinically relevant pathogen C. albicans. Our assessment, consisting of an examination of physical properties in a short term stability study and evaluation of in vitro and in vivo efficacy, suggests FmAmB-90 can simultaneously deliver both drugs in saline without sacrificing antifungal efficacy and potentially reduce host toxicity relative to current clinical preparations.
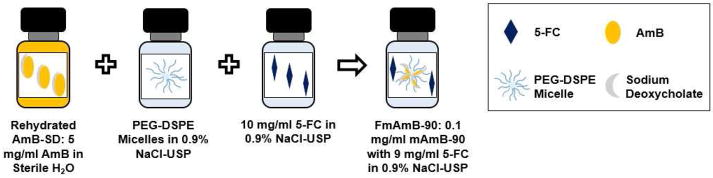
Figure 1.
Preparation of FmAmB-90: AmB-SD is reconstituted according to manufacturer’s instructions, deaggregated with a solution of PEG-DSPE micelles, and added to 5-FC in saline. This results in an aqueous solution containing 0.1 mg/ml AmB, 9 mg/ml 5-FC, and 2 mg/ml PEG-DSPE.
Materials and Methods
Preparation of FmAmB-90
Amphotericin B for Injection USP (X-GEN Pharmaceuticals, Horseheads, NY) was rehydrated with 10 ml sterile water for injection without a bacteriostatic agent USP (Baxter, Deerfield, IL), according to manufacturer’s instructions, resulting in a 5 mg/ml stock solution (Figure 1). PEG-DSPE, specifically 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-5000] (Avanti Polar Lipids, Alabaster, AL), was dissolved in 0.9% NaCl-USP (Baxter, Deerfield, IL) to 25 mg/ml. Combination of both AmB-SD and PEG-DSPE solutions resulted in a 1:90 mole ratio of AmB to PEG-DSPE at a final AmB level of 1 mg/ml. This solution was then diluted 10-fold in 10 mg/ml 5-FC dissolved in saline resulting in a solution of 0.1 mg/ml AmB, 9 mg/ml 5-FC and 2 mg/ml PEG-DSPE.
Degree of aggregation – IV/I ratio determination
The UV-visible spectrum of AmB provides insight into its degree of self-aggregation (29). To evaluate the aggregation state of AmB, samples were diluted 10-fold to a concentration of 10 μg/ml in 0.9% NaCl-USP in a quartz cuvette with a 1 mm path length (Varian, Palo Alto, CA) for UV-visible analysis using a CARY 100 Bio UV-visible spectrophotometer (Varian, Palo Alto, CA). Absorbance spectra were recorded from 300–450 nm. The monomer to aggregate ratio, also referred to as the IV/I ratio, was determined as the ratio of the fourth (~410 nm) and the first peak (~330 nm). Measurements were repeated in triplicate.
Particle size determination via dynamic light scattering
Particle size and polydispersity index (PDI), a measure of particle size distribution, were determined by dynamic light scattering (DLS) using a ZETASIZER Nano-ZS (Malvern Instruments Inc., Worcestershire, UK) with a He-Ne laser light source (4mW, 633 nm) and a 173° angle scattered light collection configuration to determine hydrodynamic diameters. Samples were diluted to a concentration of 20 μg/ml in 0.9% NaCl-USP and allowed to equilibrate to 25°C before DLS analysis. Each sample was analyzed in triplicate, and each replicate was composed of 10 measurements. In order to calculate the Z-average particle diameter and PDI from the Stokes-Einstein equation and correlation function, respectively, the Cumulants analysis method was used to curve fit the correlation function.
Short-Term Stability Study
The degree of aggregation and particle size of FmAmB-90 was monitored over 48 hours at room temperature (~25°C). Samples were stored in the dark throughout the study. Using the methods described above, the monomer to aggregate ratio and particle size of FmAmB-90 were evaluated upon preparation and 1, 24, and 48 hours after preparation. Samples were stored in the dark at room temperature during the experiment. The experiment was performed in triplicate.
Hemolysis assay
To determine the hemolytic activity of different formulations, bovine red blood cells (Innovative Research, Novi, MI) were washed three times in 1X PBS (Corning cellgro, Manassas, VA) and diluted 200-fold in PBS. The negative control (PBS) consisted of cells in a 1:1 dilution with 1X PBS. The positive control sample (TL), in which total lysis of cells was achieved, was prepared with cells in the presence of 25 μg/ml AmB-SD in PBS. The hemolytic activity of 1, 6, and 10 μg/ml AmB as AmB-SD or mAmB-90 was tested in the presence of 50 or 100 μg/ml 5-FC. These concentrations were chosen because they represent a clinically relevant range that could reasonably be detected in a patient undergoing 5-FC therapy. The hemolytic activity of 5-FC alone was also measured. Samples were incubated in a Gyromax 737 (Amerex Instruments, Inc., Concord, CA) at 37°C, shaking at 200 rpm. After 30 minutes of incubation, samples were placed on ice for 5 minutes to halt hemolysis, then centrifuged at 16,000 × g for 30 seconds. Supernatant of each sample was added in triplicate to wells of a 96-well plate and analyzed for absorbance at 405 nm. Experiments were performed in triplicate. Statistical differences between groups were compared using two-way ANOVA with Tukey’s method for multiple comparisons (GraphPad Prism 6, La Jolla, CA). Percent hemolysis was calculated using the following equation:
Candida albicans Strains and Media
Three strains of C. albicans were included in antifungal efficacy studies. One strain (K1) was originally isolated from the bloodstream of a patient suffering from disseminated candidiasis. Strains SC5314 (ATCC® MYA-2876) and Gu5 (ATCC® MYA-574), a fluconazole resistant strain, were also used in the following experiments. Resistance of Gu5 is attributed to enhanced drug efflux (30), and, given the emerging incidence of azole resistance, we felt it would be important to evaluate the efficacy of our combination against this strain. Strains were subcultured on Sabouraud dextrose agar (SDA) plates and grown in yeast peptone dextrose (YPD) medium. SDA was prepared with 1% peptone, 4% glucose, and 1.5% agar in deionized water. YPD media was prepared with 1% yeast extract, 2% peptone, and 2% glucose in deionized water. Autoclaving was used to sterilize media.
Minimum inhibitory concentration (MIC)
To determine the MIC, the lowest concentration of AmB or 5-FC that considerably inhibited visible fungal growth, CLSI M27-A3 guidelines, with modifications, were followed. To prepare the inoculum, fungal strains cultured for approximately 24 hours on SDA plates were adjusted to 1–5×106 CFU/ml in sterile normal saline and diluted 100-fold in YPD. Fungi were added to serial dilutions of drug in 96-well plates. Plates were sealed with Breathe Easier® strips, and C. albicans strains were incubated overnight at 37°C. Results were read by eye and by absorbance with a SpectraMax M2 plate reader (Molecular Devices, Sunnyvale, California) at 580 nm at 24 hours. Treatment with AmB results in obvious MICs, but 5-FC results in trailing growth that can span a large range of concentrations. For 5-FC, the concentration necessary to inhibit ≥50% as determined by absorbance is reported. Experiments were repeated in duplicate on a minimum of three separate occasions. Results are expressed as the geometric mean of the experimental outcomes.
Checkerboard Method
To define the drug interaction between 5-FC combined with either AmB-SD or mAmB-90, the checkerboard method was performed in YPD with a 96-well plate as described by White et al. (31). Columns 1 and 2 were reserved to determine the MIC of AmB and 5-FC alone, respectively. Column 3 was reserved for the media control, and the growth control was plated in column 4. Either AmB-SD or mAmB-90 was serially diluted twofold from column 5 to 12. From a separate 96-well plate containing serially diluted 5-FC, appropriate dilutions of the drug were added to rows A through H. Inoculum was prepared as described above and added to the drug plate. This resulted in concentration ranges of 2–256 μg/ml and 1.56*10−2- 2 μg/ml for 5-FC and AmB, respectively. The fractional inhibitory concentration index (FICI) was used to analyze results of the experiment. FICI was calculated as follows:
FICI values were interpreted as described in a previous study: FICI ≤ 0.5, synergistic; 0.5 < FICI ≤ 1, synergistic to additive; 1 < FICI ≤ 4, indifferent; and FICI > 4, antagonistic (32). After 24 hours of incubation, the average FIC score was calculated from the concentration of drugs present in a well containing considerably inhibited fungal growth. The absence of growth was determined by eye, and absorbance at 580 nm was used to detect wells in which fungal growth was reduced by ≥90%. Experiments were repeated on three separate occasions with distinct 24 hour C. albicans colonies and independent stocks of AmB-SD, 5-FC, and PEG-DSPE. Reported values represent the average of results detected by eye.
Time-Kill Assay
In addition to the checkerboard method, time-kill assays were used to define the relationship between 5-FC and each AmB formulation. Time-kill methods were based on previously described experiments (33). Colonies were adjusted to 1–5 × 106 CFU/ml in sterile normal saline. The yeast was diluted 10-fold in YPD alone or containing drug. Samples in 5 ml test tubes were incubated at 37°C, shaking at a speed of 200 rpm. At 0, 6, 10, 24, and 48 hours, sample was serially diluted 10-fold in sterile normal NaCl, and 20 μl of each dilution was plated on SDA. In cases where less than 12,500 CFU/ml was expected (≤ 25 CFU from the 10-fold dilution), 20 μl was plated directly from the sample tube. The theoretical limit of quantitation for these practices is 1,250 CFU/ml. Colonies were counted after 24 to 48 hours of incubation. Those with CFUs in the countable range as recommended by the United States Pharmacopeia (25–250 CFUs) were included in the dataset. Experiments were performed in duplicate on two separate occasions, and the average CFU/ml was calculated for each replicate. Drug interactions were defined as previously described: a ≥2 log10 decrease in CFU per milliliter for a combination compared to CFU reduction of the most active drug alone as synergy, a <2 log10 increase or decrease in CFU as indifference, and an increase of ≥2 log10 as antagonism (34).
In Vivo Antifungal Efficacy in a Multiple Dose Regimen
The activity of each drug, alone and in combination, was evaluated in a neutropenic mouse model of disseminated candidiasis as described previously by Andes et al (35). Treatment groups were as follows: Control (no drug), 5-FC (72 mg/kg), AmB-SD (2 mg/kg), mAmB-90 (2 mg/kg), AmB-SD+5-FC (2 mg/kg + 72 mg/kg), and mAmB-90+5-FC (2 mg/kg + 72 mg/kg). This study was approved by the Animal Research Committee of the William S. Middleton Memorial VA Hospital (Madison, WI). Briefly, three six-week-old ICR/Swiss specific-pathogen-free female mice (Harlan Sprague Dawley, Madison, WI) per treatment group were rendered neutropenic by multiple doses of cyclophosphamide. Animals were classified as neutropenic when polymorphonuclear leukocyte counts dropped below 100/mm3. Infection was established by injecting 100 μl inoculum into the lateral tail vein. The inoculum was prepared by subculturing K1 on SDA plates for 24 hours at 35°C. Six colonies were dispersed into 5 ml of sterile pyrogen-free normal saline warmed to 35°C and injected two hours prior to drug treatment. Antifungals (200 μl) were dosed intraperitoneally on the day of infection and 24 hours after the initial dose. Because 5-FC causes AmB-SD to precipitate, two separate 200 μl injections were administered to the AmB-SD+5-FC group. The animals were euthanized by CO2 asphyxiation 48 hours after the initial drug treatment. Their kidneys were immediately harvested and homogenized in 4°C sterile saline. Kidney homogenate was diluted 10-fold serially and aliquots were plated on SDA. After 24 hours of incubation at 35°C, colonies were counted. Results were expressed as the mean and standard deviation of the difference in viable fungal counts between the control group at zero hour (two hours post-infection) and each group at the 48 hour timepoint. These data represent three mice (six kidneys). Statistical differences between groups were compared using one-way ANOVA with Tukey’s multiple comparisons test (GraphPad Prism 6, La Jolla, CA). We acknowledged statistical significance when p-values were less than or equal to 0.05.
Results
Short-Term Stability Study
Deaggregation of AmB-SD by PEG-DSPE Micelles in 0.9% NaCl-USP with 5-FC
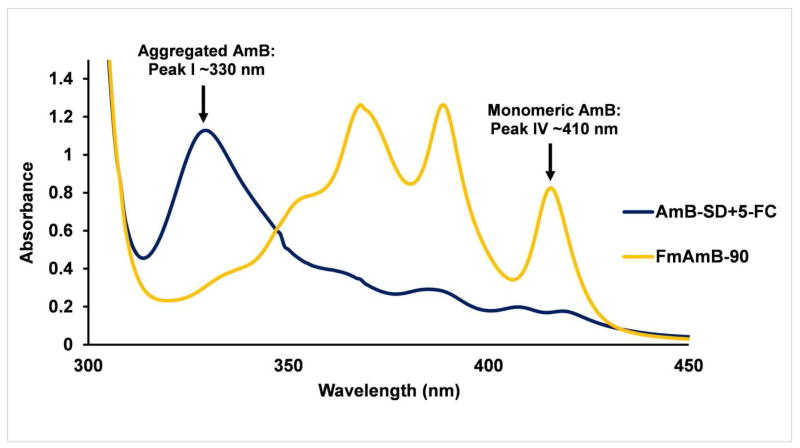
Figure 2 illustrates the absorbance spectra of AmB-SD+5-FC and FmAmB-90 upon preparation. At each time point, the average monomer to aggregate ratio of AmB-SD combined with 5-FC was less than 0.3, indicating a highly aggregated state (data not shown). Previously, we determined that the IV/I ratio of AmB-SD alone was less than 0.20 (28), so here we report a slightly less aggregated state in the presence of 9 mg/ml 5-FC. PEG-DSPE micelles deaggregated AmB-SD in normal saline containing 5-FC (Table I). At preparation, the monomer to aggregate ratio of FmAmB-90 was roughly 3.3. After one hour, the monomer to aggregate ratio rose to 3.7. A similar degree of aggregation was observed at 24 and 48 hours. These data are consistent with results from prior evaluations of mAmB-90 alone (28).
Figure 2.
Absorbance spectra of AmB-SD+5-FC and FmAmB-90. Each solution containing 0.1 mg/ml AmB and 9 mg/ml 5-FC was diluted 10-fold for UV-vis analysis.
Table I.
Physical properties of FmAmB-90 over 48 hours at 25°C
| Time (h) | Monomer to Aggregate Ratioa | Z-Average Particle Sizeb (d. nm) | PDI |
|---|---|---|---|
| 0 | 3.3 ± 0.064 | 25 ±0.6 | 0.088 |
| 1 | 3.7 ± 0.066 | 23 ±0.2 | 0.049 |
| 24 | 4.0 ± 0.16 | 24 ±0.9 | 0.15 |
| 48 | 3.9 ± 0.069 | 23 ±0.3 | 0.08 |
Sample was diluted ten-fold in saline for UV-vis analysis. Data is presented as mean ± standard deviation (n=3).
Sample was diluted five-fold in saline for DLS analysis. Data is presented as mean ± standard deviation (n=3).
Particle Size Determination
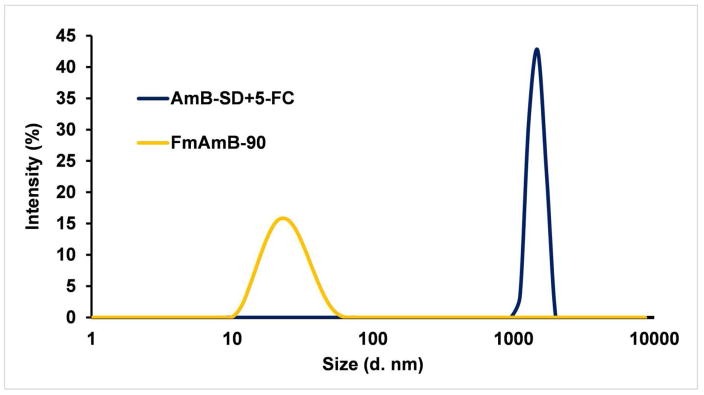
In Figure 3, we present the comparative particle sizes of AmB-SD+5-FC and FmAmB-90. At preparation, AmB-SD combined with 5-FC formed micron-sized particles (~3 μm). Addition of PEG-DSPE micelles markedly reduced particle size. The particle size of FmAmB-90 was approximately 25 nm throughout the course of 48 hours (Table I). The PDI did not exceed 0.15 at any observed timepoint throughout the stability study’s duration, indicating a narrow size distribution.
Figure 3.
Particle size of AmB-SD combined with 5-FC and FmAmB-90 upon preparation. Each sample was diluted five-fold in saline for DLS analysis.
In Vitro Hemolytic Activity
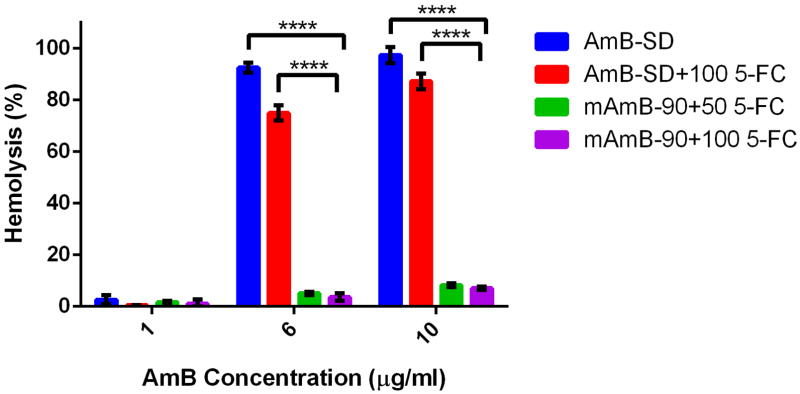
The concentration range of 5-FC from 25–100 μg/ml exhibited negligible hemolytic activity of <0.4% (data not shown). The hemolytic activity of the different AmB formulations with 5-FC is shown in Figure 4. The original formulation, AmB-SD, exhibited near total hemolysis at 6 and 10 μg/ml AmB in the presence of 100 μg/ml 5-FC. Although highly hemolytic, this was significantly less activity than AmB-SD alone (p <0.0001). Hemolytic activity did not exceed 10% at any tested concentration when 5-FC (50 and 100 μg/ml) was combined with 1, 6, and 10 μg/ml AmB as mAmB-90. The hemolytic activity of mAmB-90 with 5-FC is comparable to that of mAmB-90 alone in the same concentration range (28). At 6 and 10 ug/ml, AmB-SD with 100 μg/ml 5-FC exhibited at least 10 times the hemolytic activity measured from either combination of mAmB-90 and 5-FC (p <0.0001).
Figure 4.
Contrasted hemolytic activity after 30 min of exposure to up to 10 μg/ml AmB as either AmB-SD or mAmB-90 combined with 5-FC. Concentrations of 5-FC in are expressed in μg/ml. Data is presented as mean ± standard deviation (n=3) (p <0.0001).
In Vitro Antifungal Efficacy
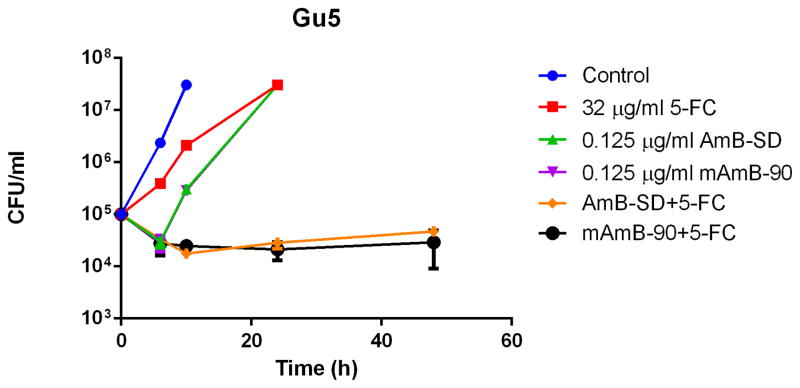
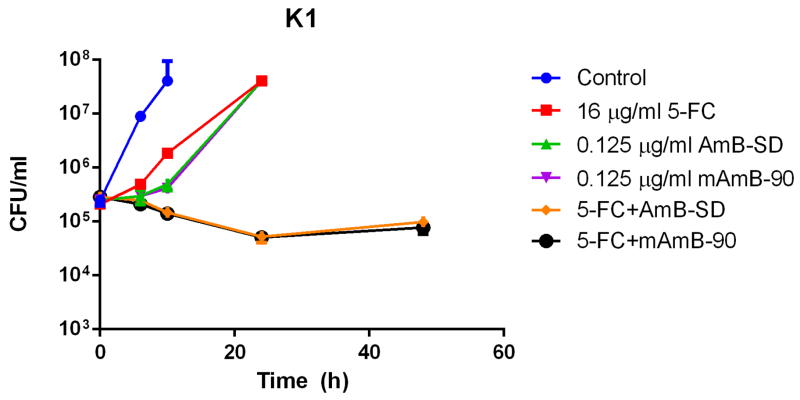
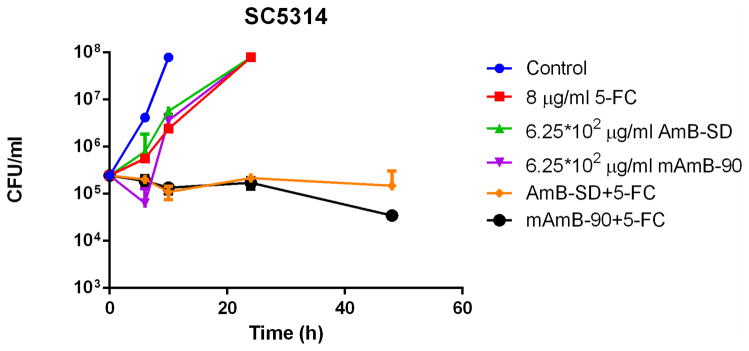
Addition of PEG-DSPE micelles to AmB-SD did not alter its fungicidal efficacy in MIC (Table II) and checkerboard experiments (Table III). An additive to synergistic relationship between both AmB formulations and 5-FC against each fungal strain in this study was indicated by FICI values of less than one. Time-kill experiments with C. albicans Gu5 (5A), K1 (5B), and SC5314 (5C) clearly showed a synergistic relationship between 5-FC and both AmB-SD and mAmB-90 (Figure 5). Similar trends were noted for each strain, suggesting usefulness of the combination against fluconazole-resistant strains. In each case, the growth control was turbid with growth at 10 hours. At 0.25 and 0.5 times the MIC of 5-FC and AmB, each single-drug treated sample was turbid with growth at 24 hours (data not shown). Combination treatment with drug concentrations equal to one-fourth of the highest documented MIC greatly reduced fungal burden.
Table II.
Minimum inhibitory concentrations of 5-FC, AmB-SD, and mAmB-90 alone or in combination against C. albicans
MIC values are expressed as the geometric mean and given in μg/ml (n=3).
Reported MIC values for 5-FC represent levels that inhibited greater than or equal to 50% of growth compared to the control (no drug).
Reported MIC values for AmB represent levels that inhibited greater than or equal to 90% of growth compared to the control.
Table III.
Fractional inhibitory concentration index of 5-FC, AmB-SD, and mAmB-90 alone or in combination against C. albicans
FICI values are expressed as the mean (n=3).
Highest tested drug levels: 2 μg/ml AmB and 256 μg/ml 5-FC
Figure 5.
Log10 CFU/milliliter versus time of C. albicans exposed to subinhibitory concentrations ¼ * MIC) of 5-FC, AmB-SD, and mAmB-90, either alone or in combination. Data is expressed as mean μ standard deviation (n=2). Strain Gu5 (5A) was exposed to 0.125 μg/ml AmB and 32 μg/ml 5-FC, K1 (5B) was treated with 0.125 μg/ml AmB and 16 μg/ml 5-FC, and SC5314 (5C) was dosed with 6.25*10−2 μg/ml AmB and 8 μg/ml 5-FC.
In Vivo Antifungal Efficacy
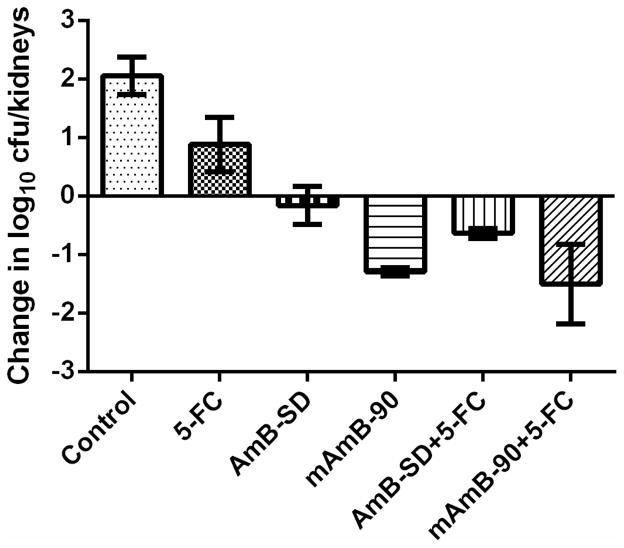
Each treatment resulted in a significantly lower fungal burden compared to the control (Figure 6). The weakest effect was noted in the group that received 72 mg/kg 5-FC. At 48 hours, the fungal burden of the control group was 1.2 times that of the 5-FC treated group (p=0.027). The greatest reduction in fungal burden was caused by treatment with mAmB-90+5-FC, which had a mean difference from the control of approximately 3.56 log10 CFUs (p <0.0001). This greatest mean difference was seconded by mAmB-90, with a mean difference of 3.35 log10 CFUs (p <0.0001). Therefore, treatment with mAmB-90 was not significantly different from combination therapy using our reformulation, mAmB-90 (p=0.98). Similarly, treatment with AmB-SD+5-FC did not result in a significantly different fungal burden relative to treatment with AmB-SD alone (p=0.67). Using the aforementioned guide to determining drug interactions, the relationship between AmB and 5-FC was defined as indifferent at the tested concentrations, as the difference in fungal burden between the most active drug (AmB) and combination treatment was less than 2 log10 CFU.
Figure 6.
Relative to the control at zero hour (two hours post-infection), change in log10 CFU/kidneys of C. albicans K1 in a neutropenic murine model of disseminated candidiasis is expressed in response to no drug (Control) and five treatment groups: 5-FC (72 mg/kg), AmB-SD (2 mg/kg), mAmB-90 (2 mg/kg), 5-FC+AmB-SD (72 mg/kg + 2 mg/kg), and 5-FC+mAmB-90 (72 mg/kg + 2 mg/kg). Results presented as mean ± standard deviation (n=3).
However, mAmB-90+5-FC was more effective than AmB-SD monotherapy. Viable colonies after AmB-SD therapy exceeded those detected after mAmB-90+5-FC treatment by a factor of 1.5 (p=0.011). Interestingly, mAmB-90 was significantly more effective than AmB-SD at the same dose of 2 mg/kg. At the 48 hour endpoint, the fungal burden of AmB-SD was 1.4 times that of mAmB-90 (p=0.034). Treatment with AmB-SD+5-FC resulted in a fungal burden 1.3 times that following mAmB-90+5-FC treatment. Combination therapy with mAmB-90 reduced fungal burden to 2 log10 CFUs, the lowest detected colony count in the data set. However, data collected from mAmB-90+5-FC treated group was the most variable, with the highest range in the dataset of 1.3 log10 CFUs. In this study, combination treatment with mAmB-90 was not significantly different from AmB-SD combined with 5-FC (p=0.14).
Discussion
Intravenous Coadministration of AmB and 5-FC
Herein, we have studied the effect of 5-FC on the antifungal efficacy and in vitro toxicity of mAmB-90. Additionally, we introduced FmAmB-90, an intravenous formulation that concurrently delivers 5-FC, monomeric AmB, and saline for sodium supplementation, a clinical measure practiced to mitigate AmB-related renal toxicity. As mentioned previously, 5-FC is orally administered in the United States, but an infusion solution for IV administration is approved for use in Europe. Each delivery mode poses challenges to safe and effective therapy. Diverse health profiles of patient populations lead to variation in oral bioavailability, while cost and strict storage requirements (18–25°C) are issues that complicate use of IV 5-FC in resource-poor settings. Furthermore, as toxicity is associated with 5-FC serum concentrations upwards of 100 mg/L, time-consuming and costly serum concentration monitoring is recommended (36–38). Any desire to combine the currently available formulations is rightfully discouraged by AmB-SD and 5-FC preparation instructions that clearly state each infusion solution should not be mixed with other drugs. From DLS measurements, we show that AmB-SD precipitates in the presence of 5-FC in saline (Figure 3), resulting in micron-sized particles that could cause an embolism. However, deaggregation of AmB and a particle size of less than 30 nm were maintained when our reformulation of AmB-SD was combined with 5-FC (28).
Although costly, IV delivery of 5-FC offers distinct advantages over oral administration. Systemic fungal infections affect a diverse patient population with great variability in underlying host immunity and disease. In otherwise healthy patient populations, oral bioavailability is quite high. However, different disease states can influence the oral bioavailability of 5-FC. For instance, people living with HIV/AIDS are especially prone to developing life-threatening fungal infections. The common incidence of achlorhydria, the reduction or absence of hydrochloric acid secretion in the stomach, in HIV/AIDS patients can affect drug absorption. The relatively low bioavailability of 5-FC (45%) reported by a study on treatment options for HIV patients suffering from cryptococcal meningitis may be attributed to achlorhydria (39). The same study reported detection of 5-fluorouracil more frequently in patients given the oral dosage form than those who received IV 5-FC, supporting the hypothesis that the prodrug can be metabolized by gut microorganisms. Delivering 5-FC via the IV route prevents unpredictable bioavailability, and, at certain doses in patients without renal insufficiency, could remove the need for serum concentration monitoring (39). It also presents an alternative option to treat neonates, patients who are unable to swallow medication, and patients who are allergic to excipients in Ancobon, i.e. patients to whom oral delivery may not be feasible.
Short-Term Stability
PEG-DSPE micelles deaggregated AmB-SD in the presence of saline and 5-FC to a similar degree of mAmB-90 without 5-FC (average monomer to aggregate ratio of approximately 3.5). To put this degree of aggregation into context, completely monomeric AmB in DMSO has a monomer to aggregate ratio of approximately 7. Thus, 5-FC addition did not impact the ability of PEG-DSPE micelles to deaggregate AmB-SD. Interestingly, FmAmB-90 continued to deaggregate over time. Previously, we proposed that PEG-DSPE micelles interact with soluble aggregates of AmB-SD, forming mixed micelles with sodium deoxycholate that sequester monomeric AmB to the micelle core containing DSPE. As 5-FC is highly hydrophilic and has a low molecular weight (129 g/mol), it is unlikely that the drug disrupts this process; it is most likely outside the micelles or perhaps interacts with the micelle corona containing the hydrophilic PEG brush and polar region of sodium deoxycholate. Further supporting our assertion that 5-FC interacts largely with the hydrophilic regions of the system, addition of 5-FC did not impact the size of mAmB-90. An identical study was attempted at the elevated temperature of 45°C (data not shown). After only one hour, FmAmB-90 became highly aggregated, with an average monomer to aggregate ratio of <0.9. Strengthened hydrophobic interaction at this high temperature may explain the return to the aggregated state.
In Vitro Hemolysis Assay
Addition of 5-FC did not alter the hemolytic profiles of mAmB-90 at 1, 6, or 10 μg/ml AmB. However, reduced hemolytic activity was noted when 100 μg/ml 5-FC was added to AmB-SD, possibly reflecting precipitated AmB that is non-hemolytic. The hemolytic activity of mAmB-90 alone was comparable to that combined with 50 and 100 μg/ml 5-FC.
Antifungal Efficacy
The MICs of 5-FC we report are higher than those determined in the CLSI-recommended medium RPMI (40). As Steier et al. suggested, this may stem from competition for cytosine in YPD (41). As our primary purpose for studying in vitro antifungal activity was to compare different formulations, not identify clinical breakpoints of susceptibility and resistance, YPD was a suitable alternative for our studies. Addition of PEG-DSPE micelles did not affect the antifungal activity of AmB-SD alone or in combination with 5-FC in in vitro experiments. No differences in activity were seen in long-term 24 hour end-point assays, i.e. MIC and the checkerboard experiment. Although the mean fungal burden is consistently lower after treatment with mAmB-90 in time-kill experiments with 48 hours of drug exposure, the difference in fungicidal activity between mAmB-90 and AmB-SD is not significant in most cases. A limitation to these in vitro studies is that cells experience equal exposure of each antifungal drug. The half-life of each drug is very different in humans, resulting in long-term exposure to AmB and relatively short-term exposure to 5-FC due to rapid renal clearance.
The static activity of AmB-SD in vivo against K1 is consistent with previous findings of Andes et al. In a study relating dosing intervals to efficacy, the fungistatic dose range associated with a 24 hour dosing interval was 2.01 to 2.79 mg/kg AmB-SD (35). The enhanced activity of mAmB-90 in vivo compared to AmB-SD is surprising but is possibly explained by the aggregation state hypothesis. The enhanced potency of deaggregated AmB may stem from a contribution of each of the following explanations: 1. Monomeric AmB is selective for ergosterol, meaning it avoids nonspecific interactions with mammalian cells; 2. In addition to not engaging with mammalian cells, monomeric AmB does not heavily associate with itself, potentially leading to a higher effective concentration, as more drug molecules are available to bind to ergosterol. Additionally, it has been posited that monomeric AmB interacts with serum components differently from the aggregated form, the implications of which may lead to altered pharmacokinetics (42).
In a similar in vivo study with the infection model presented here, Adams et al. compared the fungicidal activity of AmB-SD with that of AmB encapsulated in poly(ethylene oxide)-block-poly(N-hexyl-L-aspartamide)-stearic acid micelles. Relative to AmB-SD, the micelle formulation was only slightly less aggregated, with a IV/I ratio of approximately 0.8. No significant difference in efficacy was detected between either formulation at 0.2, 0.3, and 0.6 mg/kg AmB (43). Keeping in mind that the monomer to aggregate ratio of mAmB-90 is roughly 3.5, the aggregation state of the two micellar formulations is quite different. It is possible that the extent of aggregation influences efficacy and that formulations with similar aggregation states will exert comparable antifungal activity in vivo. Additionally, differences in efficacy may not have been detectable at these doses.
Previously, Diezi and Kwon reported the physical characteristics of a PEG-DSPE micelle loaded with cholesterol and AmB in a 20:5:1 mole ratio (44). This formulation, referred to from here on as MAmB, was predominately monomeric, similar to mAmB-90. In an animal study identical to the one presented, two doses of 150 mg/kg 5-FC and 2.7 m/kg MAmB separated by 24 hours reduced K1 by a mean 3.9 log10 CFUs relative to the control at zero hour. In two out of three animals infected with either K1 or 98–17, another bloodstream isolate of C. albicans, viable fungal growth was reduced below the limit of detection, 2 log10 CFUs (unpublished data).
Although different doses and dosing frequencies are examined in a similar infection model, previous work by Andes et al. provides a useful context in which to interpret these data. The pharmacodynamic parameter indicative of best outcome, i.e. greatest extent of infection clearance, was determined for 5-FC (40) and AmB (35). Time above the MIC was the optimal parameter for 5-FC, suggesting a frequent dosing regimen. In K1-infected mice, the greatest reduction in fungal burden (about 2.3 log10 CFUs) resulted from a total dose 400 mg/kg 5-FC at 24 hours with a 3 hour dosing interval. The parameter predictive of best outcome from AmB therapy was the ratio of peak serum concentration to the MIC, indicating a dosing schedule of large doses separated by long intervals. At a 72 hour endpoint, fungal burden dropped to the limit of detection after one dose in excess of 10 mg/kg AmB.
Combination therapy may alter the parameter best predictive of treatment success. It is also possible that infection clearance by suboptimal doses, administered at an interval unrepresentative of best outcomes, may be attributed to enhanced activity of monomeric AmB or an additive to synergistic drug relationship. The possibility remains that, had a higher dose of 5-FC been administered along with mAmB-90, an additive or synergistic relationship may have been determined in the present study. In the future, we are interested in further exploring the antifungal efficacy of combination therapy by varying exposure times of AmB and 5-FC in an in vitro dynamic time-kill model and, potentially, identify exposure times of each drug that result in optimal fungicidal activity.
Conclusions
Instead of three separate infusions, FmAmB-90 allows for delivery of both antifungals and sodium supplementation in a single infusion. Our reformulation is quickly and simply prepared by mixing aqueous solutions. Theoretically, it can be easily reproduced in any compounding pharmacy. Although storage requirements and necessity for equipment to safely administer FmAmB-90 are barriers to use in resource-poor settings, IV delivery of 5-FC is an attractive treatment option in that it offers predictable bioavailability and, at appropriate doses, has potential to reduce the incidence of hepatotoxicity and myelosuppression. Potent fungicidal activity, ease of preparation, and commercial availability of formulation components indicate potential for evaluation in clinical trials.
Acknowledgments
This work was supported by the NIH (R01 AI-43346). C. Alvarez was supported by the NSF-GRFP. C. Krug was supported by the Youth Apprenticeship Program in Biotechnology, an important workforce development initiative of Dane County. Special thanks to Drs. Tim Bugni and Christina Hull for providing C. albicans K1 and C. albicans Gu5 and SC5314, respectively.
Abbreviations
- 5-FC
5-fluorocytosine
- AmB
Amphotericin B
- AmB-SD
Fungizone
- FICI
Fractional Inhibitory Concentration Index
- MIC
Minimum Inhibitory Concentration
- PEG-DSPE
Poly(ethylene glycol)-distearoylphosphatidylethanolamine
- PDI
Polydispersity Index
- mAmB-90
Reformulated Fungizone
- FmAmB-90
Reformulated Fungizone Combined with 5-fluorocytosine
- SDA
Sabouraud Dextrose Agar
- SD
Sodium Deoxycholate
- TL
Total Lysis
- YPD
Yeast Peptone Dextrose
References
- 1.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of america. clinical infectious diseases. 2016;62(4):409–17. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medoff G, Comfort M, Kobayashi GS. Synergistic action of amphotericin B and 5-fluorocytosine against yeast-like organisms. Proceedings of the Society for Experimental Biology and Medicine. 1971;138(2):571. doi: 10.3181/00379727-138-35943. [DOI] [PubMed] [Google Scholar]
- 3.Montgomerie JZ, Edwards JE, Guze LB. Synergism of amphotericin B and 5-fluorocytosine for Candida species. Journal of Infectious Diseases. 1975;132(1):82–6. doi: 10.1093/infdis/132.1.82. [DOI] [PubMed] [Google Scholar]
- 4.Odds FC. Interactions among amphotericin B, 5-fluorocytosine, ketoconazole, and miconazole against pathogenic fungi in vitro. Antimicrobial Agents and Chemotherapy. 1982;22(5):763–70. doi: 10.1128/aac.22.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scalarone GM, Mikami Y, Kurita N, Ichihara Y, Yazawa K, Miyaji M. Turbidometric characterization of the postantifungal effect - Comparative studies with amphotericin B, 5-fluorocytosine and miconazole on Candida albicans. Mycoses. 1991;34(7–8):297–302. doi: 10.1111/j.1439-0507.1991.tb00663.x. [DOI] [PubMed] [Google Scholar]
- 6.Scalarone GM, Mikami Y, Kurita N, Yazawa K, Miyaji M. Comparative studies on the postantifungal effect produced by the synergistic interaction of flucytosine and amphotericin B on Candida albicans. Mycopathologia. 1992;120(3):133–8. doi: 10.1007/BF00436389. [DOI] [PubMed] [Google Scholar]
- 7.Larsen RA, Leal MAE, Chan LS. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS - A randomized trial. Annals of Internal Medicine. 1990;113(3):183–7. doi: 10.7326/0003-4819-113-3-183. [DOI] [PubMed] [Google Scholar]
- 8.Stamm AM, Diasio RB, Dismukes WE, Shadomy S, Cloud GA, Bowles CA, et al. Toxicity of amphotericin B plus flucytosine in 194 patients with cryptococcal meningitis. American Journal of Medicine. 1987;83(2):236–42. doi: 10.1016/0002-9343(87)90691-7. [DOI] [PubMed] [Google Scholar]
- 9.Bennett JE. Flucytosine. Annals of Internal Medicine. 1977;86(3):319–22. doi: 10.7326/0003-4819-86-3-319. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum MA. Combination treatment of invasive fungal infections. Clinical Microbiology Reviews. 2005;18(1):163–94. doi: 10.1128/CMR.18.1.163-194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(7):2234–9. doi: 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medoff G, Kobayash Gs, Venkov P, Schlessi D, Kwan CN. Potentiation of rifampicin and 5-fluorocytosine as antifungal antibiotics by amphotericin B. Proceedings of the National Academy of Sciences of the United States of America. 1972;69(1):196–9. doi: 10.1073/pnas.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beggs WH, Sarosi GA. Further evidence for sequential action of amphotericin B and 5-fluorocytosine against Candida albicans. Chemotherapy. 1982;28(5):341–4. doi: 10.1159/000238101. [DOI] [PubMed] [Google Scholar]
- 14.Harris BE, Manning BW, Federle TW, Diasio RB. Conversion of 5-fluorocytosine to 5-fluorouracil by human intestinal microflora. Antimicrobial Agents and Chemotherapy. 1986;29(1):44–8. doi: 10.1128/aac.29.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermes A, Kuijper EJ, Guchelaar HJ, Dankert J. An in vitro study on the active conversion of flucytosine to fluorouracil by microorganisms in the human intestinal microflora. Chemotherapy. 2003;49(1–2):17–23. doi: 10.1159/000069784. [DOI] [PubMed] [Google Scholar]
- 16.Vermes A, Guchelaar HJ, van Kuilenburg ABP, Dankert J. 5-fluorocytosine-related bone-marrow depression and conversion to fluorouracil: A pilot study. Fundamental & Clinical Pharmacology. 2002;16(1):39–47. doi: 10.1046/j.1472-8206.2002.00064.x. [DOI] [PubMed] [Google Scholar]
- 17.Barwicz J, Christian S, Gruda I. Effects of the aggregation state of amphotericin B on its toxicity to mice. Antimicrobial Agents and Chemotherapy. 1992;36(10):2310–5. doi: 10.1128/aac.36.10.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DRJ, et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clinical Infectious Diseases. 2001;32(5):686–93. doi: 10.1086/319211. [DOI] [PubMed] [Google Scholar]
- 19.Ostrosky-Zeichner L, Marr KA, Rex JH, Cohen SH. Amphotericin B: Time for a new “gold standard”. Clinical Infectious Diseases. 2003;37(3):415–25. doi: 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- 20.Gallis HA, Drew RH, Pickard WW. Amphotericin-B - 30 years of clinical experience. reviews of infectious diseases. 1990;12(2):308–29. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 21.Gruda I, Dussault N. Effect of the aggregation state of amphotericin B on its interaction with ergosterol. Biochemistry and Cell Biology-Biochimie Et Biologie Cellulaire. 1988;66(3):177–83. doi: 10.1139/o88-024. [DOI] [PubMed] [Google Scholar]
- 22.Gruda I, Milette D, Brother M, Kobayashi GS, Medoff G, Brajtburg J. Structure-activity study of inhibition of amphotericin B (Fungizone) binding to sterols, toxicity to cells, and lethality to mice by esters of sucrose. Antimicrobial Agents and Chemotherapy. 1991;35(1):24–8. doi: 10.1128/aac.35.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tancrede P, Barwicz J, Jutras S, Gruda I. the effect of surfactants on the aggregation state of amphotericin B. Biochimica Et Biophysica Acta. 1990;1030(2):289–95. doi: 10.1016/0005-2736(90)90305-8. [DOI] [PubMed] [Google Scholar]
- 24.Diezi TA, Takemoto JK, Davies NM, Kwon GS. Pharmacokinetics and nephrotoxicity of amphotericin B-incorporated poly(ethylene glycol)-block-poly(n-hexyl stearate l-aspartamide) micelles. Journal of Pharmaceutical Sciences. 2011;100(6):2064–70. doi: 10.1002/jps.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu BG, Okano T, Kataoka K, Sardari S, Kwon GS. In vitro dissociation of antifungal efficacy and toxicity for amphotericin B-loaded poly(ethylene oxide)-block-poly( beta-benzyl-L-aspartate) micelles. Journal of Controlled Release. 1998;56(1–3):285–91. doi: 10.1016/s0168-3659(98)00095-9. [DOI] [PubMed] [Google Scholar]
- 26.Branch RA. Prevention of amphotericin B induced renal impairment - A review on the use of sodium supplementation. Archives of Internal Medicine. 1988;148(11):2389–94. [PubMed] [Google Scholar]
- 27.Llanos A, Cieza J, Bernardo J, Echevarria J, Biaggioni I, Sabra R, et al. Effect of salt supplementation on amphotericin B nephrotoxicity. Kidney International. 1991;40(2):302–8. doi: 10.1038/ki.1991.214. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez C, Shin DH, Kwon GS. Reformulation of fungizone by PEG-DSPE micelles: deaggregation and detoxification of amphotericin B. Pharm Res. 2016;33(9):2098–106. doi: 10.1007/s11095-016-1948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolard J, Seigneuret M, Boudet G. Interaction between phospholipid-bilayer membranes and the polyene antibiotic amphotericin B - lipid state and cholesterol content dependence. Biochimica Et Biophysica Acta. 1980;599(1):280–93. doi: 10.1016/0005-2736(80)90074-7. [DOI] [PubMed] [Google Scholar]
- 30.Franz R, Ruhnke M, Morschhauser J. Molecular aspects of fluconazole resistance development in Candida albicans. Mycoses. 1999;42(7–8):453–8. doi: 10.1046/j.1439-0507.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- 31.White RL, Burgess DS, Manduru M, Bosso JA. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E test. Antimicrobial Agents and Chemotherapy. 1996;40(8):1914–8. doi: 10.1128/aac.40.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arikan S, Lozano-Chiu M, Paetznick V, Rex JH. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrobial Agents and Chemotherapy. 2002;46(1):245–7. doi: 10.1128/AAC.46.1.245-247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klepser ME, Ernst EJ, Lewis RE, Ernst ME, Pfaller MA. Influence of test conditions on antifungal time-kill curve results: Proposal for standardized methods. Antimicrobial Agents and Chemotherapy. 1998;42(5):1207–12. doi: 10.1128/aac.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canton E, Peman J, Gobernado M, Viudes A, Espinel-Ingroff A. Synergistic activities of fluconazole and voriconazole with terbinafine against four Candida species determined by checkerboard, time-kill, and Etest methods. Antimicrobial Agents and Chemotherapy. 2005;49(4):1593–6. doi: 10.1128/AAC.49.4.1593-1596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andes D, Stamsted T, Conklin R. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrobial Agents and Chemotherapy. 2001;45(3):922–6. doi: 10.1128/AAC.45.3.922-926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodwin ML, Drew RH. Antifungal serum concentration monitoring: an update. Journal of Antimicrobial Chemotherapy. 2008;61(1):17–25. doi: 10.1093/jac/dkm389. [DOI] [PubMed] [Google Scholar]
- 37.Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: Established and emerging indications. Antimicrobial Agents and Chemotherapy. 2009;53(1):24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasqualotto AC, Howard SJ, Moore CB, Denning DW. Flucytosine therapeutic monitoring: 15 years experience from the UK. Journal of Antimicrobial Chemotherapy. 2007;59(4):791–3. doi: 10.1093/jac/dkl550. [DOI] [PubMed] [Google Scholar]
- 39.Brouwer AE, van Kan HJM, Johnson E, Rajanuwong A, Teparrukkul P, Wuthiekanun V, et al. Oral versus intravenous flucytosine in patients with human immunodeficiency virus-associated cryptococcal meningitis. Antimicrobial Agents and Chemotherapy. 2007;51(3):1038–42. doi: 10.1128/AAC.01188-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andes D, van Ogtrop M. In vivo characterization of the pharmacodynamics of flucytosine in a neutropenic murine disseminated candidiasis model. Antimicrobial Agents and Chemotherapy. 2000;44(4):938–42. doi: 10.1128/aac.44.4.938-942.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steier Z, Vermitsky JP, Toner G, Gygax SE, Edlind T, Katiyar S. Flucytosine antagonism of azole activity versus candida glabrata: Role of transcription factor Pdr1 and multidrug transporter Cdr1. Antimicrobial Agents and Chemotherapy. 2013;57(11):5543–7. doi: 10.1128/AAC.02394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aramwit P, Bong GY, Lavasanifar A, Samuel J, Kwon GS. The effect of serum albumin on the aggregation state and toxicity of amphotericin B. J Pharm Sci. 2000;89(12):1589–93. doi: 10.1002/1520-6017(200012)89:12<1589::aid-jps10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Adams ML, Andes DR, Kwon GS. Amphotericin B encapsulated in micelles based on poly(ethylene oxide)-block-poly(L-amino acid) derivatives exerts reduced in vitro hemolysis but maintains potent in vivo antifungal activity. Biomacromolecules. 2003;4(3):750–7. doi: 10.1021/bm0257614. [DOI] [PubMed] [Google Scholar]
- 44.Diezi TA, Kwon G. Amphotericin B/sterol co-loaded peg-phospholipid micelles: Effects of sterols on aggregation state and hemolytic activity of amphotericin B. Pharmaceutical Research. 2012;29(7):1737–44. doi: 10.1007/s11095-011-0626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]